Introduction
Environmental fluctuation is a major factor influencing the growth, development and sur-vival of terrestrial plants (Cramer et al. 2011; Kumar et al. 2012; Pandey et al. 2017). For instance, an increase in the air temperature surrounding plants can lead to physiological defects especially in reproductive processes (Barnabas et al. 2008; Zinn et al. 2010; Hartfield and Prueger 2015). However, stress-tolerance mechanisms can allow plants to acclimate to such environmental stresses (Nakashima et al. 2014; Rejeb et al. 2014; Zhu 2016; Sade et al. 2017; Vishwakarma et al. 2017). Indeed, abiotic stres-ses often bring about modulations of morpho-genic responses, with inhibition or promotion of
growth and development as a strategy for stress acclimation (Potters et al. 2007).
As opposed to terrestrial plants, seaweeds are aquatic multicellular sessile organisms that ex-ist at the intertidal and subtidal zones. The conditions in the intertidal zone especially ex-hibit drastic changes at both daily and yearly scales (Rawlings 1999; Helmuth and Hofmann 2001; Eckersley and Scrosati, 2012). Thus, sea-weeds also are exposed to temperature fluctua-tions that influence their growth and develop-ment (Wiencke and Dieck 1989; Dudgeen et al. 1995; Kubler and Davison 1993, 1995; Nejrup et al. 2013; De Silva and Asaeda 2017; Martins et al. 2017; Mikami et al. 2018; for review, Agrawal 2012; Singh and Singh 2015). Temperature is one of the most variable factors in their
environ-Temperature promoting the asexual life cycle program in
Bangia fuscopurpurea (Bangiales, Rhodophyta) from
Esashi in the Hokkaido Island, Japan
Koji MIKAMI
1* and Ikuya KISHIMOTO
2Abstract : In the asexual life cycle of the marine red seaweed Bangia fuscopurpurea gametophytic thalli produce multiple monospores that develop into thalli as clones. We investigated the effects of heat stress on the production and release of monospores in B. fuscopurpurea from Esashi, in northern Hokkaido Island of Japan. Non-lethal high tem-peratures of 25℃ and 28℃ strongly promoted monospore discharge, whereas no spore release was observed at 30℃, the limiting growth temperature of Esashi B. fuscopurpurea. These findings differed from previous reports using B. fuscopurpurea collected at Fukaura, the northern Japan, and at the Fujan province of southern China, for which growth and monospore release were observed at 30℃. Thus, the temperature range promoting asex-ual propagation with monospore discharge in B. fuscopurpurea varies and appears to be unrelated to the thermal conditions of harvesting areas. Since each B. fuscopurpurea strain had a unique upper-limit temperature for survival and release of monospores was accelerated under non-lethal high temperature conditions, the temperature range ena-bling the asexual life cycle program seems to be restricted by the degree of heat stress tolerance of the B. fuscopurpurea strains themselves.
Keywords : asexual propagation, Bangia fuscopurpurea, heat stress, life cycle
1Faculty of Fisheries Sciences, Hokkaido University, 3-1-1 Minato-cho, Hakodate 041-8611, Japan
2Graduate school of Fisheries Sciences, Hokkaido University, 3-1-1 Minato-cho, Hakodate 041-8611, Japan *Corresponding author : Tel / fax: +81-138-40-8899, e-mail: komikami@fish.hokudai.ac.jp
ment, and seaweeds have acquired tolerance against temperature changes (Andersen et al. 2013; Smolina et al. 2016). However, it is un-clear how heat stress-tolerance is regulated, although effects of temperature changes on compositions of amino acids and membrane fatty acids have been demonstrated (Mikami et al. 2011, 2018; de Costa et al. 2018; Song et al. 2018), and transcriptional analyses have identi-fied a number of genes whose expression is up-or down-regulated by heat stress in various seaweeds (Collen et al. 2007; Heinrich et al. 2012; Choi et al. 2013; Im et al. 2015; Sun et al. 2015; Fan et al., 2017; Wang et al. 2018).
Seaweeds exhibit sexual reproduction with isomorphic or heteromorphic diploid-haploid life cycle (Thornber 2006; Coelho et al. 2007; Cock et al. 2014; Liu et al. 2017). Among the Bangiales, the life cycle consists of a visible leafy thallus and a microscopic filamentous conchocelis as a haploid gametophyte and a diploid sporophyte, respectively, both of which are multicellular and live independently in different seasons of the year, and sexual reproduction is based on the fertilization of male and female gametes pro-duced in a gametophytic thallus to produce carpospores that develop into a sporophytic conchocelis (Blouin et al. 2011; Mikami et al. 2012; Takahashi and Mikami 2017). Since the rate of production of male and female gametes on gametophytes for sexual propagation is enhanced by increased temperature and photo-period (Kakinuma et al. 2006), it is possible that the sexual life cycle program is positively regu-lated in part by heat stress in Bangiales, which would be similar to the green alga Volvox carteri (Kirk and Kirk 1986) and different from the negative effects of heat stress on reproduc-tion in terrestrial plants (Barnabas et al. 2008; Zinn et al. 2010; Hartfield and Prueger 2015). Therefore, effects of heat stress on the sexual reproductive process might differ between ter-restrial plants and algae.
As a remarkable characteristic, the life cycle of certain species of Pyropia, Porphyra, and Bangia in Bangiales includes an asexual pro-gram via production of haploid neutral spores or monospores from thalli (Blouin et al. 2011; Mikami et al. 2012; Takahashi and Mikami
2017). The asexual propagation in Bangiales has been studied mainly in Pyropia yezoensis and Banga fuscopurpurea to date. In P. yezoensis, which is a major cultivated species for nori production in Japan (Blouin et al. 2011), the production and release of monospores is accel-erated by a reduction in the extracellular calcium ion concentration (Takahashi et al. 2010) and treatment of thalli with hydrogen peroxide (Takahashi and Mikami 2017). In addition, asexual reproduction in thalli of P. yezoensis is also accelerated by irradiance (Li 1984) and fragmentation associated with woun-ding (Hafting 1999). The effects of environmen-tal stresses on the asexual reproduction in B. fuscopurpurea have been examined only for changes in temperature and photoperiod. These studies indicated that heat stress stimulates the formation and discharge of monospores (Sommerfeild and Nichols 1973; Notoya and Iijima 2003; Wang et al. 2008). Therefore, woun-ding and heat stress might potentially act as triggers of the asexual life cycle program in Bangiales, suggesting that both biotic and abi-otic stresses are key regulators of both sexual and asexual life cycle programs in red sea-weeds.
Notoya and Iijima (2003) compared the opti-mum temperature for growth and monospore discharge and found differences in these pa-rameters for B. fuscopurpurea strains collected from Fukaura and Enoshima, located northern and central Japan, respectively. The Fukaura B. fuscopurpurea was able to grow and release monospores at 30℃, whereas 25℃ was the upper limit temperature for growth and mono-spore discharge for the Enoshima strain. Inter-estingly, B. fuscopurpurea from Fujan province in southern China was similar to the Fukaura species [compare Notoya and Iijima (2003) and Wang et al. (2008)]. These observations suggest that the prevailing temperature conditions in the living areas do not correlate with the preferred temperature range for vegetative growth and the occurrence of asexual reproduction in B. fuscupurpurea.
To address this possibility, we investigated whether B. fuscopurpurea collected at Esashi on the northern Hokkaido Island of Japan exhibits
difference in temperature range for survival and monospore discharge compared to those col-lected in Fukaura, Enoshima, and Fujian.
Materials and Methods
Algal materialNaturally growing B. fuscopurpurea gameto-phytic thalli were collected at Esashi, Hokkaido, Japan on 14 May 2010. A clean single thallus whose sex was unknown was cultured in enriched sea life (ESL) medium (Kitade et al. 2002), which is made by dissolving commercially available SEALIFE powder (Marintech Co. Ltd., Japan) in distilled water (DW) with the addi-tion of ESS2 solution, at 15℃ under irradiation
of 60 mol m-2 s-1 provided by cool white
fluo-rescent lamps (Neorumi super FL40SW, Mitsu-bishi, Japan) with a photo-period of 10 h light: 14 h dark. The medium was bubbled continu-ously with filter-sterilized air and changed weekly. Resultant unisexual B. fuscopurpurea gametophytes were subcultured continually as an experimental line.
Determination of viability and the number of discharged monospores
Each 0.1 g (fresh weight) of B. fuscopurpurea gametophytic thalli was cultured in dishes (Azunoru dish 90 × 20 mm height, As One) containing 50 ml of the ESL medium under the same conditions mentioned above except for temperature at 20, 25, 28, 30, 32, and 34℃, while control experiments were performed at 15℃. Under these various temperature conditions, viability and the number of released
monos-pores were determined daily for 7 days.
To test viability, cells in thalli treated with various temperatures for one to three weeks were visualized daily by staining with ESL medium containing 0.01% erythrosine (Wako Pure Chemical Industries, Japan). After stai-ning for 5 min at room temperature, thalli were gently rinsed with ESL medium to remove excess erythrosine and mounted on a slide with ESL medium. Thalli were observed and photo-graphed using an Olympus IX73 light micro-scope equipped with an Olympus DP22 cam-era, where cells stained by the dye were de-fined as dead cells. Viability was calculated by counting the living and dead cells using micro-graphs.
The number of monospores discharged and adhered on the bottom of a 6 well-microplate with lid (Iwaki, Asahi Glass Co. Ltd., Japan) was counted daily for a week under the Olympus IX73 light microscope.
Results
Effects of heat stress on cellular viability in ga-metophytes
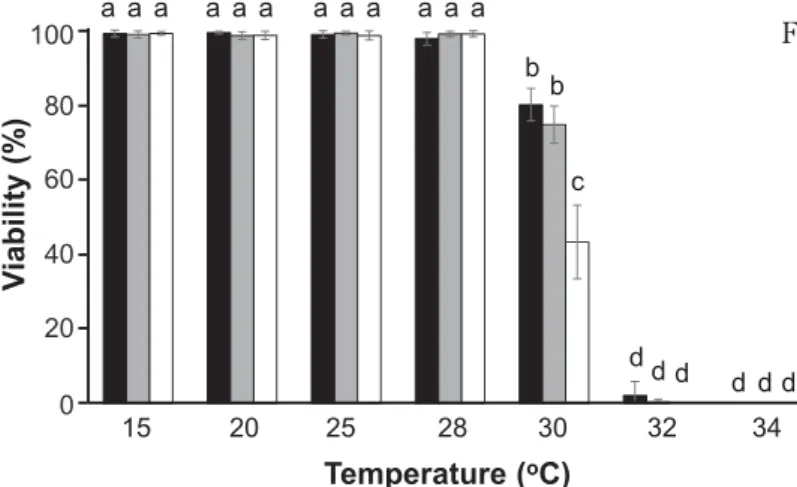
When B. fuscopurpurea gametophytes collect-ed at Esashi were incubatcollect-ed at 15, 20, 25 and 28℃, cells in every thallus were alive (Fig. 1). However, incubation at 30℃ reduced viability, such that ca. 50% of cells were dead after 3 weeks of cultivation (Fig. 1). Thalli of this northern strain of B. fuscopurpurea did not survive at 32℃ and 34℃ (Fig. 1). These results indicated that 30℃ is the upper limit or par-tially lethal temperature for survival of thalli,
Fig. 1. Effects of temperature on the viability of Bagia fuscopurpurea gametophytic thalli. Samples of the laboratory-maintained culture strain were incubated at 15, 20, 25, 28, 30, 32, and 34℃ for 1 week (black bar), 2 weeks (gray bar) and 3 weeks (white bar) and then cells were stained with 0.01% erythrosine to calculate via-bility. Mean values (n = 3 ) ± SD are shown. Letters denote statistically sig-nificant differences (p < 0.05) as deter-mined by one-way ANOVA as described in Mikami et al. (2018).
meaning that 25℃ and 28℃ act as non-lethal temperatures.
Effects of heat stress on discharge of monos-pores from gametophytic thalli
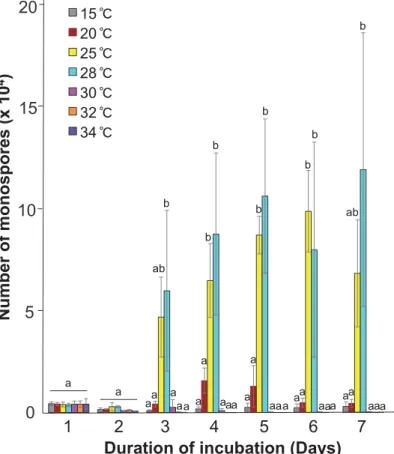
Monospores were discharged from thalli (Fig. 2A) and then developed into gametophytes (Fig. 2B). Thus, we next examined the effects of high temperature on discharge of monospores. Dis-charge of monospores was clearly observed 3 days after the temperature shift from 15℃ to 25℃ and 28℃, and continued during further incubation under these temperatures, although the number of released monospores varied among triplicated experiments (Fig. 3). By con-trast, 15℃ and 20℃ conditions had only a weak effect on monospore release, and no accumula-tion of monospores was observed upon incuba-tion at over 30℃ (Fig. 3). Together with the data shown in Fig. 1, we conclude that non-lethal, but not non-lethal, high temperature can stimulate asexual propagation in the northern B. fuscopurpurea.
Discussion
The aim of the present study was to address
the relationship between environmental thermal conditions and specification of the temperature range promoting the asexual life cycle in the red seaweed B. fuscopurpurea. Thus, we examined the effects of temperature on vegetative cell survival and asexual life cycle propagation in B. fuscopurpurea collected at Esashi in northern Japan to compare with established results from Notoya and Iijima (2003) and Wang et al. (2008). We demonstrated that the upper limit for viability of thalli was 30℃ and that non-lethal high temperatures such as 25℃ and 28℃ pro-moted the asexual life cycle program in Esashi B. fuscopurpurea (Figs. 1 and 3). These values were similar to those reported for Enoshima
Fig. 3. Promotion of the asexual life cycle by non-lethal high temperature. Gametophytic thalli were incubated at 15, 20, 25, 28, 30, 32, and 34℃ for 1 week. The number of re-leased monospores was counted every day under the microscope. Mean values (n = 3 ) ± SD per 0.1 g of fresh weight samples were calculated from results of three independ-ent experimindepend-ents. Letters denote statistically significant differences (p < 0.05) as deter-mined by one-way ANOVA as described in Mikami et al. (2018).
Fig. 2. Early stages of the asexual life cycle program of Bangia fuscopurpurea. (A) Monospores discharged from a thallus. A cast-off uniseriate ga-metophyte remained as a cell wall skeleton after complete release of monospores. (B) Growing germlings of the two-cell stage. Scale bar=50
B. fuscopurpurea (see Notoya and Iijima 2003) and different from B. fuscopurprea strains collected at Fukaura and Fujian, both of which were able to grow and release monospores at 30℃ (Notoya and Iijima 2003; Wang et al. 2008). Together, these results indicate that there is no correlation between thermal conditions of liv-ing areas and the preferred temperature range for vegetative growth and asexual propagation; however, it was found a relationship between growth under the non-lethal temperature and promotion of asexual propagation. Therefore, we hypothesize that temperature ranges enabling vegetative growth and asexual life cycle propa-gation in B. fuscopurpurea are determined by the degree of thermotolerance in each organ-ism.
Our results clearly demonstrated the heat stress-inducible promotion of the asexual prop-agation in B. fuscopurpurea; however, the ef-fects of heat stress on vegetative growth have not been clarified, as we tested only survival rates of cells in gametophytic thalli. To per-form a full comparison with previous reports (Notoya and Iijima 2003; Wang et al. 2008), as-sessment of the effects of heat stress on growth of monospore germlings should be performed in the northern B. fuscopurpurea. These experi-ments should help clarify the relationship be-tween heat stress tolerance and promotion of asexual propagation in B. fuscopurpurea. Recent phylogenetic analyses show that sea-weeds of the genus Bangia are highly diver-gent and are classified into at least 4 distinct clades, based on combinational analysis of se-quences of the plastid Rubisco large subunit and nuclear small-subunit ribosomal RNA genes (Sutherland et al. 2011; Sanchez et al. 2014). These results suggest that the original genus Bangia might be more correctly classified into different novel genera, although it is unclear whether differences in heat sensitivities of the asexual life cycle programs of each B. fuscopurpurea strains correspond to differences among clades of the phylogenetic trees. Thus, it is necessary to elucidate the relationship be-tween heat stress sensitivity and phylogenetic position in B. fuscopurpurea collected in vari-ous areas, which is an important approach to
judge whether the classification of Bangia spe-cies into one genus should be revised.
As far as we know, heat stress-inducible promotion of the asexual life cycle is unique to B. fuscopurpurea. Indeed, our preliminary ex-periments showed that discharge of monos-pores from thalli of the marine red seaweed Pyropia yezoensis and the fresh water red alga B. atropurpurea was not significantly accelerated under heat stress conditions (data not shown). In addition, the regulatory mechanisms for initiation of the asexual propagation in B. fuscopurpurea collected in different areas might have distinct temperature sensitivities. Al-though the overall machinery regulating pro-duction and release of monospores is thought to be common in Bangiales, it is possible that there are components whose heat sensitivities differ among species. How the strength of heat stress tolerance is differently determined among B. fuscopurpurea strains from various areas has not been elucidated. Thus, studies on regulato-ry mechanisms and heat stress-sensitive com-ponents for formation and release of monos-pores are necessary to understand how the degree of thermotolerance is determined and why the strength of heat stress tolerance varies among organisms collected in different areas. Moreover, results from these studies could help to judge the probability of the presence of taxonomic variation in the genus Bangia.
Acknowledgments
We thank Ryunosuke Irie for his assistance for data analyses. This study was supported in part by KAKENHI (15H04539).
References
Agrawal SC. Factors controlling induction of reproduction in algae--review: the text. Re-view article. Folia Microbiol. 2012; 57 : 87-407. Andersen GS, Pedersen MF, Nielsen SL. Tem-perature acclimation and heat tolerance of photosynthesis in Norwegian Saccharina latissima (Laminariales, Phaeophyceae). J. Phycol. 2013 ; 49 : 689-700.
drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008 ; 31 : 11-38.
Blouin NA, Brodie JA, Grossman AC, Xu P, Brawley SH. Porphyra: a marine crop shaped by stress. Trends Plant Sci. 2011 ; 16 : 29-37. Choi S, Hwang MS, Im S, Kim N, Jeong W-J, Park E-J, Gong Y-G, Choi D-W. Transcrip-tome sequencing and comparative analysis of the gametophyte thalli of Pyropia tenera under normal and high temperature condi-tions. J. Appl. Phycol. 2013 ; 25 : 1237-1246. Cock JM, Godfroy O, Macaisne N, Peters AF,
Coelho SM. Evolution and regulation of complex life cycles: a brown algal perspec-tive. Curr. Opin. Plant Biol. 2014 ; 17 : 1-6. Coelho SM, Peters AF, Charrier B, Roze D,
Destombe C, Valero M, Cock JM. Complex life cycles of multicellular eukaryotes: new approaches based on the use of model organisms. Gene 2007 ; 406 : 152-170.
Collen J, Guisle-Marsollier I, Leger JJ, Boyen C. Response of the transcriptome of the in-tertidal red seaweed Chondrus crispus to controlled and natural stresses. New Phy-tol. 2007 ; 176 : 45-55.
da Costa E, Azevedo V, Melo T, Rego AM, V Evtuguin D, Domingues P, Calado R, Pereira R, Abreu MH, Domingues MR. High-resolution lipidomics of the early life sta-ges of the red seaweed Porphyra dioica. Molecules 2018 ; 23. doi: 10.3390 / molecules 23010187
Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K. Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol. 2011 ; 11 : 163. doi:10.1186 / 1471-2229-11-163
De Silva HCC, Asaeda T. Effects of heat stress on growth, photosynthetic pigments, oxida-tive damage and competioxida-tive capacity of three submerged macrophytes. J. Plant Interact. 2017 ; 12 : 228-236.
Dudgeon SR, Kubler JE, Vadas RL, Davison IR. Physiological responses to environmental variation in intertidal red algae: Does thal-lus morphology matter? Mar. Ecol. Prog. Ser. 1995 ; 117 : 193-205.
Eckersley LK, Scrosati, RA. Temperature,
des-iccation, and species performance trends along intertidal elevation gradient. Curr. Dev. Oceanogr. 2012 ; 5 : 59-73
Fan M, Sun X, Xu N, Liao Z, Li Y, Wang J, Fan Y, Cui D, Peng Li P, Zengliang Miao Z. Integration of deep transcriptome and pro-teome analyses of salicylic acid regulation high temperature stress in Ulva prolifera. Sci. Rep. 2017 ; 7 : 11052. doi: org / 10. 1038 / s 41598-017-11449-w
Hafting JT. A novel technique for propagation of Porphyra yezoensis Ueda blades in sus-pension cultures via monospores. J. Appl. Phycol. 1999 ; 11 : 361-367.
Hatfield JL, Prueger JH. Temperature ex-tremes: Effect on plant growth and devel-opment. Weather Climate Extremes 2015;10: 4-10.
Heinrich S, Valentin K, Frickenhaus S, John U, Wiencke C. Transcriptomic analysis of ac-climation to temperature and light stress in Saccharina latissima (Phaeophyceae). PLoS ONE 2012 ; 7 : e44342. doi: org / 10. 1371 / jour-nal. pone. 0044342
Helmuth BS, Hofmann GE. Microhabitats, ther-mal heterogeneity, and patterns of physio-logical stress in the rocky intertidal zone. Biol. Bull. 2001 ; 201 : 374-384.
Im S, SanChoi S, Hwang MS, Park E-J, Jeong W-J, Choi D-W. De novo assembly of tran-scriptome from the gametophyte of the marine red algae Pyropia seriata and iden-tification of abiotic stress response genes. J. Appl. Phycol. 2015 ; 27 : 1343-1353.
Kakinuma M, Kaneko I, Coury DA, Suzuki T, Amano H. Isolation and identification of gametogenesis-related genes in Porphyra yezoensis (Rhodophyta) using subtracted cDNA libraries. J. Appl. Phycol. 2006;18:489-496.
Kirk DL, Kirk MM. Heat shock elicits produc-tion of sexual inducer in Volvox. Science 1986 ; 231 : 51-54.
Kitade Y, Fukuda S, Nakajima M, Watanabe T, Saga N. Isolation of a cDNA encoding a homologue of actin from Porphyra yezoensis (Rhodophyta). J. Appl. Phycol. 2002 ; 14 : 135-141.
light-use characteristics of Chondrus crispus (Rhodophyta). Eur. J. Phycol. 1995 ; 30 : 189-195.
Kumar AA, Mishra P, Kumari K, Panigrahi KC. Environmental stress influencing plant de-velopment and flowering. Front. Biosci. (Schol Ed). 2012 ; 4 : 1315-1324.
Li SY. The ecological characteristics of monos-pores of Porphyra yezoensis and their use in cultivation. Hydrobiologia 1984 ; 116-117 : 255-543.
Liu X, Bogaert K, Engelen AH, Leliaert F, Roleda MY, De Clerck O. Seaweed repro-ductive biology: environmental and genetic controls. Bot. Mar. 2017 ; 60 : 89-108.
Martins N, Tanttu H, Pearson GA, Serrao EA, Bartsch I. Interactions of daylength, tem-perature and nutrients affect thresholds for life stage transitions in the kelp Laminaria digitata (Phaeophyceae). Bot. Mar. 2017 ; 60 : 109-121.
Mikami K, Ito M, Taya K, Kishimoto I, Koba-yashi T, Itabashi Y, Tanaka R. Parthenos-porophytes of the brown alga Ectocarpus siliculosus exhibit sex-dependent differences in thermotolerance as well as fatty acid and sterol composition. Mar. Environ. Res. 2018 ; 137 : 188-195.
Mikami K, Li L, Takahashi M. Monospore-based asexual life cycle in Porphyra yezoensis. In: Mikami K (ed). Porphyra yezoensis: Fron-tiers in Physiological and Molecular Bio-logical Research. Nova Science Publishers, New York, 2012 ; 15-37.
Mikami K, Takahashi M, Hirata R, Yokoyama T, Taniguchi M, Saga N Mori, T. Alanine is a possible compatible solute involved in cold acclimation in the marine red alga Porphyra yezoensis. In: Pomin VH (ed). Sea-weed: Ecology, Nutrient Composition and Medicinal Uses. Nova Science Publishers, New York, 2011 ; 1-14.
Nakashima K, Yamaguchi-Shinozaki K, Shino-zaki K. The transcriptional regulatory net-work in the drought response and its crosstalk in abiotic stress responses inclu-ding drought, cold, and heat. Front. Plant Sci. 2014 ; 5 : 170. doi: 10.3389/fpls.2014.00170
Nejrup LB, Staehr PA, Thomsen AS.
Temperature- and light-dependent growth and metabolism of the invasive red algae Gracilaria vermiculophylla - a comparison with two native macroalgae. Eur. J. Phycol. 2013 ; 48 : 295-308.
Notoya M, Iijima N. Life history and sexuality of archeospore and apogamy of Bangia atropurpurea (Roth) Lyngbye (Bangiales, Rhodophyta) from Fukaura and Enoshima, Japan. Fish. Sci.: 2003 ; 69 : 799-805.
Pandey P, Irulappan V, Bagavathiannan MV, Senthil-Kumar M. Impact of combined abi-otic and biabi-otic stresses on plant growth and avenues for crop improvement by exploi-ting physio-morphological traits. Front. Plant Sci. 2017 ; 8 : 537. doi: 10. 3389 / fpls. 2017. 00537 Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MA. Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci. 2007 ; 12 : 98-105.
Rawlings TA. Adaptations to physical stresses in the intertidal zone: The egg capsules of neogastropod molluscs. Amer. Zool. 1999;39: 230-243.
Rejeb IB, Pastor V, Mauch-Mani B. Plant re-sponses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants (Basel) 2014 ; 3 : 458-475.
Sade N, Del Mar Rubio-Wilhelmi M, Umnajki-tikorn K, Blumwald E. Stress-induced se-nescence and plant tolerance to abiotic stress. J. Exp. Bot. 2018 ; 69 : 845-853.
Sanchez N, Verges A, Peteiro C, Sutherland JE, Brodie J. Diversity of bladed Bangiales (Rhodophyta) in western Mediterranean: recognition of the genus Themis and de-scriptions of T. ballesterosii sp. nov., T. iberica sp. nov., and Pyropia parva sp. nov. J. Phycol. 2014 ; 50 : 908-929.
Singh SP, Singh P. Effect of temperature and light on the growth of algae species: A review. Renew. Sust. Energ. Rev. 2015 ; 50 : 431-444.
Smolina I, Kollias S, Jueterbock A, Coyer JA, Hoarau G. Variation in thermal stress re-sponse in two populations of the brown seaweed, Fucus distichus, from the Arctic and subarctic intertidal. R. Soc. Opensci. 2015 ; 3 : 150429. doi: 10.1098/rsos.150429
Sommerfeld MR, Nichols HW. The life cycle of Bangia fuscopurpurea in culture I. Effects of temperature and photoperiod on the mor-phorogy and reproduction of the Bangia phase. J. Phycol. 1973 ; 9 : 205-210.
Song Y, Zhao J, Chen J, Luo Q, Yang R, Xu J, Chen H, Yan X. Heat shock induced meta-bolic conversion of membrane lipids, fatty acids and volatile organic compounds of Pyropia haitanensis under different heat shock time. Phycol. Res. 2018 ; 66 : 89-99. Sun P, Mao Y, Li G, Cao M, Kong F, Wang L,
Bi G. Comparative transcriptome profiling of Pyropia yezoensis (Ueda) M.S. Hwang & H. G. Choi in response to temperature stresses. BMC Genomics 2015 ; 16 : 463. doi: org / 10. 1186 / s12864-015-1586-1
Sutherland JE, Lindstrom SC, Nelson WA, Brodie J, Lynch MD, Hwang MS, Choi HG, Miyata M, Kikuchi N, Oliveira MC, Farr T, Neefus C, Mols-Mortensen A, Milstein D, M. A new look at ancient order: Generic revi-sion of the Bangiales (Rhodophyta). J Phy-col. 2011 ; 47 : 1131-1151.
Takahashi M, Mikami K. Oxidative stress pro-motes asexual reproduction and apogamy in the red seaweed Pyropia yezoensis. Front. Plant Sci. 2017 ; 8 : 62. doi: 10. 3389 / fpls. 2017. 00062
Takahashi M, Saga N, Mikami K. Photosynthesis-dependent extracellular Ca2+
influx triggers an asexual reproductive cycle in the ma-rine red macroalga Porphyra yezoensis, Amer. J. Plant Sci. 2010 ; 1 : 1-11. doi: 10. 4236 / ajps. 2010. 11001
Thornber CS. Functional properties of the iso-morphic biphasic algal life cycle. Integr. Comp. Biol. 2006 ; 46 : 605-614
Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S. Abscisic acid signaling and abi-otic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017 ;8 : 161. doi: 10.3389/fpls. 2017.00161
Wang W, Lin Y, Teng F, Ji D, Xu Y, Chen C, Xie C. Comparative transcriptome analysis between heat-tolerant and sensitive Pyropia
haitanensis strains in response to high temperature stress. Algal Res. 2018 ; 29 : 104-112.
Wang W-J, Zhu J-Y, Xu P, Xu JR, Lin XZ, Huang CK, Song WL, Peng G, Wang GC. Characterization of the life history of Bangia fuscopurpurea (Bangiaceae, Rhodo-phyta) in connection with its cultivation in China. Aquaculture 2008 ; 278 : 101-109. Wiencke C, tom Dieck I. Temperature
require-ments for growth and temperature toler-ance of macroalgae endemic to the Antarc-tic region. Mar. Ecol. Prog. Ser. 1989;54 : 189-197.
Zinn KE, Tunc-Ozdemir M, Harper JF. Tem-perature stress and plant sexual reproduc-tion: uncovering the weakest links. J. Exp. Bot. 2010 ; 61 : 1959-1968.
Zhu, ZK. Abiotic stress signaling and respon-ses in plants. Cell 2016 ; 167 : 313-324.
Received 6 July 2018 Accepted 8 August 2018