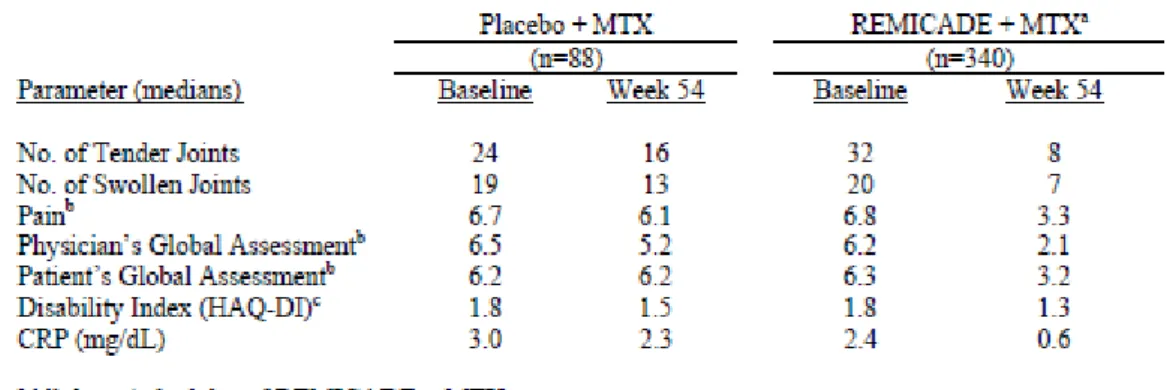レミケード点滴静注用 100
に関する資料
本資料に記載された情報に係る権利及び内容の責任は田辺
三菱製薬株式会社に帰属するものであり,当該情報を適正使
用以外の営利目的に利用することはできません.
田辺三菱製薬株式会社
1.5 起原又は発見の経緯及び開発の経緯
レミケード
Ⓡ
点滴静注用
100
製造販売承認事項一部変更承認申請書
添付資料
第
1 部(モジュール 1)
1.5 起原又は発見の経緯及び開発の経緯
田辺三菱製薬株式会社
1.5 起原又は発見の経緯及び開発の経緯
略語・略号一覧
略語・略号
略していない表現(英語)
略していない表現(日本語)
ATI
Antibodies to infliximab
抗インフリキシマブ抗体
CRP C-Reactive
Protein
C-反応性蛋白
CT Computed
tomography
コンピューター断層検査
ESR
Erythrocyte sedimentation rate
赤血球沈降速度
HLA Human
Leukocyte
Antigen
ヒト白血球抗原
IgG Immunoglobulin
G
免疫グロブリン
G
IL-6 Interleukin-6
インターロイキン
-6
MICA
MHC class I polypeptide-related sequence A
-
MRI
Magnetic resonance imaging
核磁気共鳴画像法
MTX Methotrexate
メトトレキサート
PET
Positron emission tomography
ポジトロン断層法
QOL
Quality of life
生活の質
SF-36
36-item short form health survey
-
TNFα
Tumor necrosis factor-alpha
腫瘍壊死因子
α
VAS Visual
Analog
Scale
-
1.5 起原又は発見の経緯及び開発の経緯
1.5 起原又は発見の経緯及び開発の経緯
1.5.1 起原又は発見の経緯
インフリキシマブ(以下,本剤)は米国セントコア社(現ヤンセンバイオテック社)によ
り遺伝子組換え技術を用いて創製された抗ヒト腫瘍壊死因子(以下,TNF)α モノクローナ
ル抗体であり,ヒト
TNFα に特異的なマウス由来の抗原結合可変領域と,ヒト IgG1,κ アイ
ソタイプ抗体の定常領域を有する.本剤は
TNFα が関与する種々の炎症性疾患に対して,
TNFα の中和及び膜結合型 TNFα の発現細胞の傷害により効果を発揮する.
本剤は
1998 年以降,クローン病,関節リウマチ,強直性脊椎炎,尋常性乾癬,乾癬性関節
炎,膿疱性乾癬,乾癬性紅皮症,潰瘍性大腸炎及びベーチェット病による難治性網膜ぶどう
膜炎の治療薬として,
2014 年 8 月時点で本邦を含め世界 105 ヶ国で承認され,約 221 万人に
投与されている.国内では
2002 年 1 月にクローン病寛解導入について承認されて以降,2003
年
7 月に関節リウマチ,2007 年 1 月にベーチェット病による難治性網膜ぶどう膜炎,2007
年
11 月にクローン病維持療法,2009 年 7 月に関節リウマチにおける関節の構造的損傷の防
止,2010 年 1 月に乾癬,2010 年 4 月に強直性脊椎炎,2010 年 6 月に潰瘍性大腸炎の治療薬
として承認されている.
更に,関節リウマチについては 2009 年 7 月に 10 mg/kg までの増量
及び
4 週間隔までの投与間隔の短縮,クローン病については 2011 年 8 月に 10 mg/kg までの
増量が承認されている.
近年,欧米では既に使用が認められているが,国内では承認されていない医薬品や適応(以
下「未承認薬・適応外薬」
)について,いわゆるドラッグラグの解消が望まれていた.
このドラッグラグ解消の一つの施策として,これら未承認薬・適応外薬に対して要望の公
募が行われ,これらの医療上の必要性を評価するとともに,企業に対して現時点でのエビデ
ンスによる公知申請の該当性や承認申請のために追加で実施が必要な試験計画を提出させ,
その妥当性を確認する「医療上の必要性の高い未承認薬・適応外薬検討会議」
(以下,検討会
議)が発足し,製薬企業による未承認薬・適応外薬の開発促進が図られることになった.
ベーチェット病の治療については生活指導と共に,薬物療法としては治療の対象になる病
態の重症度及び後遺症を残す可能性の有無により,治療の優先順位を決め治療法を選択する
こととされており,腸管型ベーチェット病,神経型ベーチェット病及び血管型ベーチェット
病ではいろいろな後遺症を残すことがあるため,それぞれの病型に応じた治療がなされてい
る
[1]
.一方,既存治療では効果不十分な患者が存在し,治療に難渋しているのが現状であ
る.また,広く使用されている副腎皮質ホルモン剤(以下,ステロイド)についてはステロ
イド依存例も存在しており,ステロイド長期投与について臨床上の問題となっている.以上
のように,腸管型,神経型及び血管型ベーチェット病を取り巻く治療環境は十分ではなく,
このような背景より,本剤に対し厚生労働省難治性疾患克服事業 ベーチェット病に関する
調査研究班などより「ベーチェット病の特殊型(腸管型,神経型,血管型)
」の効能で開発の
要望がなされた.
1.5 起原又は発見の経緯及び開発の経緯
本要望に対して,
「医療上の必要性に係る基準」への該当性に関する専門作業班では,
適
応疾患の重篤性及び医療上の有用性について,ベーチェット病の特殊型(腸管型,神経型,
血管型)は生命に重大な影響がある疾患(致死的な疾患)であり,既存の療法が国内にない
と評価された.
この専門作業班の評価を基に,第
6 回検討会議で医療上の必要性が高いと評価され,医療
現場で早期に使用することができるように
2010 年 12 月 13 日付で開発要請「未承認薬・適応
外薬の開発の要請について」
(平成
22 年 12 月 13 日付医政研発 1213 第 1 号,薬食審査発 1213
第
1 号)を受けたことから腸管型,神経型及び血管型ベーチェット病患者を対象とした臨床
試験を計画した.
1.5.1.1 腸管型,神経型及び血管型ベーチェット病の病態とその治療
1.5.1.1.1 病態
1.5.1.1.1.1 概念
ベーチェット病は,原因不明の多臓器侵襲性の炎症性疾患である.
4 つの主症状(口腔粘
膜の再発性アフタ性潰瘍,皮膚症状,眼症状,外陰部潰瘍)と
5 つの副症状(変形や硬直を
伴わない関節炎,副睾丸炎,回盲部潰瘍で代表される消化器病変,血管病変,中等度以上の
中枢神経病変)からなっており,経過中に
4 つの主症状が出現したものは完全型とされてい
る.経過中に
3 主症状,あるいは 2 主症状と 2 副症状が出現したもの,経過中に定型的眼症
状とその他の
1 主症状,あるいは 2 副症状が出現したものが不全型とされている
[
2]
.副症
状のうち,腸管,中枢神経,血管の病変が前面に立ち,治療上も優先度が高い場合はそれぞ
れ腸管型,神経型,血管型ベーチェット病とされ,予後が悪いことが知られている
[
3]
.
1.5.1.1.1.2 病因
難病情報センターのホームページによると,ベーチェット病の病因は以下のように考えら
れている.
病因は未だ不明であるが,疾患の発症には遺伝素因と環境因子(外因)の双方が重要であ
ると考えられている.
トルコを中心に家族内集積も報告されているが,
ほとんどが孤発例で,
日本では家族例が特に多いわけではない.
ベーチェット病ではヒト白血球抗原
: Human Leukocyte Antigen (以下,HLA)-B51 の陽性
率が高く,発病に
HLA-B51 そのもの,あるいはこれに連鎖する素因の役割が重視されてい
る.実際,日本人の
HLA-B51 保有者でも,ベーチェット病に罹患する相対危険率は 7.9 と極
めて高い.
HLA-B51 以外にも,HLA-A26 ほか MHC class I polypeptide-related sequence A (以
下,
MICA)などいくつかの遺伝子多型と疾患の関連が報告されている.
1.5 起原又は発見の経緯及び開発の経緯
免疫異常や好中球機能過剰をはじめとした自然免疫系の異常を引き起こし,発症にいたると
考えられている
[
1]
.
また,ベーチェット病と
TNFα に関して,ベーチェット病患者の末梢血単核球及び結節性
紅斑様皮疹の病変部,腸管型ベーチェット病患者の腸管生検組織で
TNFα 産生が亢進してい
ることや,神経型ベーチェット病患者では髄液中の
TNFα 濃度が高値を示すことが報告され
ており,ベーチェット病の病態に共通して
TNFα が関与していることが示唆されている
[
4]
[
5]
.
なお,抗ヒト
TNFα モノクローナル抗体製剤である本剤は,ベーチェット病による難治性
網膜ぶどう膜炎について医薬品製造販売承認されている.
1.5.1.1.1.3 症状・診断
ベーチェット病は多臓器侵襲性の疾患であり,症状や臨床経過は多様である.難病情報セ
ンターのホームページによると,腸管型,神経型及び血管型ベーチェット病についても,以
下のとおり症状や臨床経過は多様である.腸管型,神経型及び血管型ベーチェット病につい
ては,現在,ベーチェット病に関する調査研究班により,それぞれ腸管ベーチェット病診療
コンセンサス・ステートメント(原因不明小腸潰瘍症の実態把握,疾患概念,疫学,治療体
系の確率に関する研究班との共同作成)
,神経ベーチェット病の診療のガイドライン及び血管
型ベーチェット病診療ガイドラインステートメント案が作成されているが,国際的な診断基
準はない
[
1][6][7][8]
.
1.5.1.1.1.3.1 消化器病変
腹痛,下血,下痢などが主な症状である.病変の好発部位は回盲部末端から盲腸にかけて
であり,打ち抜き型の潰瘍性病変を特徴とし,多発することが多い.このほか食道から直腸
にいたるまでどこにでも病変が生じうる.鑑別診断としてクローン病などの炎症性腸疾患が
特に問題となる.腸管穿孔,腸管出血,時に穿孔性腹膜炎により,緊急手術を要することも
ある.また,術後の縫合不全など合併症や病変の再発を起こしやすく,手術を繰り返し,短
腸症候群となることがあり,予後は悪い
[
1][6][9]
.
1.5.1.1.1.3.2 中枢神経病変
ベーチェット病の症状の中で最も遅発性で男性に多い.大きく髄膜炎,脳幹脳炎として急
性型と片麻痺,小脳症状,錐体路症状など神経症状に認知症などの精神症状をきたす慢性進
行型に大別される.また,急性型で発症し,慢性進行型へ移行する場合もある.
MRI で脳幹
部,大脳皮質などに病変を認め,髄液所見では細胞増多,蛋白増加を認める.一般に神経症
状は遅発性とされてきたが,最近増加しているシクロスポリン治療に伴う急性型は,比較的
発症早期にも出現する
[
1]
.
1.5 起原又は発見の経緯及び開発の経緯
急性型は発熱を伴った髄膜脳炎の型をとり,片麻痺や脳神経麻痺など様々な脳局所徴候を
伴うことが多く,急性発作の治療と予防が重要となる.障害部位は
MRI の T2 強調画像やフ
レアー画像において高信号域として抽出される.
慢性進行型は治療反応性に乏しく,若年で認知症や性格変化をきたし,認知症・精神症状
や構語障害,運動失調が出現し,これが徐々に進行し,廃人同様となることがあり,悲惨な
転帰をたどるため,予後は不良である.
MRI では局所の T2 高信号域は必ずしも認められず,
大脳,小脳及び脳幹の萎縮を認めることが特徴的である.脳幹の萎縮は診断から
2 年以内に
顕著に進行することが報告されており,発症後期と比較して発症早期に急速に進行すること
が示唆されている
[
10][11]
.
1.5.1.1.1.3.3 血管病変
病変は静脈系,動脈系のいずれにも生じる.静脈病変のうち,頻度の高い表在性血栓性静
脈炎は皮膚症状に含まれるため,静脈病変として取り上げられるのは深部静脈血栓である.
症状としては血栓による還流障害が主体で,上大静脈症候群や
Budd-Chiari 症候群,時に肺塞
栓が発生することがあり,致死的な転帰をたどることもある.動脈病変としては大動脈をは
じめ中型から大型の動脈に血栓性閉塞や動脈瘤を形成する.また,肺循環系にも病変は出現
し,肺動脈瘤は致命的な喀血の原因となりうる.動脈病変のうち,動脈瘤を形成した場合,
ベーチェット病による血管の脆弱性により動脈瘤は破裂を起こすことがあり,その際は緊急
手術となる.緊急手術時のリスクは,胸部大動脈瘤や腹部大動脈瘤では死亡率は高く,四肢
の動脈瘤では死亡率は低いものの四肢切断の可能性がある.また,吻合部に動脈瘤を形成す
る頻度が高く,手術後にもリスクがあり,予後は不良である
[
1][12]
.
1.5.1.1.2 治療
腸管型,神経型及び血管型ベーチェット病では,国際的な治療方針はないが,それぞれの
病型における治療指針がベーチェット病に関する調査研究班により示されている.
1.5.1.1.2.1 腸管型ベーチェット病の治療
腹痛,下痢,下血などの消化器症状及び全身症状の強い場合,内視鏡や
X 線造影で深掘れ
の潰瘍が確認された場合,寛解導入療法としてステロイドの投与を考慮する.あるいはイン
フリキシマブ,アダリムマブの投与を行う.軽症~中等症の患者には
5-アミノサリチル酸製
剤(以下,5-ASA 製剤)が有効な場合がある.維持療法としては 5-ASA 製剤やコルヒチンを
使用してもよい.治療抵抗性の患者に対してはアザチオプリンなどの免疫調節薬の投与を考
慮する.そのほか,全身症状が強い症例や経口摂取が困難な症例に対しては完全静脈栄養療
法が用いられる.外科治療は内科治療で改善が期待できない病態に適応がある
[6]
.
1.5 起原又は発見の経緯及び開発の経緯
1.5.1.1.2.2 神経型ベーチェット病の治療
神経型(急性型)ベーチェット病の急性期の治療として中等量以上のステロイド投与が行
われる.シクロスポリン投与によって誘発された神経型(急性型)ベーチェット病はシクロ
スポリン中止でほぼ完全に抑制される.シクロスポリンと無関係に生じた神経型(急性型)
ベーチェット病の発作予防としてはコルヒチンが推奨される.ベーチェット病に関する調査
研究班によるガイドラインでは,インフリキシマブの発作予防効果については今後検討する
必要があるとされている.
神経型(慢性進行型)ベーチェット病に対しては,メトトレキサート(以下,
MTX)を使
用し,効果不十分の場合にはインフリキシマブを追加併用する.ステロイド,アザチオプリ
ン,シクロホスファミド,コルヒチンはいずれも無効である
[
7]
.
1.5.1.1.2.3 血管型ベーチェット病の治療
致死的となりうる動脈瘤破裂,肺血管よりの出血を回避し,血管病変に伴う諸症状を緩和
することを治療目標とする.動脈瘤,肺動脈瘤の急性期には高用量のステロイドを使用し,
症状の軽快を確認しつつ減量する.免疫調節薬併用による治療成績の向上が報告されており,
シクロホスファミド,MTX,アザチオプリン,シクロスポリンなどを積極的に併用する.生
物学的製剤による治療は症例報告レベルで有効例が散見されており,今後の治療オプション
として期待される.外科的手術は術後合併症が少なくないことから,免疫抑制療法を優先す
るが,救命的緊急手術として実施される
[8]
.
1.5.1.1.2.4 治療のまとめ
以上,これら腸管型,神経型及び血管型ベーチェット病に対する治療は,ステロイドや免
疫調節薬などが中心的に使用されている.ベーチェット病に対し,ステロイドは適応を有し
ており,アダリムマブは腸管ベーチェット病に適応を有しているが神経型ベーチェット病及
び血管型ベーチェット病に対する適応は有していない.シクロホスファミド及びアザチオプ
リンは「難治性リウマチ性疾患」の効能があるため適応を有していると考えられるが,多く
の薬剤が適応外使用されているのが現状である.また,既存治療の効果が十分であるとは言
えず,十分な治療効果が得られない患者や,忍容性に問題があるために治療を受けられない
患者が存在することは重大な問題である.以上のように,重篤な疾患である腸管型,神経型
及び血管型ベーチェット病に対して治療環境が整備されているとは言い難い(
表 1.5-1
)
.
ベーチェット病は,眼症状や腸管病変,中枢神経病変,血管病変を有している場合には特
に予後が悪い.眼症状については本剤が承認されたことにより視力予後の改善が期待される
が,腸管型,神経型及び血管型ベーチェット病については十分な治療法が存在せず種々の後
1.5 起原又は発見の経緯及び開発の経緯
1.5 起原又は発見の経緯及び開発の経緯
表
1.5-1 腸管型,神経型及び血管型ベーチェット病の治療及び承認状況
[
6][7][8]
薬剤
承認状況
備考
腸管型
薬物療法
メサラジン
適応外
コルヒチン
保険適応
「保険診療における医薬品の取扱い
について(昭和
55 年 9 月 3 日付保発
第
51 号厚生省保険局長通知)」に基づ
く保険適応あり
プレドニゾロン(経口)
保険適応
「ベーチェット病(眼症状のない場
合)
」として承認
アザチオプリン
保険適応
「難治性リウマチ性疾患」として承認
アダリムマブ
保険適応
「腸管型ベーチェット病」として承認
インフリキシマブ
適応外
「ベーチェット病による難治性網膜
ぶどう膜炎」の承認あり
栄養療法
完全静脈栄養
適応外
経腸栄養
適応外
神経型
急性型
プレドニゾロン(静注)
適応外
プレドニゾロン(経口)
保険適応
ベーチェット病(眼症状のない場合)
として承認
コルヒチン
保険適応
「保険診療における医薬品の取扱い
について(昭和
55 年 9 月 3 日付保発
第
51 号厚生省保険局長通知)」に基づ
く保険適応あり
慢性進行型
MTX
適応外
インフリキシマブ
適応外
「ベーチェット病による難治性網膜
ぶどう膜炎」の承認あり
血管型
プレドニゾロン(経口)
保険適応
「ベーチェット病(眼症状のない場
合)
」として承認
シクロホスファミド
保険適応
「難治性リウマチ性疾患」として承認
アスピリン
適応外
ワルファリン
適応外
MTX
適応外
アザチオプリン
保険適応
「難治性リウマチ性疾患」として承認
1.5.2 開発の経緯
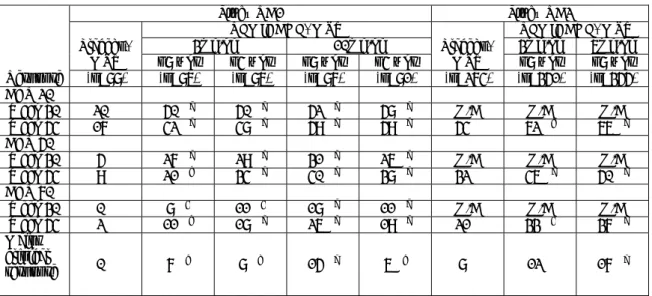
本剤のベーチェット病における臨床試験と承認状況を
図 1.5-1
に,品質・非臨床試験及び
ベーチェット病以外の臨床試験と承認状況を
図 1.5-2
に,本申請に関するベーチェット病臨
床試験の概要を
表 1.5-2
に示す.
本申請に際し,品質に関する試験,薬物動態試験及び毒性試験は新たに実施していない.
なお本剤は,2012 年 9 月 13 日に特殊型ベーチェット病(腸管型,神経型,血管型)に対
し希少疾病用医薬品に指定されている.
1.5 起原又は発見の経緯及び開発の経緯
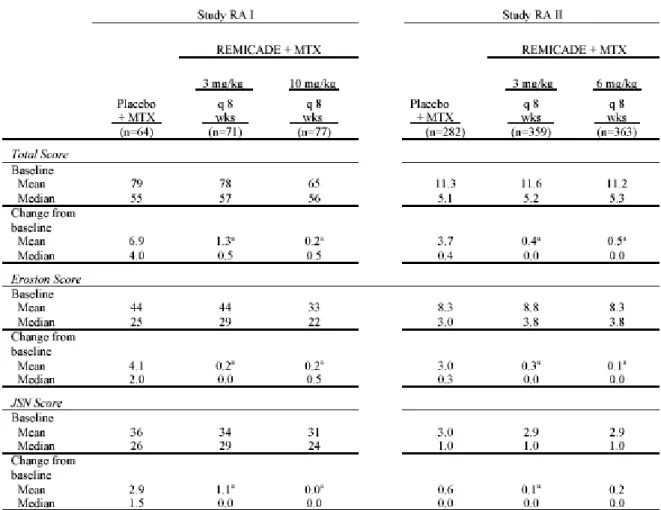
1.5.2.2.2 有効性及び安全性のまとめ
本治験より,腸管型,神経型及び血管型ベーチェット病患者に対する本剤の有効性及び安
全性について,臨床的に意義のある効果及び忍容性が確認され,有用であると考えられた.
また,効果減弱が認められた患者において
10 mg/kg を 8 週間隔で投与した場合に改善が認め
られ,5 mg/kg 投与時の安全性を大きく上回ることはなく,増量投与の意義が確認されてい
る関節リウマチ及びクローン病と同様に,本治験でも有用であると考えられた.
腸管型,神経型及び血管型ベーチェット病患者に対する本剤使用の症例報告及び公表文献
等においても,有用性が確認された[
表
2.5.4.5―2
][
表
2.5.4.5―4
][
表
2.5.4.5―6
].
1.5.2.2.3 薬物動態
次に,本治験における薬物動態の検討を行った.
腸管型,神経型及び血管型ベーチェット病患者の血清中インフリキシマブ濃度は既承認疾
患と比較して若干高いものの,個別値はいずれも,国内で実施された臨床試験で投与量及び
投与間隔が本治験と同じ他疾患(クローン病,乾癬,潰瘍性大腸炎及びベーチェット病によ
る難治性網膜ぶどう膜炎)における濃度範囲内(最小値~最大値)であった.また,有害事
象の発現率は既承認と比較して大きな差は認められなかった.
30 週以降に効果の減弱が認め
られ,本剤の投与が
5 mg/kg から 10 mg/kg に増量された被験者では,いずれの被験者におい
ても,投与終了
1 時間後及びトラフにおける血清中インフリキシマブ濃度は増量することに
より用量に依存して上昇した.
更に,ATI については,94.4%(17/18)の被験者において定量限界値以上の血清中インフ
リキシマブが検出されたため評価することができなかった.評価することができた
1 名は陰
性であり,陽性は認められなかった.
以上のことから,既承認である他疾患と同様であり,薬物動態による新たな安全性上の懸
念はないと考えられた.
1.5.3 申請効能・効果及び用法・用量
以上のように,腸管型,神経型及び血管型ベーチェット病患者に対し,本剤治療により臨
床的に意義のある効果が示され,安全性や薬物動態の点からも既承認疾患と同程度の忍容性
が示されたことから,以下の効能・効果,用法・用量で本剤の製造販売承認事項一部変更承認
申請を行うこととした.
1.5 起原又は発見の経緯及び開発の経緯
効能・効果 既存治療で効果不十分な下記疾患
腸管型ベーチェット病、神経型ベーチェット病、血管型ベーチェット病
用法・用量
<腸管型ベーチェット病、神経型ベーチェット病、血管型ベーチェット病>
通常、体重
1kg 当たり 5mg を 1 回の投与量とし点滴静注する。初回投与後、2 週、6
週に投与し、以後
8 週間の間隔で投与を行うこと。なお、6 週の投与以後、効果が不十
分又は効果が減弱した場合には、体重
1kg 当たり 10mg を 1 回の投与量とすることがで
きる。
1.5.4 参考文献
[
1] 難病情報センター[Internet]. 東京: 公益財団法人 難病医学研究財団. 1997[cited 2014
Sep 3]. Available from: http://www.nanbyou.or.jp/entry/330[
資料番号:
5.4.3
]
[
2] 石ヶ坪良明,岳野光洋,黒沢美智子,大野重昭,蕪城俊克,水木信久,他.臨床調査
個人票の改定案:厚生労働科学研究費補助金 難治性疾患克服研究事業 ベーチェッ
ト病に関する調査研究 平成
20~22 年度 総括・分担研究報告書.2011 Mar. p. 29-42.
[
資料番号:
5.4.1
]
[
3] ベーチェット病研究班[Internet]. 横浜: 厚生労働省科学研究費補助金 ベーチェット
病 に 関 す る 調 査 研 究 事 務 局
. [cited 2014 Oct 2]. Available from:
http://www-user.yokohama-cu.ac.jp/~behcet/patient/behcet/care.html[
資料番号:
5.4.2
]
[
4] 鈴木登. ベーチェット病の病態形成における Th1 細胞の関与に関する研究:厚生労
働科学研究費補助金(難治性疾患克服研究事業)ベーチェット病に関する調査研究
平成
16 年度 総括・分担研究報告書. 2005 Mar. p. 35-8. [
資料番号:
5.4.9
]
[
5] 鈴木登, 黒川真奈絵, 奈良和彦, 吉川英志, 松田隆秀, 野中信宏, 他. ベーチェット病
における免疫異常及び炎症病態に関する研究
: 厚生労働科学研究費補助金(難治性疾
患克服研究事業)ベーチェット病に関する調査研究 平成
17~19 年度総合研究報告
書
. 2008 Mar. p. 43-51.[
資料番号:
5.4.10
]
[
6] 厚生労働科学研究費補助金(難治性疾患克服研究事業)ベーチェット病に関する調査
研究班,厚生労働科学研究費補助金(難治性疾患克服研究事業)原因不明の小腸潰瘍
症の実態把握,疾患概念,疫学,治療体系の確立に関する研究班
. 腸管ベーチェット
病診療コンセンサス・ステートメント.
2013 年 9 月 1 日改訂.[
資料番号:
5.4.4
]
[
7] 厚生労働科学研究費補助金(難治性疾患等克服研究事業(難治性疾患克服研究事業))
ベーチェット病に関する調査研究班
. 神経ベーチェット病の診療のガイドライン.平
成
25 年 12 月.[
資料番号:
5.4.11
]
[
8] 厚生労働科学研究難治性疾患等克服研究事業(難治性疾患克服研究事業)ベーチェッ
ト病に関する調査研究班
. 血管型ベーチェット病診療ガイドラインステートメント
案.平成
26 年 2 月 23 日.[
資料番号:
5.4.12
]
1.5 起原又は発見の経緯及び開発の経緯
[
9] 鈴木温,関下芳明,塩野恒夫,藤森勝,加藤紘之. 穿孔性腹膜炎を呈した腸管ベーチ
ェット病の
3 手術例-特にステロイド使用,腸瘻増設の有用性について-. 日消外会
誌
. 2002;35(12): 1817-20. [
資料番号:
5.4.5
]
[
10] 廣畑俊成. 炎症性疾患に伴う神経障害 ベーチェット病. Clinical Neuroscience.
2011;29:234-5. [
資料番号:
5.4.6
]
[11] Kikuchi H, Takayama M, Hirohata S. Quantitative analysis of brainstem atrophy on magnetic
resonance imaging in chronic progressive neuro-Behçet’s disease. J Neurol Sci. 2014;
337:80-5. [
資料番号:
5.4.7
]
レミケード
Ⓡ
点滴静注用
100
製造販売承認事項一部変更承認申請書
添付資料
第
1 部(モジュール 1)
1.6 外国における使用状況等に関する資料
田辺三菱製薬株式会社
1.6 外国における使用状況等に関する資料
2
目次
1.6
外国における使用状況等に関する資料
... 3
1.6.1 外国における使用状況 ... 3
1.6.2 米国添付文書 ... 4
1.6.2.1 米国添付文書 原文 ... 5
1.6.2.2 米国添付文書 和訳概要 ... 21
1.6.3 EU 添付文書... 54
1.6.3.1 EU 添付文書 原文 ... 55
1.6.3.2 EU 添付文書 和訳概要 ... 91
1.6.4 Company Core Data Sheet ... 135
1.6 外国における使用状況等に関する資料
1.6 外国における使用状況等に関する資料
1.6.1
外国における使用状況
本剤は,2014 年 8 月現在,本邦を含む 105 ヶ国において承認を取得している.
これらの国のうち,米国の添付文書の原文と和訳概要を
1.6.2.1
及び
1.6.2.2
に,また
EU の
添付文書の原文と和訳概要を
1.6.3.1
及び
1.6.3.2
に示す.
また,ヤンセンバイオテック社による本剤の
Company Core Date Sheet を
1.6.4
に示す.
なお,現時点で,海外において腸管型,神経型及び血管型ベーチェット病の効能・効果で
承認を取得している又は開発を行っている国又は地域はない.
1.6 外国における使用状況等に関する資料
4
1.6.2 米国添付文書
1.6 外国における使用状況等に関する資料
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use REMICADE®
safely and effectively. See full prescribing information for REMICADE. REMICADE (infliximab)
Lyophilized Concentrate for Injection, for Intravenous Use Initial U.S. Approval: 1998
---RECENT MAJOR
CHANGES---WARNING: SERIOUS INFECTIONS and MALIGNANCY 01/2015 Warnings and Precautions: Serious Infections (5.1) 01/2015 Warnings and Precautions: Malignancies (5.2) 01/2015
---INDICATIONS AND
USAGE---REMICADE is a tumor necrosis factor (TNF) blocker indicated for:
Crohn’s Disease (1.1):
• reducing signs and symptoms and inducing and maintaining clinical remission in adult patients with moderately to severely active disease who have had an inadequate response to conventional therapy.
• reducing the number of draining enterocutaneous and rectovaginal fistulas and maintaining fistula closure in adult patients with fistulizing disease.
Pediatric Crohn’s Disease (1.2):
• reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients with moderately to severely active disease who have had an inadequate response to conventional therapy.
Ulcerative Colitis (1.3):
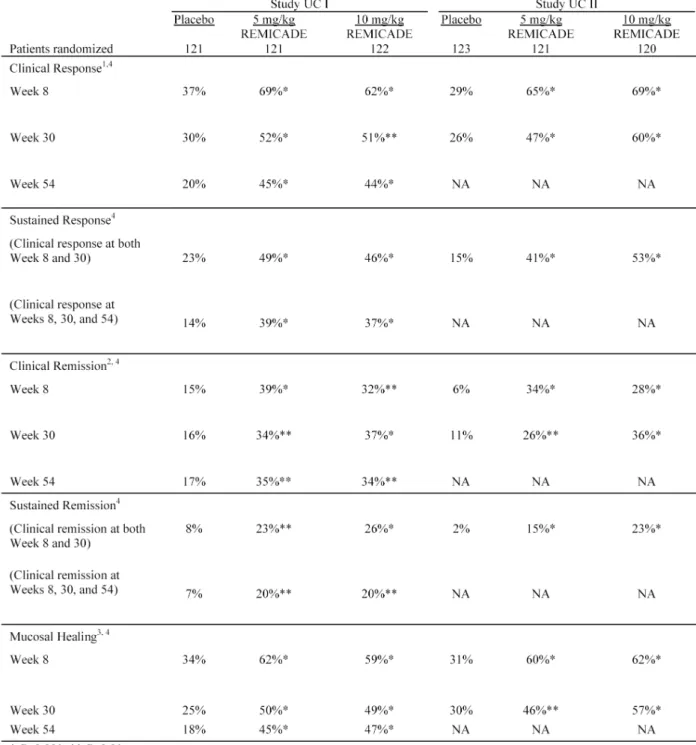
• reducing signs and symptoms, inducing and maintaining clinical remission and mucosal healing, and eliminating corticosteroid use in adult patients with moderately to severely active disease who have had an inadequate response to conventional therapy.
Pediatric Ulcerative Colitis (1.4):
• reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients with moderately to severely active disease who have had an inadequate response to conventional therapy.
Rheumatoid Arthritis (1.5) in combination with methotrexate:
• reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active disease.
Ankylosing Spondylitis (1.6):
• reducing signs and symptoms in patients with active disease.
Psoriatic Arthritis (1.7):
• reducing signs and symptoms of active arthritis, inhibiting the progression of structural damage, and improving physical function.
Plaque Psoriasis (1.8):
• treatment of adult patients with chronic severe (i.e., extensive and/or disabling) plaque psoriasis who are candidates for systemic therapy and when other systemic therapies are medically less appropriate.
---DOSAGE AND
ADMINISTRATION---REMICADE is administered by intravenous infusion over a period of not less than 2 hours.
Crohn’s Disease (2.1)
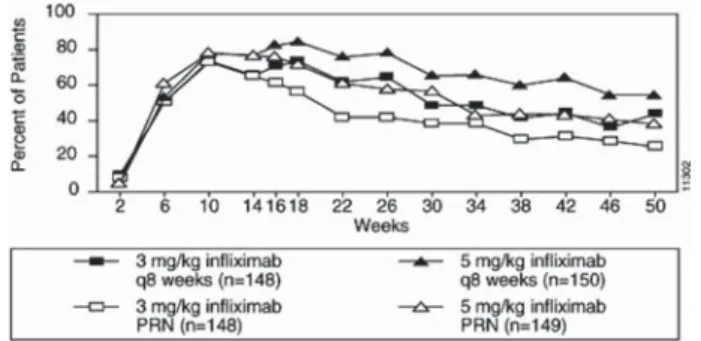
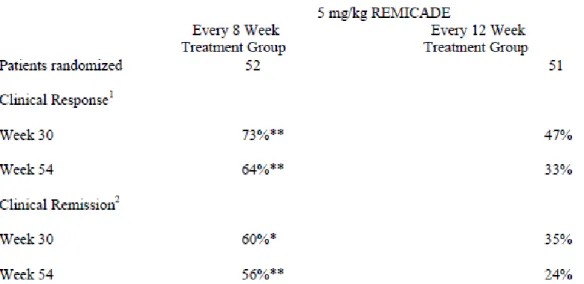
• 5 mg/kg at 0, 2 and 6 weeks, then every 8 weeks. Some adult patients who initially respond to treatment may benefit from increasing the dose to 10 mg/kg if they later lose their response.
Pediatric Crohn’s Disease (2.2)
• 5 mg/kg at 0, 2 and 6 weeks, then every 8 weeks.
Ulcerative Colitis (2.3)
• 5 mg/kg at 0, 2 and 6 weeks, then every 8 weeks.
Pediatric Ulcerative Colitis (2.4)
• 5 mg/kg at 0, 2 and 6 weeks, then every 8 weeks.
Rheumatoid Arthritis (2.5)
• In conjunction with methotrexate, 3 mg/kg at 0, 2 and 6 weeks, then every 8 weeks. Some patients may benefit from increasing the dose up to 10 mg/kg or treating as often as every 4 weeks.
Ankylosing Spondylitis (2.6)
• 5 mg/kg at 0, 2 and 6 weeks, then every 6 weeks.
Psoriatic Arthritis (2.7) and Plaque Psoriasis (2.8)
• 5 mg/kg at 0, 2 and 6 weeks, then every 8 weeks.
---DOSAGE FORMS AND
STRENGTHS---100 mg of lyophilized infliximab in a 20 mL vial for intravenous infusion. (3)
---CONTRAINDICATIONS---• REMICADE doses >5 mg/kg in moderate to severe heart failure. (4)
• Previous severe hypersensitivity reaction to REMICADE or known hypersensitivity to inactive components of REMICADE or to any murine proteins. (4)
---WARNINGS AND
PRECAUTIONS---• Serious infections – do not give REMICADE during an active infection. If an infection develops, monitor carefully and stop REMICADE if infection becomes serious. (5.1)
• Invasive fungal infections – for patients who develop a systemic illness on REMICADE, consider empiric antifungal therapy for those who reside or travel to regions where mycoses are endemic (5.1)
• Malignancies – the incidence of malignancies including lymphoma was greater in REMICADE treated patients than in controls. Due to the risk of HSTCL carefully assess the risk/benefit especially if the patient has Crohn’s disease or ulcerative colitis, is male, and is receiving azathioprine or 6-mercaptopurine treatment. (5.2) • Hepatitis B virus reactivation – test for HBV infection before starting REMICADE. Monitor HBV carriers during and several months after therapy. If reactivation occurs, stop REMICADE and begin anti-viral therapy. (5.3)
• Hepatotoxicity – rare severe hepatic reactions, some fatal or necessitating liver transplantation. Stop REMICADE in cases of jaundice and/or marked liver enzyme elevations. (5.4)
• Heart failure – new onset or worsening symptoms may occur. (4, 5.5) • Cytopenias – advise patients to seek immediate medical attention if signs and
symptoms develop, and consider stopping REMICADE. (5.6)
• Hypersensitivity – serious infusion reactions including anaphylaxis or serum sickness-like reactions may occur. (5.7)
• Demyelinating disease – exacerbation or new onset may occur. (5.8) • Lupus-like syndrome – stop REMICADE if syndrome develops. (5.13)
• Live vaccines or therapeutic infectious agents – should not be given with REMICADE. Bring pediatric patients up to date with all vaccinations prior to initiating REMICADE. (5.14)
---ADVERSE
REACTIONS---Most common adverse reactions (>10%) – infections (e.g. upper respiratory, sinusitis, and pharyngitis), infusion-related reactions, headache, and abdominal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. ---DRUG
INTERACTIONS---• Use with anakinra or abatacept– increased risk of serious infections (7.1)
---USE IN SPECIFIC
POPULATIONS---• Pediatric Use – REMICADE has not been studied in children with Crohn’s disease or ulcerative colitis <6 years of age. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and MEDICATION GUIDE. Revised: January 2015 WARNING: SERIOUS INFECTIONS and MALIGNANCY
See full prescribing information for complete boxed warning SERIOUS INFECTIONS
• Increased risk of serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial sepsis, invasive fungal infections (such as histoplasmosis) and infections due to other opportunistic pathogens.
• Discontinue REMICADE if a patient develops a serious infection. • Perform test for latent TB; if positive, start treatment for TB prior to starting
REMICADE. Monitor all patients for active TB during treatment, even if initial latent TB test is negative. (5.1)
MALIGNANCY
• Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with tumor necrosis factor (TNF) blockers, including REMICADE.
• Postmarketing cases of fatal hepatosplenic T-cell lymphoma (HSTCL) have been reported in patients treated with TNF blockers including REMICADE. Almost all had received azathioprine or 6-mercaptopurine concomitantly with a TNF-blocker at or prior to diagnosis. The majority of REMICADE cases were reported in patients with Crohn’s disease or ulcerative colitis, most of whom were adolescent or young adult males. (5.2)
REMICADE®(infliximab)
FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: SERIOUS INFECTIONS AND MALIGNANCY 1 INDICATIONS AND USAGE
1.1 Crohn’s Disease 1.2 Pediatric Crohn’s Disease 1.3 Ulcerative Colitis 1.4 Pediatric Ulcerative Colitis 1.5 Rheumatoid Arthritis 1.6 Ankylosing Spondylitis 1.7 Psoriatic Arthritis 1.8 Plaque Psoriasis
2 DOSAGE AND ADMINISTRATION
2.1 Crohn’s Disease 2.2 Pediatric Crohn’s Disease 2.3 Ulcerative Colitis 2.4 Pediatric Ulcerative Colitis 2.5 Rheumatoid Arthritis 2.6 Ankylosing Spondylitis 2.7 Psoriatic Arthritis 2.8 Plaque Psoriasis
2.9 Monitoring to Assess Safety
2.10 Administration Instructions Regarding Infusion Reactions 2.11 General Considerations and Instructions for Preparation and
Administration
3 DOSAGE FORMS AND STRENGTHS 4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS (see Boxed WARNINGS)
5.1 Serious Infections 5.2 Malignancies
5.3 Hepatitis B Virus Reactivation 5.4 Hepatotoxicity
5.5 Patients with Heart Failure 5.6 Hematologic Reactions 5.7 Hypersensitivity 5.8 Neurologic Reactions 5.9 Use with Anakinra 5.10 Use with Abatacept
5.11 Concurrent Administration with other Biological Therapeutics 5.12 Switching Between Biological Disease-Modifying Antirheumatic Drugs
(DMARDs) 5.13 Autoimmunity
5.14 Live Vaccines/Therapeutic Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience 6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Use with Anakinra or Abatacept 7.2 Use with Tocilizumab
7.3 Use with Other Biological Therapeutics
7.4 Methotrexate (MTX) and Other Concomitant Medications 7.5 Immunosuppressants
7.6 Cytochrome P450 Substrates
7.7 Live Vaccines/Therapeutic Infectious Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy 8.3 Nursing Mothers 8.4 Pediatric Use 8.5 Geriatric Use 10 OVERDOSAGE 11 DESCRIPTION 12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action 12.2 Pharmacodynamics 12.3 Pharmacokinetics 13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Crohn’s Disease 14.2 Pediatric Crohn’s Disease 14.3 Ulcerative Colitis 14.4 Pediatric Ulcerative Colitis 14.5 Rheumatoid Arthritis 14.6 Ankylosing Spondylitis 14.7 Psoriatic Arthritis 14.8 Plaque Psoriasis
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING 17 PATIENT COUNSELING INFORMATION
17.1 Patient Counseling
*Sections or subsections omitted from the full prescribing information are not listed
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE 1.1 Crohn’s Disease
REMICADE is indicated for reducing signs and symptoms and inducing and maintaining clinical remission in adult patients with moderately to severely active Crohn’s disease who have had an inadequate response to conventional therapy. REMICADE is indicated for reducing the number of draining enterocutaneous and rectovaginal fistulas and maintaining fistula closure in adult patients with fistulizing Crohn’s disease.
1.2 Pediatric Crohn’s Disease
REMICADE is indicated for reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients 6 years of age and older with moderately to severely active Crohn’s disease who have had an inadequate response to conventional therapy.
1.3 Ulcerative Colitis
REMICADE is indicated for reducing signs and symptoms, inducing and maintaining clinical remission and mucosal healing, and eliminating corticosteroid use in adult patients with moderately to severely active ulcerative colitis who have had an inadequate response to conventional therapy.
1.4 Pediatric Ulcerative Colitis
REMICADE is indicated for reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients 6 years of age and older with moderately to severely active ulcerative colitis who have had an inadequate response to conventional therapy.
1.5 Rheumatoid Arthritis
REMICADE, in combination with methotrexate, is indicated for reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in patients with moderately to severely active rheumatoid arthritis.
1.6 Ankylosing Spondylitis
REMICADE is indicated for reducing signs and symptoms in patients with active ankylosing spondylitis.
1.7 Psoriatic Arthritis
REMICADE is indicated for reducing signs and symptoms of active arthritis, inhibiting the progression of structural damage, and improving physical function in patients with psoriatic arthritis.
WARNING: SERIOUS INFECTIONS and MALIGNANCY SERIOUS INFECTIONS
Patients treated with REMICADE®are at increased risk for developing serious
infections that may lead to hospitalization or death [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)]. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
REMICADE should be discontinued if a patient develops a serious infection or sepsis.
Reported infections include:
• Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. Patients should be tested for latent tuberculosis before REMICADE use and during therapy.1,2Treatment for latent infection
should be initiated prior to REMICADE use.
• Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Empiric anti-fungal therapy should be considered in patients at risk for invasive fungal infections who develop severe systemic illness. • Bacterial, viral and other infections due to opportunistic pathogens,
including Legionella and Listeria.
The risks and benefits of treatment with REMICADE should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with REMICADE, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
MALIGNANCY
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including REMICADE [see Warnings and Precautions (5.2)].
Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers including REMICADE. These cases have had a very aggressive disease course and have been fatal. Almost all patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with a TNF-blocker at or prior to diagnosis. The majority of reported REMICADE cases have occurred in patients with Crohn’s disease or ulcerative colitis and most were in adolescent and young adult males.
1.8 Plaque Psoriasis
REMICADE is indicated for the treatment of adult patients with chronic severe (i.e., extensive and/or disabling) plaque psoriasis who are candidates for systemic therapy and when other systemic therapies are medically less appropriate. REMICADE should only be administered to patients who will be closely monitored and have regular follow-up visits with a physician [see Boxed Warnings, Warnings and Precautions (5)].
2 DOSAGE AND ADMINISTRATION 2.1 Crohn’s Disease
The recommended dose of REMICADE is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of adults with moderately to severely active Crohn’s disease or fistulizing Crohn’s disease. For adult patients who respond and then lose their response, consideration may be given to treatment with 10 mg/kg. Patients who do not respond by Week 14 are unlikely to respond with continued dosing and consideration should be given to discontinue REMICADE in these patients.
2.2 Pediatric Crohn’s Disease
The recommended dose of REMICADE for pediatric patients 6 years and older with moderately to severely active Crohn’s disease is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks.
2.3 Ulcerative Colitis
The recommended dose of REMICADE is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of adult patients with moderately to severely active ulcerative colitis.
2.4 Pediatric Ulcerative Colitis
The recommended dose of REMICADE for pediatric patients 6 years and older with moderately to severely active ulcerative colitis is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks.
2.5 Rheumatoid Arthritis
The recommended dose of REMICADE is 3 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 3 mg/kg every 8 weeks thereafter for the treatment of moderately to severely active rheumatoid arthritis. REMICADE should be given in combination with methotrexate. For patients who have an incomplete response, consideration may be given to adjusting the dose up to 10 mg/kg or treating as often as every 4 weeks bearing in mind that risk of serious infections is increased at higher doses [see Adverse Reactions (6.1)].
2.6 Ankylosing Spondylitis
The recommended dose of REMICADE is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 6 weeks thereafter for the treatment of active ankylosing spondylitis.
2.7 Psoriatic Arthritis
The recommended dose of REMICADE is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of psoriatic arthritis. REMICADE can be used with or without methotrexate.
2.8 Plaque Psoriasis
The recommended dose of REMICADE is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of chronic severe (i.e., extensive and/or disabling) plaque psoriasis.
2.9 Monitoring to Assess Safety
Prior to initiating REMICADE and periodically during therapy, patients should be evaluated for active tuberculosis and tested for latent infection [see Warnings and Precautions (5.1)].
2.10 Administration Instructions Regarding Infusion Reactions
Adverse effects during administration of REMICADE have included flu-like symptoms, headache, dyspnea, hypotension, transient fever, chills, gastrointestinal symptoms, and skin rashes. Anaphylaxis might occur at any time during REMICADE infusion. Approximately 20% of REMICADE-treated patients in all clinical trials experienced an infusion reaction compared with 10% of placebo-treated patients [see Adverse Reactions (6.1)]. Prior to infusion with REMICADE, premedication may be administered at the physician’s discretion. Premedication could include antihistamines (anti-H1 +/- anti-H2), acetaminophen and/or corticosteroids. During infusion, mild to moderate infusion reactions may improve following slowing or suspension of the infusion, and upon resolution of the reaction, reinitiation at a lower infusion rate and/or therapeutic administration of antihistamines, acetaminophen, and/or corticosteroids. For patients that do not tolerate the infusion following these interventions, REMICADE should be discontinued.
During or following infusion, patients who have severe infusion-related hyper sensitivity reactions should be discontinued from further REMICADE treatment. The management of severe infusion reactions should be dictated by the signs and symptoms of the reaction. Appropriate personnel and medication should be available to treat anaphylaxis if it occurs.
2.11 General Considerations and Instructions for Preparation and Administration
REMICADE is intended for use under the guidance and supervision of a physician. The reconstituted infusion solution should be prepared by a trained medical professional using aseptic technique by the following procedure:
1. Calculate the dose, total volume of reconstituted REMICADE solution required and the number of REMICADE vials needed. Each REMICADE vial contains 100 mg of the infliximab antibody.
2. Reconstitute each REMICADE vial with 10 mL of Sterile Water for Injection, USP, using a syringe equipped with a 21-gauge or smaller needle as follows: Remove the flip-top from the vial and wipe the top with an alcohol swab. Insert the syringe needle into the vial through the center of the rubber stopper and direct the stream of Sterile Water for Injection, USP, to the glass wall of the vial. Gently swirl the solution by rotating the vial to dissolve the lyophilized powder. Avoid prolonged or vigorous agitation. DO NOT SHAKE. Foaming of the solution on reconstitution is not unusual. Allow the reconstituted solution to stand for 5 minutes. The solution should be colorless to light yellow and opalescent, and the solution may develop a few translucent particles as infliximab is a protein. Do not use if the lyophilized cake has not fully dissolved or if opaque particles, discoloration, or other foreign particles are present.
3. Dilute the total volume of the reconstituted REMICADE solution dose to 250 mL with sterile 0.9% Sodium Chloride Injection, USP, by withdrawing a volume equal to the volume of reconstituted REMICADE from the 0.9% Sodium Chloride Injection, USP, 250 mL bottle or bag. Slowly add the total volume of reconstituted REMICADE solution to the 250 mL infusion bottle or bag. Gently mix. The resulting infusion concentration should range between 0.4 mg/mL and 4 mg/mL. 4. The REMICADE infusion should begin within 3 hours of reconstitution and dilution.
The infusion must be administered over a period of not less than 2 hours and must use an infusion set with an in-line, sterile, non-pyrogenic, low-protein-binding filter (pore size of 1.2 μm or less). The vials do not contain antibacterial preservatives. Therefore, any unused portion of the infusion solution should not be stored for reuse. 5. No physical biochemical compatibility studies have been conducted to evaluate the co-administration of REMICADE with other agents. REMICADE should not be infused concomitantly in the same intravenous line with other agents. 6. Parenteral drug products should be inspected visually before and after
reconstitution for particulate matter and discoloration prior to administration, whenever solution and container permit. If visibly opaque particles, discoloration or other foreign particulates are observed, the solution should not be used.
3 DOSAGE FORMS AND STRENGTHS
100 mg vial: 100 mg lyophilized infliximab in a 20 mL vial for injection, for intravenous use.
4 CONTRAINDICATIONS
REMICADE at doses >5 mg/kg should not be administered to patients with moderate to severe heart failure. In a randomized study evaluating REMICADE in patients with moderate to severe heart failure (New York Heart Association [NYHA] Functional Class III/IV), REMICADE treatment at 10 mg/kg was associated with an increased incidence of death and hospitalization due to worsening heart failure [see Warnings and Precautions (5.5) and Adverse Reactions (6.1)].
REMICADE should not be re-administered to patients who have experienced a severe hypersensitivity reaction to REMICADE. Additionally, REMICADE should not be administered to patients with known hypersensitivity to inactive components of the product or to any murine proteins.
5 WARNINGS AND PRECAUTIONS 5.1 Serious Infections
Patients treated with REMICADE are at increased risk for developing serious infections involving various organ systems and sites that may lead to hospitalization or death.
Opportunistic infections due to bacterial, mycobacterial, invasive fungal, viral, or parasitic organisms including aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, histoplasmosis, legionellosis, listeriosis, pneumocystosis and tuberculosis have been reported with TNF-blockers. Patients have frequently presented with disseminated rather than localized disease.
Treatment with REMICADE should not be initiated in patients with an active infection, including clinically important localized infections. Patients greater than 65 years of age, patients with co-morbid conditions and/or patients taking concomitant immunosuppressants such as corticosteroids or methotrexate may be at greater risk of infection. The risks and benefits of treatment should be considered prior to initiating therapy in patients:
• with chronic or recurrent infection; • who have been exposed to tuberculosis; • with a history of an opportunistic infection;
• who have resided or traveled in areas of endemic tuberculosis or endemic mycoses, such as histoplasmosis, coccidioidomycosis, or blastomycosis; or • with underlying conditions that may predispose them to infection. Tuberculosis
Cases of reactivation of tuberculosis or new tuberculosis infections have been observed in patients receiving REMICADE, including patients who have previously received treatment for latent or active tuberculosis. Cases of active tuberculosis have also occurred in patients being treated with REMICADE during treatment for latent tuberculosis.
REMICADE®(infliximab) REMICADE®(infliximab)
Patients should be evaluated for tuberculosis risk factors and tested for latent infection prior to initiating REMICADE and periodically during therapy. Treatment of latent tuberculosis infection prior to therapy with TNF blocking agents has been shown to reduce the risk of tuberculosis reactivation during therapy. Induration of 5 mm or greater with tuberculin skin testing should be considered a positive test result when assessing if treatment for latent tuberculosis is needed prior to initiating REMICADE, even for patients previously vaccinated with Bacille Calmette-Guerin (BCG).
Anti-tuberculosis therapy should also be considered prior to initiation of REMICADE in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent tuberculosis but having risk factors for tuberculosis infection. Consultation with a physician with expertise in the treatment of tuberculosis is recommended to aid in the decision whether initiating anti-tuberculosis therapy is appropriate for an individual patient.
Tuberculosis should be strongly considered in patients who develop a new infection during REMICADE treatment, especially in patients who have previously or recently traveled to countries with a high prevalence of tuberculosis, or who have had close contact with a person with active tuberculosis.
Monitoring
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with REMICADE, including the development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy. Tests for latent tuberculosis infection may also be falsely negative while on therapy with REMICADE.
REMICADE should be discontinued if a patient develops a serious infection or sepsis. A patient who develops a new infection during treatment with REMICADE should be closely monitored, undergo a prompt and complete diagnostic workup appropriate for an immunocompromised patient, and appropriate antimicrobial therapy should be initiated.
Invasive Fungal Infections
For patients who reside or travel in regions where mycoses are endemic, invasive fungal infection should be suspected if they develop a serious systemic illness. Appropriate empiric antifungal therapy should be considered while a diagnostic workup is being performed. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. When feasible, the decision to administer empiric antifungal therapy in these patients should be made in consultation with a physician with expertise in the diagnosis and treatment of invasive fungal infections and should take into account both the risk for severe fungal infection and the risks of antifungal therapy.
5.2 Malignancies
Malignancies, some fatal, have been reported among children, adolescents and young adults who received treatment with TNF-blocking agents (initiation of therapy ≤ 18 years of age), including REMICADE. Approximately half of these cases were lymphomas, including Hodgkin’s and non-Hodgkin’s lymphoma. The other cases represented a variety of malignancies, including rare malignancies that are usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months (range 1 to 84 months) after the first dose of TNF blocker therapy. Most of the patients were receiving concomitant immunosuppressants. These cases were reported post-marketing and are derived from a variety of sources, including registries and spontaneous postmarketing reports.
Lymphomas
In the controlled portions of clinical trials of all the TNF-blocking agents, more cases of lymphoma have been observed among patients receiving a TNF blocker compared with control patients. In the controlled and open-label portions of REMICADE clinical trials, 5 patients developed lymphomas among 5707 patients treated with REMICADE (median duration of follow-up 1.0 years) vs. 0 lymphomas in 1600 control patients (median duration of follow-up 0.4 years). In rheumatoid arthritis patients, 2 lymphomas were observed for a rate of 0.08 cases per 100 patient-years of follow-up, which is approximately three-fold higher than expected in the general population. In the combined clinical trial population for rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, and plaque psoriasis, 5 lymphomas were observed for a rate of 0.10 cases per 100 patient-years of follow-up, which is approximately four-fold higher than expected in the general population. Patients with Crohn’s disease, rheumatoid arthritis or plaque psoriasis, particularly patients with highly active disease and/or chronic exposure to immunosuppressant therapies, may be at a higher risk (up to several fold) than the general population for the development of lymphoma, even in the absence of TNF-blocking therapy. Cases of acute and chronic leukemia have been reported with postmarketing TNF-blocker use in rheumatoid arthritis and other indications. Even in the absence of TNF blocker therapy, patients with rheumatoid arthritis may be at a higher risk (approximately 2-fold) than the general population for the development of leukemia.
Hepatosplenic T-cell lymphoma (HSTCL)
Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers including REMICADE. These cases have had a very aggressive disease course and have been fatal. Almost all patients had received treatment with the immunosuppressants azathioprine or 6-mercaptopurine concomitantly with a TNF-blocker at or prior to diagnosis. The majority of reported REMICADE cases have occurred in patients with
Crohn’s disease or ulcerative colitis and most were in adolescent and young adult males. It is uncertain whether the occurrence of HSTCL is related to TNF-blockers or TNF-blockers in combination with these other immunosuppressants. When treating patients, consideration of whether to use REMICADE alone or in combination with other immunosuppressants such as azathioprine or 6-mercaptopurine should take into account a possibility that there is a higher risk of HSTCL with combination therapy versus an observed increased risk of immunogenicity and hypersensitivity reactions with REMICADE monotherapy from the clinical trial data [see Warnings and Precautions (5.7) and Adverse Reactions (6.1)].
Skin cancer
Melanoma and Merkel cell carcinoma have been reported in patients treated with TNF blocker therapy, including REMICADE [see Adverse Reactions (6.2)]. Periodic skin examination is recommended for all patients, particularly those with risk factors for skin cancer.
Other Malignancies
In the controlled portions of clinical trials of some TNF-blocking agents including REMICADE, more malignancies (excluding lymphoma and nonmelanoma skin cancer [NMSC]) have been observed in patients receiving those TNF-blockers compared with control patients. During the controlled portions of REMICADE trials in patients with moderately to severely active rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, and plaque psoriasis, 14 patients were diagnosed with malignancies (excluding lymphoma and NMSC) among 4019 REMICADE-treated patients vs. 1 among 1597 control patients (at a rate of 0.52/100 patient-years among REMICADE-treated patients vs. a rate of 0.11/100 patient-years among control patients), with median duration of follow-up 0.5 years for REMICADE-treated patients and 0.4 years for control patients. Of these, the most common malignancies were breast, colorectal, and melanoma. The rate of malignancies among REMICADE-treated patients was similar to that expected in the general population whereas the rate in control patients was lower than expected.
In a clinical trial exploring the use of REMICADE in patients with moderate to severe chronic obstructive pulmonary disease (COPD), more malignancies, the majority of lung or head and neck origin, were reported in REMICADE-treated patients compared with control patients. All patients had a history of heavy smoking [see Adverse Reactions (6.1)]. Prescribers should exercise caution when considering the use of REMICADE in patients with moderate to severe COPD.
Psoriasis patients should be monitored for nonmelanoma skin cancers (NMSCs), particularly those patients who have had prior prolonged phototherapy treatment. In the maintenance portion of clinical trials for REMICADE, NMSCs were more common in patients with previous phototherapy [see Adverse Reactions (6.1)]. The potential role of TNF-blocking therapy in the development of malignancies is not known [see Adverse Reactions (6.1)]. Rates in clinical trials for REMICADE cannot be compared to rates in clinical trials of other TNF-blockers and may not predict rates observed in a broader patient population. Caution should be exercised in considering REMICADE treatment in patients with a history of malignancy or in continuing treatment in patients who develop malignancy while receiving REMICADE.
5.3 Hepatitis B Virus Reactivation
Use of TNF blockers, including REMICADE, has been associated with reactivation of hepatitis B virus (HBV) in patients who are chronic carriers of this virus. In some instances, HBV reactivation occurring in conjunction with TNF blocker therapy has been fatal. The majority of these reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to HBV reactivation. Patients should be tested for HBV infection before initiating TNF blocker therapy, including REMICADE. For patients who test positive for hepatitis B surface antigen, consultation with a physician with expertise in the treatment of hepatitis B is recommended. Adequate data are not available on the safety or efficacy of treating patients who are carriers of HBV with anti-viral therapy in conjunction with TNF blocker therapy to prevent HBV reactivation. Patients who are carriers of HBV and require treatment with TNF blockers should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy. In patients who develop HBV reactivation, TNF blockers should be stopped and antiviral therapy with appropriate supportive treatment should be initiated. The safety of resuming TNF blocker therapy after HBV reactivation is controlled is not known. Therefore, prescribers should exercise caution when considering resumption of TNF blocker therapy in this situation and monitor patients closely.
5.4 Hepatotoxicity
Severe hepatic reactions, including acute liver failure, jaundice, hepatitis and cholestasis, have been reported rarely in postmarketing data in patients receiving REMICADE. Autoimmune hepatitis has been diagnosed in some of these cases. Severe hepatic reactions occurred between 2 weeks to more than 1 year after initiation of REMICADE; elevations in hepatic aminotransferase levels were not noted prior to discovery of the liver injury in many of these cases. Some of these cases were fatal or necessitated liver transplantation. Patients with symptoms or signs of liver dysfunction should be evaluated for evidence of liver injury. If jaundice and/or marked liver enzyme elevations (e.g., ≥ 5 times the upper limit of normal) develop, REMICADE should be discontinued, and a thorough investigation of the abnormality should be undertaken. In clinical trials, mild or moderate elevations of ALT and AST have been observed in patients receiving REMICADE without progression to severe hepatic injury [see Adverse Reactions (6.1)].