札幌の醸造所における人家性ショウジョウバエの分布と季節的消長
全文
(2) Journal of Hokkaido University of Education (Section II B) Vol. 34, No. 2 March, 1984. ^•mi^±^&^ (Hi2g[SB) ^ 34^ ^2^- Bg%59^3^. Microdistribution and Phenology in Domestic Species of Drosopkila in and near a Brewery in Sapporo, Northern Japan.. Hide-aki WATABE Biological Laboratory, Sapporo College, Hokkaido University of Education, Sapporo 064. ^ ^ ^ B§ : ^^@a?tH<T^Jott^A^|4'>3^'>'3'J7-. ^^N7<h^gp?fIN. WMM^:ANL^W^f;riNS^. Abstract Weekly collections were made in and near a beer brewery in Sapporo, in order to study the microniche and phenology of domestic species of Drosophila. Data obtained were analysed by the methods of Kimoto (1976) and Colwell and Futuyma (1971). D. melanogaster had a broad niche, being abundant at all trap-spots, while its sibling species, D. simulans, was found exclusively at a small garden near the brewery. D. virilis was rather common at sites associated with beer. Differences were also found in the phenology of the domestic species. D. melanogaster, D. simulans, D. immigrans and D. busckii, which are considered to be species of tropical origin, have never or scarcely ever been collected in the spring months. D. virilis, a temperate species, was more evenly distributed over the whole year except for the snowy. months, and would have a superior ability to tolerate cold than the above four species.. Introduction According to the drosophilid list published recently (Wheeler, 1981), about 2600 species have been located throughout the world. A great majority of them are peculiar to particular geographical areas of the earth, but ten species of the genus Drosophila are well known as domestic species which adapt to artificial environments and have a wide range of distribution (Carson, 1965 ; Dobzhansky, 1965 ; Throckmorton, 1975). As domestic Drosophila, for example. (7).
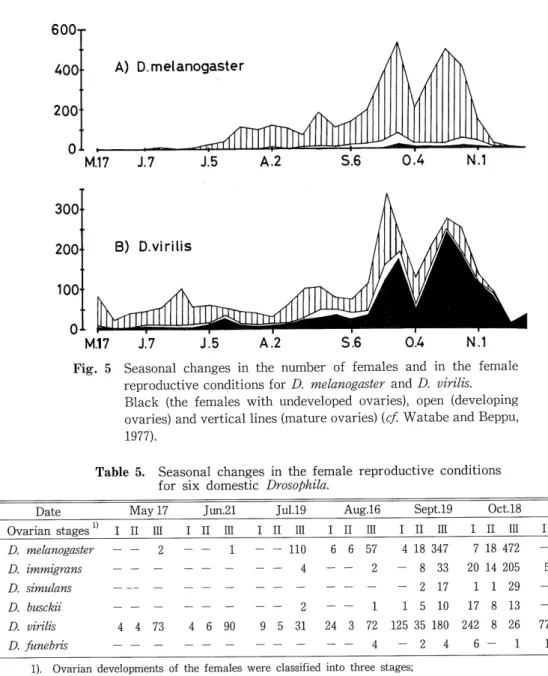
(3) 42. Hide-aki. WATABE. D. melanogaster, can easily be cultured in the laboratory, they have been genetically studied in detail. Considering the number of genetic studies written about these species, there is still a relatively lack of information on their ecology in their natural environments (cf. Mather, 1956 ; Nozawa, . 1956 ; Shorrocks, 1973 ; Atkinson and Shorrocks, 1977). In Hokkaido seven domestic Drosophila spp. have been recorded, all of which coexist within a small area (Kaneko et al., 1966 ; Minami et al., 1979 ; Watabe, 1979). Moreover, no diapause has been found for any of the domestic Drosophila so far studied (Lumme, 1978 ; Watabe, 1979), and most of them are thought to be unable to spend the winter outdoors in cool and cold regions (Takada and Toyofuku, 1960 ; Chiang et al., 1962 ; Watabe, 1979). The present paper reports on the microniche and phenology of domestic Drosophila inhabiting one particular habitat-a brewery-on the basis of weekly collection data.. Collection Methods and Trap-spots The ecological study was made from M.ay 5 to November 22 in 1977, at a beer brewery in the center of Sapporo City. The meteorological data for Sapporo are given in Table 1. Collections were periodically carried out using eight "retainer" traps baited with fermenting banana (Toda, 1977). Samples were fixed with Kahle's solution, removed to the laboratory at weekly intervals and examined under a binocular microscope. The number of specimens collected was recorded both for species and trap-spot separately.. The traps were set up at different sites from the ecological point of view regarding the light conditions and the presence or absence of alcohol. The microenvironments of each trap-spot are. investigated in Table 2. Table 1. Monthly mean temperature (A), the highest (B) and lowest (C) temperatures in each month, and rainfall (D) in 1977, in Sapporo. Month Jan.. A: Mean. -7.5. Feb.. Mar.. —5.. 5. -0.1. Apr.. May. Jun.. Jul.. Aug.. 5.2. 11.2. 15.9. 21.3. 20.3. Sept.. Oct.. 17.4. 11.3. Nov. 5.2. Dec, -0.. 6. B: Highest. 0.3. 5.. 6. 10.0. 18.1. 27.0. 27.1. 34.0. 29.4. 29.3. 22.4. 13.5. 10.. 8. c: Lowest. -19.1. -17.. 9. -13.7. -2.2. 0.4. 7.7. 12.7. 11.8. 5.6. 0.1. -4.0. -11.. 7. 86. 0. 74.0. 131.0. 75.0. 7.5. 58.0. 190.5. 99.0. 20.5. 151.5. D: Rainfall. 94.0. Temperature (°C), rainfall (mm).. (8). 115. 5.
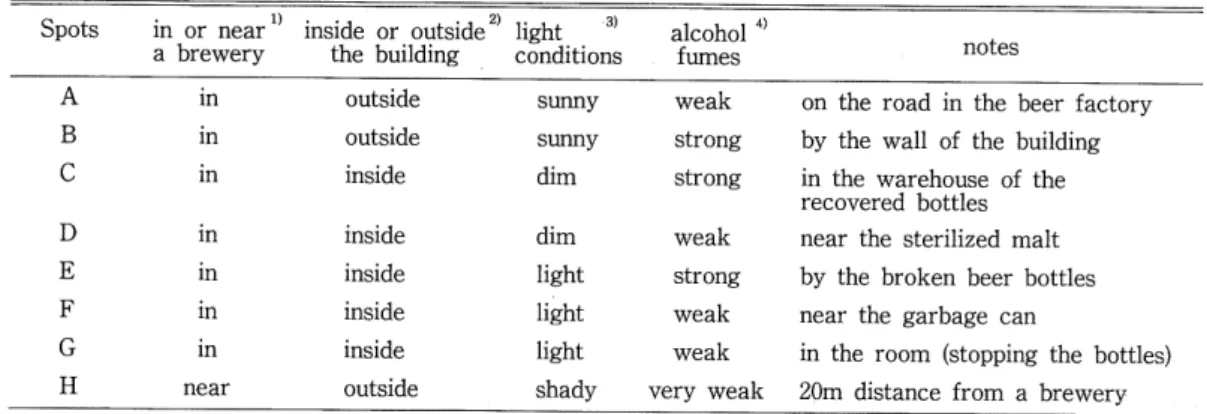
(4) 43. Microdistritution and Phenology in Drosophila. Table 2. Microenvironments of trap-spots. Spots. inside or outside the building. in or near a brewery. light. ^ 3. alcohol. conditions. notes. fumes. A. in. outside. sunny. weak. on the road in the beer factory. B. in. outside. sunny. strong. by the wall of the building. in. inside. dim. strong. D. in. inside. dim. weak. near the sterilized malt. E. in. inside. light. strong. by the broken beer bottles. F. in. inside. light. weak. near the garbage can. in. inside. light. weak. in the room (stopping the bottles). outside. shady. c. G. H. near. very weak. in the warehouse of the. recovered bottles. 20m distance from a brewery. 1). Trap H was set up at a small garden with several trees of aspen and birch. 2). Collection at spot A was done untill the beginning the re-construction of the road on September 13. 3). Trap G was set up under the fluoresecent lamps. 4). Traps 6, C and E were within one meter distance from the alcohol resource (beer).. Analysis Methods for Microdistribution The similarities between the traps were calculated by the method of Kimoto's CTT index (Kimoto, 1976). Cn=. 22 nij'mj'. (O^C.^1). (27r2y+2^2/)^,-^,'. V 7T2 1=. ,<_i /t y —. 2 n</. w. 2^2/. 2 m/ -^7~. {Nj and Nj' indicate the total individual number at trap ; and /, respectively, nij and nij' are the number of individuals of species i at trap j and ;, respectively.) The average of all original CTT values was calculated, and this value was adopted as a criterion of the relative heterogeneity of the trap-spots. A matrix based on the above calcu-. lations was then subjected to a cluster analysis, UPGMA method {cf. M.inami et al., 1979). The similarity of microdistribution for each species was also calculated by Colwell and Futuyma's niche overlap method (y) (Colwell and Futuyma, 1971). ,^•'=1—^-2 djk\pij-pi'j\, ptj=N^~'. Ni = 2 dj k nij, d, = -^s- , J. ^—|Uj J. A^(logM--logAO-2 nij\og{ni,lNi) 8j=. ^N,\ogN,-N\ogN. (9).
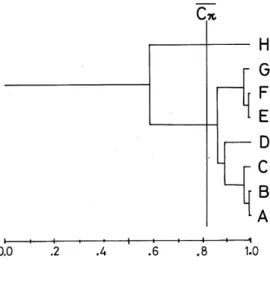
(5) 44. Hide-aki WATABE. ('absolute weighting factor') (N, total individual number of all species ; Ni, total individual number of species i. ) Rare species, which were considered rare if they numbered less than the trap number, were. excluded from the analysis. According to the procedure of UPGMA, groupings were also made.. Results The results are divided into two parts, the first part analysing the micro-habitat of each domestic species and the second part dealing with the seasonal changes of population sizes and of age constitutions for six common species.. 1. Micro distribution patterns in and near the brewery. Table 3 shows the number of both species and individuals obtained at each trap-spot separately, together with a diversity index of each spot and the niche breadth of the species. A total number of 20292 samples, belonging to 3 genera and 24 species, were collected. About 95 percent of the assemblage was accounted for by seven domestic Drosophila : D. melanogaster. (47.7% of total samples), D. virilis (31.0%), D. immigrans (10.4%), D. busckii (2,0%), D. simulans (1.7%), D. funebris (1.2%) and D. hydei (0.1%). The wild species were collected mostly at spot H, being 73.0 percent of the total wild flies. As the brewery itself is like a huge bait-trap, the common occurrence of the wild species suggests a continual migration from their natural. habitats to this brewery. Table 4 and Fig. 1 give the similarity of each trap-spot according to Kimoto's CTT index.. Except for trap H, all the traps showed a high degree of similarity to each other. In this. H. analysis the average of all original CTT values was adopted as a criterion for the heterogeneous environmental structures. Basically, two. major groups were distinguished among eight traps. One group included traps A~G and the. other only trap H. A diversity index value (/lj) of trap-spot H was the lowest (Table 3), which means a high degree of heterogeneity in the environments. These results also suggest either. that the environmental structure in the brewery is very simple or that there exists a strong. 0.0. .2. .4. .6. 1.0. factor which influences the distribution and abundance of the species.. Fig.. On the whole, domestic Drosophila spp. were present at all spots, but the niche breadth value {/3i) varied with species (Table 3). The. (10). 1 Dendrogram resulting from the. cluster analysis (UPGMA) based on. the similarity matrix of Table 4..
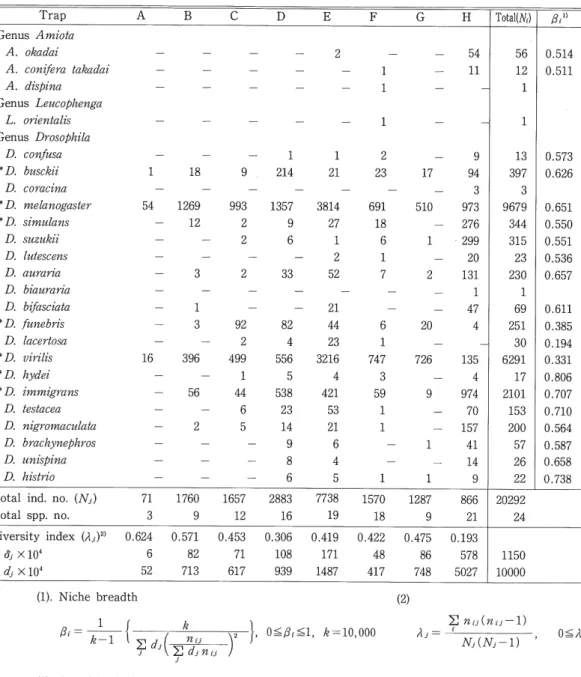
(6) 45. Microdistritution and Phenology in Drosophila. higher value of this index indicates the broader-niche species, while the narrow-niche species will yield restricted value. Among the abundant species, D. melanogaster and D. immigrans. showed a high value, but D. virilis and D. funebris a low value. Fig. 2 shows the niche overlap dendrogram for each species, calculated by the method of Colwell and Futuyma (1971), The dendrogram, which shows the ecological distance between species with regard to trap-spots, is. a convenient place to help clarify the relationships and characteristics of these domestic species. Four groups among the species seem to emerge- the first group containing only D. Table 3. Drosophilid flies collected in and near a brewery in Sapporo.. A. Trap. c. B. D. Genus Amiota. 2. A. okadai. A. dispina Genus Lencophenga. D. confnsa * D. biisckii D. coracina * D, melanogaster. 1. 18. 9. 1 214. 1 21. 2 23. 54. 1269 12. 993 2 2. 1357 9 6. 2. 33. 691 18 6 1 7. 510. 3. 3814 27 1 2 52. 92 2 499 1 44 6 5. 82 4 556 5 538 23 14 9 8 6. 21 44 23 3216 4 421 53 21 6 4 5. 6 1 747 3 59 1 1. 20. * D, simnlans D. suznkii D. hitescens D. anraria D. bianraria. 1 3. D. bifasciata *D, fnnebris D. lacertosa. 16. *D. virilis. 396. *D. hydei. 56. * D, immigrans D. testacea. 2. D. nigromactdata D. brachynephros D, tinispina. D. histrio. 17. 1 2. 9 94 3 973 276 299 20 131 1 47 4. 1. 1. 135 4 974 70 157 41 14 9. 726 9. 1. 71 3. 1760 9. 1657 12. 2883 16. 7738 19. 1570 18. 1287 9. 866 21. 0.624. 0.571. 0.453. 0.306. 0.419. 0.422. 0.475. 0.193. Total ind. no. (Nj) Total spp. no.. 6 52. ^ x l04 d, X 104. 82 713. 71 617. 108 939. 171 1487. 48 417. 56 12 1. i3.l). 0.514 0.511. 86 748. 578 5027. 13 397 3 9679 344 315 23 230 1 69 251 30 6291 17 2101 153 200 57 26 22 20292 24. 0.573 0.626 0.651 0.550 0.551 0.536 0.657 0.611 0.385 0.194 0.331 0.806 0.707 0.710 0.564 0.587 0.658 0.738. 1150 10000. (2). (1). Niche breadth. 2^-. Total(^). 1. 1. L. orientalis. Genus Drosophila. ?-1. 54 11. 1 1. A. conifera takadai. Diversity index (/tj)2>. H. G. F. E. , 0^,<1, ^=10,000 2^-ny 3. (3). Asterisks indicate domestic species of Drosphila.. (11). Aj^. 2n,v(n,,-l). NAN,-1). O^Aj^l.
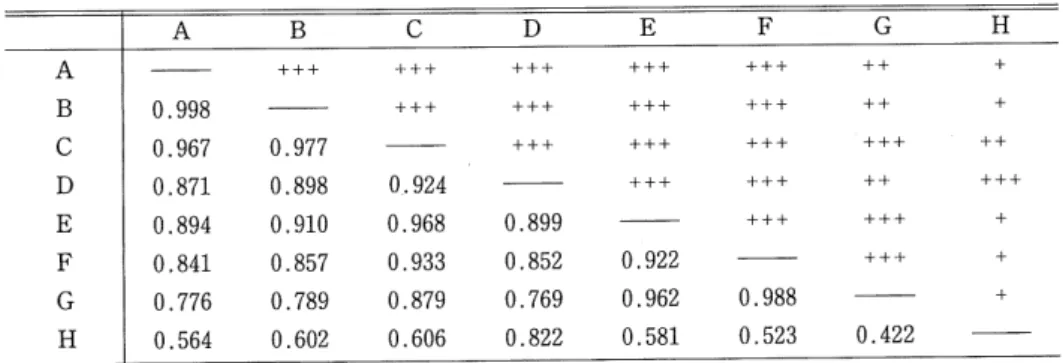
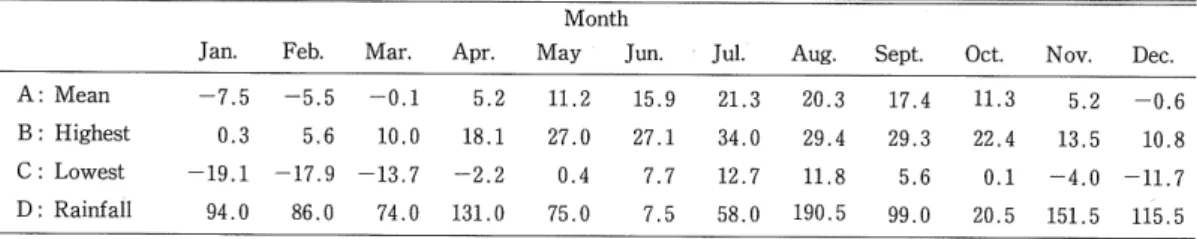
(7) 46. Hide-aki WATABE. Table 4. Similarity matrix among the traps.. A. A B. c. D. +++. +++. +++. +++. ++. +++. +++. +++. +++. ++. +++. +++. +++. +++. ++. +++. +++. ++. +++. +++. +++. +. +++. +. 0.998. c. H. B 4-++. 0.967. 0.977. D. 0.871. 0.898. 0.924. E. 0.894. 0.910. 0.968. 0.899. F. 0.841. 0.857. 0.933. 0.852. 0.922. 0.776. 0.789. 0.879. 0.769. 0.962. 0.988. 0.564. 0.602. 0.606. 0.822. 0.581. 0.523. G H. +. +. 0.422. 0.4^.+ <0.6^+ <0.8^ +++ <1.0. melanogaster, the second group also only D. funebris, the third group D. virilis and D. lacertosa and the fourth group D. immigrans, D. simulans, D. busckii, D. hydei as well as the wild species. D. melanogaster was abundant at all spots, but D. funebris was rather common at spots C and. D, both of which were dim and relatively cool. D. virilis was more frequent at spot E associated with beer. The species in the fourth group were dominant at spot H.. 2. Seasonal changes of population sizes and of age constitutions. Fig. 3 shows the seasonal fluctuations of population sizes for six domestic species. No attempt was made to estimate actual population numbers, and the numerical estimates of the populations are therefore of course not abso- ~r=o.w. lute. However, as all domestic Drosophila had. D.malanogaster. been very well trapped (Watabe et al., 1980) and. D.funebris D.virilis. the same sample unit has been used for each. D.lacertosa. collection, numerical changes in the trapping. D.hydei D. busckii. popultions are considered to reflect similar. D. bifasciata. changes in the order of the size of the actual. D.auraria. population. Numerical changes were more pro-. D.testacia D. histrio. nounced for D. melanogaster, D. simulans, D.. D.unispina. immigrans and D. bnsckii than for D. virilis. D.. D.immigrans. melanogaster has never or scarcely ever been. [- D.brachynephros. collected in the spring months. Of the 9697. D.nigromaculata D.confusa. trap-caught D. melanogaster adults, only three. D. lutescens. flies were captured in May. Thereafter the. D.sumulans. numbers increased and reached a peak on. A.okadai D.suzukii. September 27.. A.conifera takadai. D. simulans, D. immigrans, D. busckii and D.. ftinebris behaved in a similar fashion. Fig. 4 investigates the seasonal changes in the proportions of the three commonest species. D. melanogaster was the most abundant during the. (12). Fig. 2 Niche overlap dendrogram for 20 species collected in and near the brewery..
(8) 47. Microdistritution and Phenology in Drosophila. 1200. 600+ A) D.melanogaster. 800. 400. 400. B) D.virilis. 200. MAY I JUN UUL IAUG I SEPT I OCT iNOVl ' IMAY IJU N JJUL FAUG ISEPT IOCT INOV 400. 80+. C) D.immigrans. 200. 40. E) D.simulans. 40. D) D. busckii. w[ F)D.funebris -^ 0. Fig.3 Seasonal changes in the number of Drosophila species. Open circles indicate that none of specimens was collected.. 100C. "HTfKl 3.melanog. 50. ; D.virilis. J7. J5. 56. A2. 04. N1. Fig.4 Seasonal changes in the proportions of the three commonest species of Drosophila in the brewery.. summer and D. immigrans became dominant in the fall. D. virilis was more evenly distributed. over the whole year except for the snowy months, but the size of its population reached a maximum in late September. Fig. 5 and Table 5 show the seasonal changes in the female age structures for domestic species. The high ratio of sexually mature females during the warm. months suggests the active breeding of the species. Two types were distinguished in the female reproductive conditions of fall Drosophila populations (Fig. 5). Most females of D. melanogaster, D. immigmns, D. simulans and D. busckii had mature ovaries with eggs during all the active. (13).
(9) Hide-aki WATABE. 600r. 400. A) D.melanogaster. 200 0^. M.17 J.7 15 A.2. 5.6 0.4 N.1. o1. M.17. Fig. 5. Seasonal changes in the number of females and in the female reproductive conditions for D. melanogaster and D. virilis. Black (the females with undeveloped ovaries), open (developing ovaries) and vertical lines (mature ovaries) {cf. Watabe and Beppu,. 1977).. Table 5. Seasonal changes in the female reproductive conditions for six domestic Drosophila. Date Ovarian stages. D. D. melanogaster. May 17 I II III. Jun.21. Jul.19. I II III. I II III. 2 - -. - 110. Aug.16. I ii in. I 11 III. I ii m. 6. 57. 4 18 347. 7 18 472. 2. 8. 33. 20 14 205. 2. 17. 1. 1. 5. 10. 17. 72. 125 35 180. 242. 4. 2. 4. 6. 6. 4. D. immigmns D. simulans. 2. D. biisckii D. virilis. 4 4 73 4 6 90. 5 31. D. fimebris. Oct. 18. Sept.19. 1 24. 3. 1. Nov.8. I 11 III 3. 21. 6. 23. 29. 3. 7. 8. 13. 1. 8. 26. 77. 1. 1. 5. 6. 5. 1). Ovarian developments of the females were classified into three stages; Stage I (undeveloped ovaries), Stage II (developing), Stage III (mature) (Watabe and Beppu, 1977).. months, even in the fall. The fall populations of D. virilis (Fig. 5) and probably D. fzmebris also (Table 5) were dominated by females with undeveloped ovaries.. (14).
(10) Microdistritution and Phenology in Drosophila 49. Discussion Though domestic species of Drosophila have been found all over the world, they are closely adapted to a specific niche that man has created (Carson, 1965 ; Dobzhansky, 1965). The socalled "domestic environment" has been discussed as a whole in comparison with the "natural environment" , it consists of various ecological factors, e. g., food, humidity, temperature and. light conditions (cf. McKenzie, 1974 ; Shorrocks, 1974 ; Atkinson and Shorrocks, 1977). Atkinson and Shorrocks (1977) have demonstrated that domestic Drosophila utilize the different resources at their main breeding sites. A slight differnce in microenvironments may influence. the species composition of Drosophila and allow them to coexist in nature (IVterrell, 1951 ; McKenzie, 1974). D. melanogaster and D. simulans are siblings with considerable morphological and genetic similarity. In Japan, D. simulans is a recently introduced colonizer (Watanabe and Kawanishi,. 1976 ; Watabe et al., 1980) and coexist with D. melanogaster in many places. In the brewery these two species were present at many trap-spots. A relatively higher photo-preference of D.. simidans adults than D. melanogaster has bden reported (Kawanishi and Watanabe, 1978). This explains the absence or poverity of D. smwlans inside houses which are relatively darker than the open air. It is also known that D. melanogaster is more resistant to the presence of alcohol. than its sibling (McKenzie and Parsons, 1974). Pipkin and Hewitt (1972) have reported that the specific activity of ADH (alcohol dehydrogenase) for D. melanogaster was much higher than that of D. simulans. IVIcKenzie (1974) has observed that during the vintage season in Australia D. simulans is only collected outside the cellars seperate from the alcohol resource. In the. brewery it was also absent at site B, which was a sunny place affected by strong alcohol fumes (Table 2, 3). This indicates that the narrow niche of D. simnlans in the brewery would be primarily due to the presence of the alcohol resource.. D. virilis is considered to be native to East Asia (Patterson and Stone, 1952 ; Throckmorton, 1975 ; Watabe and Higuchi, 1979), but it has never been collected in natural environments, at least in Japan. Its large populations are restricted to two kinds of specific. habitat; both at breweries and timberyards in Honshu (Saito et al., 1962) and only at the former in Hokkaido (Kaneko et al., 1966 ; Watabe, Unpubl.). During the survey a large number of the D. virilis larvae were collected in recovered beer bottles. This species is associated with beer.. D. immigrans had the broad niche, but it became more abundant in the fall, unlike D. melanogaster. This species is rather common in the sparce forests or orchards near human. habitations. It has a tendency to invade natural forests (Toda, 1973, 1974) and to immigrate at high elevations during a dry summer in southern Japan (Hirumi, 1961). Among domestic species of Drosophila, D. immigrans is a only species collected in natural forests in Hokkaido. D. biisckii was collected abundantly at garbages by a net-sweeping. This suggests that its food preference is likely to be different from that of other domestic species {cf. Carson, 1965 ; Atkinson and Shorrocks, 1977). It is difficult to discuss the microhabitat of D. hydei and D. fzmebris because of the small samples taken. The former is more frequent in southern Japan and. (15).
(11) 50. Hide-aki. WATABE. the latter in north-eastern Hokkaido.. A difference was also found in the phenology of domestic Drosophila. Except for D. virilis, all domestic species are very rare in spring. The differences in the seasonality suggest the probable difference in their respective ability to tolerate cold stress. Yamamoto and Ohba (1982) have reported that D. virilis has greater resistance to cold than D. immigrans. This agrees with the present results obtained in the brewery. These differences in domestic species of Drosophila may have some relation to their original distributions. As we have said, domestic Drosophila are now cosmopolitan, but their wide range of distribution is due to modern artificial transportation. Doth D. melanogaster and D. simulans are probably peculiar to either Africa or. South-east Asia, while D. immigrans and D. busckii to the latter (cf. Dobzhansky, 1965 ; Throckmorton, 1975). D. virilis is native to East Asia and D: funebris probably comes from near Moscow (cf. Dubnin and Tiniakov, 1946a, b, 1947). The first four species are originally a tropical species of the Old World and the last two species are temperate species. Therefore D. virlis and probably D. funebris are also likely to have a superior ability to tolerate cold stress than the tropical origin species of Drosophila. In conclusion, the Sapporo populations of D. melanogaster, D. simzdans, D. immigrans and. D. bzisckii are constituted in the following spring by a very small number of founder individuals which have overwintered indoors and/or immigrated from warm southern districts.. Acknowledgements I wish to express my sincere thanks to Dr. Eizi Momma for his pertinent guidance in the course of the present study. Cordial thanks are due to Dr. IVCasanori J. Toda and Mr. Takashi Nagao for their kind suggestion and help in statistical treatments of data, and to the Sapporo Meteorological Observatory for offering the meteorological data.. References. Atkinson, W. and B. Shorrocks, 1977. Breeding site specificity in the domestic species of Drosophila. Oecologia (Berl.) 29 : 223-232. Carson, H. L., 1965. Chromosomal morphism in geographically widespread species of Drosophila. In The Genetics of Colonizing species (eds.H.G. Baker and G. L. Stebbins), pp. 503—531. Academic Press. New York. Chiang, H. C., D. Benoit and J. Maki, 1962. Tolerance of adult Drosophila melanogaster to sub-freezing temperatures. Can. Entmol. 94 : 722-727. Colwell, R. K. and D. J. Futuyma, 1971. On the mesurement of niche breadth and overlap. Ecology 52 : 567576. Dobzhansky, Th., 1965. "Wild" and "domestic" species of Drosophila. In The Genetics of Colonizing Species (eds. H. G. Baker and G. L. Stebbins), pp. 533—546. Academic Press. New York. Dubinin, N. P. and G. G. Tiniakov, 1946a. Natural selection and chromosomal variability in populations of Drosophila fnnebris. J. Hered. 37 : 39—44.. (16).
(12) Microdistritution and Phenology in Drosophila 51 Dubinin, N. P. and G. G. Tiniakov, 1946b. Inversion gradients and natural selection in ecological races of. Drosophila funebris. Genetics 31 : 537—545. Dubinin, N. P. and G. G. Tiniakov, 1947. Inversion gradients and selection in the ecological races of Drosophila fnnebris. Am. Nat. 81 : 148-153. Hirumi, H., 1961. Studies on the chromosomal polymorphism in natural populations of Drosophila immigrans in southerncentral districts of Japan. Jpn. J. Genet. 36 : 297—305. (In Japanese with English summary). Kaneko, A., M. Kawakami and H. Takada, 1966. Drosophila survey of Hokkaido, XXII. Drosophilid flies collected in breweries. J. Fac. Sci., Hokkaido Univ. Ser. VI (Zool.) 16 : 31—37. Kawanishi, M. and T. K. Watanabe, 1978. Difference in photopreferences as a cause of coexistence of Drosophila simnlans and D. melanogaster in nature. Jpn. J. Genet. 53 : 209—214. Kimoto, S., 1976. Methods of the ecological study, 14. Methods of ecological study on animal community, I. Diversity and constitutions of species. pp. 192. Kyoritsu Publ. Tokyo. (In Japanese). Lumme, J., 1978. Phenology and photoperiodic diapause in northern populations of Drosophila. In Evolution of Insect Migration and Diapause(ed. H. Dingle), pp. 145—170. Springer-Verlag. New York. Mather, W. B., 1956. The genus Drosophila (Diptera) in eastern Queensland. II. Seasonal changes in a natural population. Aust. J. Zool. 4 : 65-75.. McKenzie, J. A., 1974. The distribution of vineyard populations of Drosophila melanogaster and Drosophila simulans during vintage and non-vintage periods. Oecologia (Berl.) 15 : 1-16. McKenzie, J. A. and P. A. Parsons, 1974. Microdistribution in a natural population of Drosophila melanogaster to alcohol in the environment. Genetics 77 : 385—394. Merrell, D. J., 1951. Interspecific competition between Drosophila fnnebris and Drosophila melanogaster. Am. Nat. 85 : 159-169. Minami, N., M. J. Toda and K. Beppu, 1979. Ecological structure of drosophilid assemblage in Tomakomai Experimental Forests, Hokkaido University. Res. Bull. Coll. Exp. Forests, Coll. Agr., Hokkaido. Univ. 36 : 479—508. (In Japanese with English summary) Nozawa, K., 1956. A statistical study on the natural population of genus Drosophila. Jpn. J. Ecol. 6 : 1—5. (In. Japanese with English summary) Patterson, J. T. and W. S. Stone, 1952. Evolution in the genus Drosophila, pp. 610. MEacmillan Co. New York.. Pipkin, S. B. and N. E. Hewitt, 1972. Variation of alcohol dehydrogencse lebels in Drosophila species hybrids. J. Hered. 63 : 267-270. Saito, S., Y. Ohmori, S. Matsui and T. Komatsubara, 1962. Seasonal observation of Drosophila in Niigata City. Jpn. J. Sanit. Zool. 13 : 229-232. (In Japanese) Shorrocks, B., 1974. Niche parameters in domestic species of Drosophila. J. Nat. Hist. 8 : 215—222. Takada, H. and Y. Toyofuku, 1960. Notes on hibernation of Drosophilidae in Hokkaido. Zool. Mag. 69 :223—. 232. (In Japanese with English summary). Throckmorton, L. H., 1975. The phylogeny, ecology and geography of Drosophila. In Handbook of Genetics (ed. R. C. King), 3 : 421-469. Plenum Press. New York. Toda, M.. ]., 1973. Seasonal activity and microdistribution of drosophilid flies in Misumai in Sapporo. J. Fac. Sci., Hokkaido Univ. Ser. VI (Zool.) 18 : 532-550. Toda, M. J., 1974. A preliminary study on microdistribution and dispersal in drosophilid natural populations. J. Fac. Sci., Hokkaido Univ. Ser. VI (Zool.) 19 : 641-656. Toda, M. J., 1977. Two new "retainer" bait traps. Drosophila Inform. Serv. 52 : 180. Watabe, H., 1979. Drosophila survey of Hokkaido, XXXVI. Seasonal changes in the reproductive condition of wild and domestic species of Drosophila. J. Fac. Sci., Hokkaido Univ. Ser. VI (Zool.) 21 : 365— 372. Watabe, H. and K. Beppu, 1977. Drosophila survey of Hokkaido, XXXIII. Ovarian development of Drosophila in relation to wild population. J. Fac. Sci., Hokkaido Univ. Ser. VI (Zool.) 20 : 611—620.. Watabe, H., and C. Higuchi, 1979. On a new species of the virilis group of the genus Drosophila (Diptera, Drosophilidae), with revision of the geographical distribution of the group. Annot. Zool. Japan. 52 : 203-211.. (17).
(13) 52. Hide-aki. WATABE. Watabe, H., E. Momma and M. T. Kimura, 1980. Changes in drosophilid fauna at the University Botanical Garden in Sapporo, Japan. Drosophila Inform. Serv. 55 : 141—142. Watanabe, T. K. and M. Kawanishi, 1976. Colonization of Drosophila simulans in Japan. Proc. Jpn. Acad.52: 191-194. Wheeler, M.R., 1981. The Drosophilidae : A taxonomic overview. In The Genetics and Biology of Drosophila (eds. M. Ashburner,H. L. Carson and J.N. Thompson, Jr.), 3a : 1-97. Acadmic Press. New York. Yamamoto, A. and S. Ohba, 1982. Strategic differences in thermal adaptation between two Drosophila species,. D. virilis and D. immigrans. Oecologia (Berl.) 52 : 333—339.. (18).
(14)
図
関連したドキュメント
A NOTE ON SUMS OF POWERS WHICH HAVE A FIXED NUMBER OF PRIME FACTORS.. RAFAEL JAKIMCZUK D EPARTMENT OF
A lemma of considerable generality is proved from which one can obtain inequali- ties of Popoviciu’s type involving norms in a Banach space and Gram determinants.. Key words
[r]
proof of uniqueness divides itself into two parts, the first of which is the determination of a limit solution whose integral difference from both given solutions may be estimated
de la CAL, Using stochastic processes for studying Bernstein-type operators, Proceedings of the Second International Conference in Functional Analysis and Approximation The-
[3] JI-CHANG KUANG, Applied Inequalities, 2nd edition, Hunan Education Press, Changsha, China, 1993J. FINK, Classical and New Inequalities in Analysis, Kluwer Academic
When a 4-manifold has a non-zero Seiberg-Witten invariant, a Weitzenb¨ ock argument shows that it cannot admit metrics of positive scalar curvature; and as a consequence, there are
Such bounds are of interest because they can be used to improve estimates of volumes of hyperbolic manifolds in much the same way that B¨ or¨ oczky’s bounds [B¨ o1], [B¨ o2] for