お問合せ先
茨城大学学術企画部学術情報課(図書館) 情報支援係
http://www.lib.ibaraki.ac .jp/toiawas e/toiawas e.html
T itle
S pecies of Phragmidiaceae (Pucciniales) collected in the
L iupanshan, Ningxia Hui A utonomous R egion, C hina
A uthor(s )
ONO, Y oshitaka; W E NG, Y ue-T ai; X UE , Y u
C itation
茨城大学教育学部紀要. 自然科学 = B ulletin of the F aculty
of E ducation, Ibaraki University. Natural science, 67: 27-36
Is s ue D ate
2018-01-30
UR L
http://hdl.handle.net/10109/13512
R ig hts
*College of Education, Ibaraki University, Mito, 310-8215; Department of Forestry, School of Forestry, Northeast Forestry University, Harbin, Heilongjiang, 150040, P. R. China
**Department of Forestry, School of Forestry, Northeast Forestry University, Harbin, Heilongjiang, 150040, P. R. China
Species of Phragmidiaceae (Pucciniales) collected in the
Liupanshan, Ningxia Hui Autonomous Region, China
Yoshitaka Ono*, Yue-Tai Weng** and Yu Xue**
(Accepted August 31, 2017)
Abstract
During the rust fungus survey in September 2016 in the Liupanshan, Ningxia Hui Autonomous
Region, P. R. China, Gymnoconia peckiana var. verrucosa, Phragmidium barclayi and P. sikangense were found on Rubus spp., Phragmidium potentillae on Potentilla sp. and Phragmidium tuberculatum on Rosa omeiensis. These collections include a new observation of mixed infections of three rust species on a single host leaf and new host/geographic distribution records for Ningxia
Hui Autonomous Regions.
Keywords – Geographic distribution・Host preference・Potentilla・Pucciniales・Rosa・Rubus
Introduction
People’s Republic of China (inclusive of main continental part and islands) has traditionally been well
studied for rust fungi. Compilation of rust taxa from the records published by Tai (1979), Teng (1996)
and Zhuang et al. (1998, 2003, 2005, 2012) revealed that 999 species in 57 teleomorphic genera and 76 anamorphic species are distributed in China (Ono unpublished). In the Phragmidiaceae, ifty-four species in seven genera are recorded: Gerwasia (two species), Gymnoconia (one species), Hamaspora (seven species),
Kuehneola (four species), Phragmidium (thirty-seven species), Trachyspora (one species) and Xenodochus
(two species) (Li 1988; Zhuang et al. 2012). All the species occur exclusively on rosaceous plants, except for
three in Kuehneola.
By contrast, Ningxia Hui Autonomous Region has been poorly explored for rust fungi despite that the Region is diverse in geology and topography and rich in lora and vegetation. Nine national nature reserves have been designated in Ningxia Hui Autonomous Region. Ecologists have endeavored in value assessment of
ecosystem services in relation to ecological restoration and nature conservation in national nature reserves in
Ono et al.: Puragmidiaceous rust fungi in Liupanshan 27
Ningxia (Wang et al. 2014). We carried out the survey and collection of rust fungi in the Liupanshan in 8–10
September 2016 and found several rare, otherwise interesting, rust taxa. We herein report one Gymnoconia
and four Phragmidium species with new host and geographic distribution records.
Materials and Methods
Survey sites: The geography and vegetation of the Liupanshan was briely described by Ono et al. (2018).
The rust fungus survey was carried out in 8–10 September 2016. The sites surveyed were: 1) near the
summit of the Liupanshan, close to the Red Army Long March Museum (approx. 35°40´N, 106°12´E and alt.
2700–2800 m); 2) the Liupanshan National Forest, Yehegu (Wild Lotus Valley) Scenic Spot, off prefectural
road 344 (approx. 35°51´N, 106°13´E and alt. ca. 2300 m); 3) the Liupanshan National Forest, Liangdian
Gorge Area, off prefectural road 344 (approx. 35°23´N, 106°16´E and alt. 2200-2300 m); 4) the Liupanshan
National Forest, Liangdian Gorge Area, along Xiaonan River trail (approx. 35°21´N, 106°18´E and alt. ca.
2100 m); and the Liupanshan National Forest, near Lao Long Tan (Dragon King Pool) Scenic Spot (approx.
35°23´N, 106°20´E and alt. ca. 2000 m). Permission and guide were provided by the Guyuan Liupanshan
Forest Management Department, the Liupanshan National Nature Reserve Management Bureau.
Rust-infected leaves were collected, dried and preserved as herbarium specimens in the Herbarium of
Systematic Mycology, the College of Education, Ibaraki University (IBAR) and in the mycological herbarium
of the Department of Forestry, Northeast Forestry University.
Microscopy: Morphology and size of paraphyses and spores were examined under a differential interference
contrast (DIC) microscope (Olympus BX51-DIC) equipped with an Olympus DP21 digital photography
system. Spore size (20 spores/specimen) was measured with an Olympus DP21 digital photography system.
Results and Discussion
Gymnoconia peckiana (Howe) Trotter var. verrucosa (N. Zhang) J.Y. Zhuang & S.X. Wei in W.Y. Zhuang,
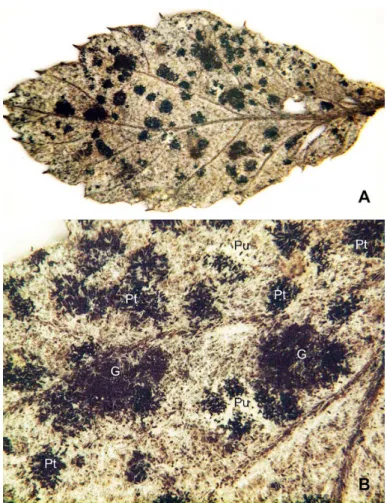
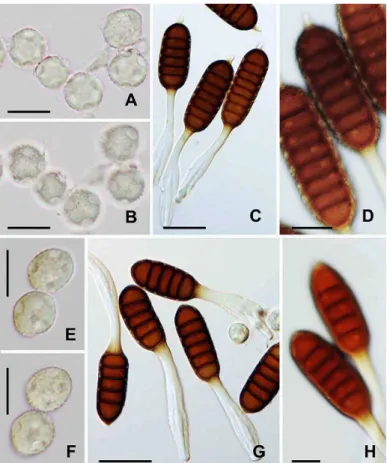
Fungi of Northwestern China, p. 242, 2005. Figs. 1 & 2A, B.
Only telia occurred on the abaxial leaf surface. The sori were subepidermal in origin, becoming erumpent,
powdery, and dark purplish brown (Fig. 1B). No paraphyses were observed in the sori. Teliospores were
two-celled, formed on a pedicel, but becoming deciduous upon maturation, variable in shape, oblong-ellipsoid,
broadly ellipsoid, obovoid or ovoid, and 36–56 × 23–35 µm in size (Fig. 2A). The wall was evenly 1.5–2.5
µm thick, dark cinnamon- or chestnut-brown, with prominent longitudinally arranged warts and ridges
(Fig. 2B). The germ pore of upper teliospore cells was located at or near the apex and of lower about a half
depressed below the septum.
Specimen examined: on Rubus erythrocarpus T.T. Yu & L.T. Lu, People’s Republic of China, Ningxia Hui Autonomous Region, Guyuan, Liupanshan, on prefectural road 415 from Longde, alt. 2700-2800 m (approx.
35°39´N and 106°12´E), 8 September 2016. Y. Ono, Y. Xue and Y.T. Weng 16CH-2 (T, IBAR-11161).
cylindrical, bearing short bristles and short slender prickles. Leaves were mostly trifoliolate, but a few imparipinnately quiquefoliate leaves were present. Petioles were 2.5–5 cm long. Petiolules of terminal lealets were 2–3.5 cm long. Lateral lealets had short petiolule of 1–3 mm long. The petiolules bore short bristles and slender prickles. Stipules were linear and 2–3 mm long. Blade of terminal lealets was ovate to ovate-lanceolate with cuneate or round base, and 3–4 × 1.5–2 cm in size. Lateral leaflets were ovate or elliptic with cuneate or round base, and 1.5–2.5 × 0.8–1.5 cm in size. The abaxial surface of the lealets was densely gray tomentose with slender prickles sparsely distributed along veins. The adaxial surface of the leaflets was pilose. Margin of the terminal lealets was coarsely incised, doubly serrate or irregularly and sharply serrate with acute or shortly acuminate apex. These morphological features agree with the description of R.
erythrocarpus (Li and Boufford 2003), except for the color of branchlets, the size of lealets and the length
Fig. 1. Mixed infections of Gymnoconia and two Phragmidium species on Rubus erythrocapus (IBAR-11161) A. Densely formed uredinia and telia on the abaxial surface of a lealet of an imparipinnately compound leaf. B. Details of sorus distribution of Gymnoconia and two Phragmidium species. G: Telia (dark purplish brown) of G. peckiana var. verrucosa. Pu: Uredinia (dull white) of Phragmidium species. Pt: Telia (black) of Phragmidium species. Note that two Phragmidium species cannot be differentiated by sorus characteristics.
of petioles and petiolules. Rubus erythrocarpus has been recorded in northwest of Yunnan Province (Li and Boufford 2003). Although our host identiication cannot be conclusive because species of the genus Rubus is prominently diverse in species and individuals of each species are also highly variable, R. erythrocarpus is the species that is most close in vegetative morphology to our collection.
This rare rust fungus was irst found on R. pileatus Focke in Pingli, Shaanxi Province (Zhang et al. 1997). An additional collection was made on R. bilorus Buch.-Ham. ex Sm. in the Liupanshan (Zhuang et al. 2012).
Rubus erythrocarpus is a new host plant of this fungus.
uredinia and blackish telia of Phragmidium species (Fig. 1B). However, the two Phragmidium species could not be differentiated by sorus features under a binocular microscope. Simultaneous infections of two or more
rust species on a single leaf of a host plant may be infrequent, if not rare (Ono 2016). When telial stages of different species are present on a single leaf, there would be no dificulty in distinguishing those species, because rust species are circumscribed mostly by teliospore morphology. However, if spermogonial-aecial
sori and/or uredinial sori are present together with separate telial sori, there is a risk at which different spore
stages of different species are erroneously connected in the life cycle. If teliospores occur in uredinia, or
urediniospores in telia, life-cycle connection between the uredinial and telial stages can be substantiated (see
below).
Arthuriomyces rubicola J.Y. Zhuang & S.X. Wei (the genus now synonymized under Gymnoconia) was once described for a fungus that was determined to occur on R. pileatus in Jiuzhaigou, Sichuan Province (Zhuang and Wei 1993). The fungus was later found to be Puccinia atragenes W. Hausm. on Clematis sp. (Zhuang et al. 2012). It was apparently accidental stray that frequently happens when a large amount of rust
collections is handled at the same time.
Phragmidium potentillae (Pers.) P. Karst., Bidr. Känn. Finl. Nat. Folk 31: 49, 1878. Fig. 2C–E.
Uredinia and telia were formed densely on the abaxial surface of lealets of trifoliate leaves. Uredinia were subepidermal in origin, becoming erumpent, densely paraphysate at periphery, powdery, and orange-yellow.
The paraphyses were cylindrical to clavate, moderately incurved, and 41–70 × 10–15 µm in size (Fig. 2E).
The wall was thin and colorless. Urediniospores were borne singly on a pedicel, soon becoming deciduous,
broadly ellipsoid to obovoid, and 19–24 × 15–18 µm in size (Fig. 2C). The wall was evenly ca. 1.5 µm thick,
colorless, and completely echinulate (Fig. 2D). No germ pore was observed. Telia were similar to, but smaller
than, uredinia, or replacing uredinia. Teliospores were three- to six-celled, borne on a persistent pedicel,
cylindrical with round or conical apex, base attenuating towards the pedicel, and 41–87 × 21–26 µm in size
(Fig. 2E). The wall was 3–4 µm thick at sides and up to 5 µm thick at the apex, sooth-surfaced, and dark
chestnut-brown. The pedicel was 46–98 µm long, 7–9 µm wide, and non-hygroscopic (Fig. 2E)
Specimen examined: on Potentilla sp., People’s Republic of China, Ningxia Hui Autonomous Region, Guyuan, Liupanshan, on prefectural road 415 from Longde, alt. 2700-2800 m (approx. 35°39´N and
106°12´E), 8 September 2016. Y. Ono, Y. Xue and Y.T. Weng 16CH-6 (U & T, IBAR-11165).
This is the irst record of P. potentillae in Ningxia Hui Autonomous Region. This rust species has been known to occur on a number of Potentilla species and widely distributed in temperate regions in the Northern Hemisphere.
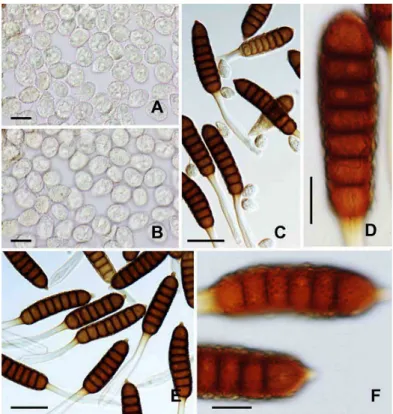
Phragmidium tuberculatum Jul. Müll., Ber. Deutsch. Bot. Ges. 3: 391, 1885. Fig. 3A–D.
Uredinia and telia occurred in dense groups on the abaxial surface of lealets. Uredinia were subepidermal in origin, becoming erumpent, densely paraphysate at periphery, powdery, and orange-yellow. The paraphyses
were cylindrical to clavate, moderately to strongly incurved, and 36–53 × 9–16 µm in size. The wall was
ventrally thin, dorsally thickened to 8 µm, and colorless. Urediniospores were borne singly on a pedicel, soon
becoming deciduous, subglobose to broadly ellipsoid, and 18–22 × 16–20 µm in size (Fig. 3A). The wall
was evenly ca. 1.5 µm thick, colorless, and completely echinulate (Fig. 3B). Germ pores were mostly six and
scattered on the wall. The wall at the germ pore intruded into the spore lumen (Fig. 3A, B). Telia were similar to, but smaller than, uredinia, or replacing uredinia. Teliospores were ive- to seven-celled, rarely eight-celled, borne on a persistent pedicel, cylindrical, round or conical at the apex, round at the base, and 65–75 × 26–36
µm in size (Fig. 3C). The wall was 3–5 µm thick, densely covered with prominent warts, and dark
chestnut-brown (Fig. 3D). The apical papilla was apparent and up to 15 µm high. The pedicel was 90–124 µm long.
The hygroscopic part at lower half of the pedicel was 15–22 µm wide (Fig. 3C).
Specimen examined: on Rosa omeiensis Roelfe, People’s Republic of China, Ningxia Hui Autonomous Region, Liupanshan National Forest, Liangdian Gorge Area, off prefectural road 344, alt. 2200–2300 m
(approx. 35°23´N and 106°16´E), 10 September 2016. Y. Ono, Y. Xue and Y.T. Weng 16CH-25 (U & T,
IBAR-11184).
This is the irst record of P. tuberculatum in Ningxia Hui Autonomous Region. This rust species has been known to occur on a number of Rosa species and widely distributed in temperate regions in the Northern Hemisphere.
Phragmidium barclayi Dietel, Hedwigia 29: 264, 1890. Figs. 1 & 3E–H.
Uredinia and telia were scattered or in dense groups on the abaxial surface of leaflets. Uredinia were
subepidermal in origin, becoming erumpent, densely paraphysate at periphery, powdery, and orange-yellow.
The paraphyses were cylindrical, moderately incurved, and 48–98 × 8–16 µm in size. The wall was thin and
colorless. Urediniospores were borne singly on a pedicel, soon becoming deciduous, subglobose to broadly
ellipsoid, and 22–27 × 17–22 µm in size (Fig. 3E). The wall was evenly ca. 1.5 µm thick, colorless, and
completely echinulate (Fig. 3F). Germ pores were mostly six and scattered on the wall. The wall at the germ
pore intruded into the spore lumen (Fig. 3E, F). Telia were similar to, but smaller than, uredinia, or replacing
uredinia. Teliospores were four- to seven-celled, rarely eight-celled, borne on a persistent pedicel, cylindrical,
round at both ends, and 58–101 × 29–38 µm in size (Fig. 3G). The wall was 3–6 µm thick at side, up to 9
µm thick at the apex, sparsely covered with small warts, and dark chestnut-brown (Fig. 3H). The pedicel was
52–134 µm long. The hygroscopic part at lower half of the pedicel was (9–) 14–22 µm wide (Fig. 3G).
Specimens examined: on Rubus erythrocarpus T.T. Yu & L.T. Lu, People’s Republic of China, Ningxia Hui Autonomous Region, Liupanshan, on prefectural road 415 from Longde, alt. 2700–2800 m (approx. 35°39´N
and 106°12´E), 8 September 2016. Y. Ono, Y. Xue and YT. Weng 16CH-2 (T, IBAR-11161); on Rubus aff.
niveus Thunb., People’s Republic of China, Ningxia Hui Autonomous Region, Liupanshan, on prefectural road 415 from Longde, alt. 2700–2800 m (approx. 35°39´N and 106°12´E), 8 September 2016. Y. Ono, Y.
Xue and Y.T. Weng 16CH-8 (U & T, IBAR-11167); Liupanshan National Forest, Yehegu (Wild Lotus Valley)
Scenic Spot, off prefectural road 344, alt. ca. 2300 m (approx. 35°51´N and 106°13´E), 9 September 2016. Y.
Ono, Y. Xue and Y.T. Weng 16CH-18 (T, IBAR-11177); Liupanshan National Forest, Liangdian Gorge Area,
off prefectural road 344, alt. 2200-2300 m (approx. 35°23´N and 106°16´E), 10 September 2016. Y. Ono, Y.
Xue and Y.T. Weng 16CH-30 (U & T, IBAR-11189).
This rust species has been reported from the Himalayas (Dietel 1890; Arthur and Cummins 1933; Sydow
and Mitter 1933; Sydow 1938; Ahmad 1956; Durrieu 1977; Ono 1992; Khalid et al. 1993; Zhuang and Wei
1994). In China, this fungus has been recorded on R. austrotibetanus T.T. Yu & L.T. Lu in Mt. Qomolangma region, Tibetan Everest Himalaya (Zhuang and Wei 1994) and on R. niveus (reported as R. foliolosus D. Don) in Yunnan Province (Kakishima et al. 2002). The host plants of the three collections (IBAR-11167, 11177
and 11189) from the Liupanshan appear to be R. niveus; however, the identiication is not conclusive because
the plants of our collections are sterile and because the species is highly variable in vegetative morphology.
Phragmidium barclayi is new to Ningxia Hui Autonomous Region and R. erythrocarpus is a new host plant of this rust species.
Phragmidium sikangense Petr., Meddn Göteb. Bot. Trädg. 17: 123, 1947. Figs. 1 & 4.
Uredinia and telia were scattered or in dense groups on the abaxial surface of leaflets. Uredinia were
subepidermal in origin, becoming erumpent, densely paraphysate at periphery, powdery, and orange-yellow.
The paraphyses were cylindrical or clavate, moderately incurved, and 49–110 × 11–22 µm in size. The wall
was thin and colorless. Urediniospores were borne singly on a pedicel, soon becoming deciduous, subglobose,
broadly ellipsoid or obovoid, and 22–29 × 15–20 µm in size (Fig. 4A). The wall was evenly ca. 1.5 µm
thick, colorless, and completely echinulate (Fig. 4B). No germ pores were observed in the urediniospores. Telia were similar to, but smaller than, uredinia, or replacing uredinia. Teliospores were ive- to eight-celled, mostly seven- or eight-celled, borne on a persistent pedicel, cylindrical, round at both ends, and 80–116 × 25–
30 µm in size (Fig. 4C, E). The apical papilla was up to 5–10 µm high. The wall was 3–5 µm thick, densely
covered with prominent warts, and dark chestnut-brown (Fig. 4D, F). The pedicel was 89–138 µm long. The
hygroscopic part at lower half was 10–18 µm wide (Fig. 4C, E).
Specimens examined: on Rubus erythrocarpus T.T. Yu & L.T. Lu, People’s Republic of China, Ningxia Hui Autonomous Region, Liupanshan, on prefectural road 415 from Longde, alt. 2700-2800 m (approx.
35º39´N and 106º12´E), 8 September 2016. Y. Ono, Y. Xue and Y.T. Weng 16CH-2 (U & T, IBAR-11161); on
Liupanshan National Forest, Liangdian Gorge Area, off prefectural road 344, alt. 2200-2300 m (approx.
35º23´N and 106º16´E), 10 September 2016. Y. Ono, Y. Xue and Y.T. Weng 16CH-36 (T, IBAR-11195).
Only telial stage has been known for P. sikangense (Wei 1988; Zhuang et al. 2012). Our collection on R.
erythrocarpus (IBAR-11161) bore teliospores morphologically the same as those on R. pungens var. oldhamii
(IBAR-11195) (Fig. 4C, D, E and F). The specimen (IBAR-11161) also bore G. peckiana var. verrucosa and P.
barclayi in addition to P. sikangense (see above, Fig. 1). The two Phragmidium species formed teliospores in separate telia or in uredinia together with urediniospores. Because the two species can easily be distinguished
by morphology of teliospores and because co-occurrence of urediniospores and teliospores in the same
sori may prove the same ontogenic origin of the two kinds of spores, the life-cycle connection between the
uredinial and telial stages of P. sikangense is substantiated. In addition, the fact that urediniospores of P. barclayi bear several distinct germ pores scattered on the spore wall (Fig. 3E, F) supports the conclusion that the urediospores without apparent germ pores (Fig. 4A, B) belong to P. sikangense.
Phragmidium sikangense has been recorded on R. inopertus (Focke) Focke in Sichuan Province (Wei 1988), R. parvifolius L. in Shaanxi Province (Tai 1979), R. pileatus Focke in the Qinling Mountains, Shaanxi Province (Cao and Zhuang 2000), R. pungens Cambess. in Sichuan Province (Tai 1979), R. pungens
var. oldhami (Miq.) Maxim. in the Qinling Mountains, Shaanxi Province (Cao and Zhuang 2000), and
R. teledapos Focke in the Qinling Mountains, Shaanxi Province (Cao and Zhuang 2000). Phragmidium sikangense on R. pungens var. oldhami is a new geographic distribution record in Ningxia Hui Autonomous Region and R. erythrocarpus is a new host plant of this fungus.
Disclosure
The authors declare no conlict of interests. This study complies with the current laws of P. R. China and Japan.
Acknowledgments
This study was supported, in part, by Grant-in-Aid from Northeast Forestry University, P. R. China.
The field survey and collecting study materials were carried out under the permission from the Guyuan
Liupanshan Forest Department, the Liupanshan National Nature Management Bureau, Forest Ministry of P. R. China. We thank Mr. Xiao-Fu Cheng, the Guyuan Liupanshan Forest Department, for his ield guide to the Liupanshan. Rust fungus specimens were imported under permission of the Ministry of Agriculture, Forestry
and Fisheries, Japan (28 Yokoshoku No. 672).
References
Ahmad S. 1956. Uredinales of West Pakistan. Biologia (Lahore) 2: 27–101.
Arthur JC, Cummins GB. 1933. Rusts of the Northwest Himalayas. Mycolgia 25: 397–406.
Cao ZM, Li ZQ, Zhuang JY. 2000. Uredinales from the Qinling mountains. I. Mycosystema 19: 13–23.
Dietel P. 1890. Uredineen aus dem Himalaya. Nova Hedwigia 29: 259–270.
Durrieu G. 1977. Les rouilles des Rubus au Népal. Trav. G. Viennot-Bourgin, Société Phytopathologie de France. pp. 103–
117.
Kakishima M, Ono Y, Wayuno D, Zhou X, Zang M. 2002. Rust fungi of Phragmidium (Uredinales) from Yunnan Province,
China, collected in 1998. Bulletin of the National Science Museum, Tokyo. Series B (Botany) 28: 11–18.
Khalid AN, Iqbal SH, Parveen B. 1993. Rust lora of Pakistan. II. Genus Phragmidium Link., on Rubus spp. Pakphyton 5: 133–136.
Li B. 1988. A taxonomic study of Puccinia on Polygonaceae from China. Mycosystema 1: 149–177.
Li L, Boufford DE. 2003. Rubus. pp. 195-285. In: Wu ZY, Raven PH, Hong DY (eds.) Flora of China. Vol. 9 (Pittosporaceae
through Connaraceae). Science Press, Beijing, and Missouri Botanical Garden Press, St. Louis.
Ono Y. 1992. Uredinales collected in the Kaghan Valley, Pakistan. pp. 217–240. In: Nakaike T, Malik S (eds.) Cryptogamic
lora of Pakistan. Vol. 1. National Science Museum. Tokyo.
Ono Y. 2016. Mixed infections of grapevine leaf rusts Phakopsora meliosmae-myrianthae and P. montana in Japan. Journal
of General Plant Pathology 82: 338–347; Doi: 10.1007/s10327-016-0655-x.
Ono Y, Weng YT, Xu Y. 2018. Puccinia rhei-palmati occurs in the Liupanshan, Ningxia Hui Autonomous Region, China.
Bulletin of the College of Education, Ibaraki University (Natural Sciences) 67: 17-26.
Sydow H. 1938. Fungi Himalayensis. Annales Mycologici 36: 437–442.
Sydow H, Mitter JH. 1933. Fungi Indici – I. Annales Mycologici 31: 84–97.
Tai FL. 1979. Sylloge Fungorum Sinicorum. Science Press, Peking.
Teng SC. 1996. Fungi of China. Mycotaxon, Ltd. Ithaca.
Wang Y, Gao J, Wang J. Qiu J. 2014. Value assessment of ecosystem services in nature reserves in Ningxia, China: A
response to ecological restoration. PLoS ONE 9(2): e89174. doi:10.1371/journal.pone.0089174.
Wei SX. 1988. A taxonomic study of the genus Phragmidium of China. Mycosystema 1: 179–210.
Zhang N, Zhuang JY, Wei SX. 1997. Fungal lora of the Daba Mountains: Uredinales. Mycotaxon 61: 49–79.
Zhuang JY, Wei SX. 1993. Materials for study on rust fungi of eastern rimland of Quinghai-Xizang (Tibet) Plateau. IV. An
unusual puccinioid rust on Rubus. Mycosystema 6: 47–50.
Zhuang JY, Wei SX. 1994. An annotated checklist of rust fungi from the Mt. Qomolangma region (Tibetan Everest
Himalaya). Mycosystema 7: 37–87.
Zhuang JY, Wei SX. 2005. Urediniomycetes, Uredinales. pp. 233–290. In: Zhuang WY (ed.) Fungi of Northwestern China.
Mycotaxon, Ltd., Ithaca.
Zhuang JY, Wei SX, Wang YC. 1998. Flora fungorum sinicorum. Vol. 10. Uredinales (I). Science Press, Beijing.
Zhuang JY, Wei SX, Wang YC. 2003. Flora fungorum sinicorum. Vol. 19. Uredinales (II). Science Press, Beijing.
Zhuang JY, Wei SX, Wang YC. 2005. Flora fungorum sinicorum. Vol. 25. Uredinales (III). Science Press, Beijing.
Zhuang JY, Wei SX, Wang YC. 2012. Flora fungorum sinicorum. Vol. 41. Uredinales (IV). Science Press. Beijing.