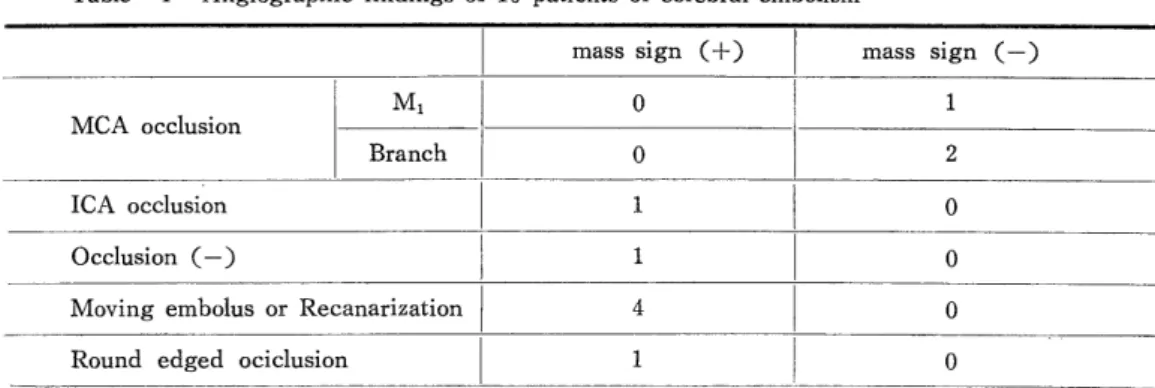
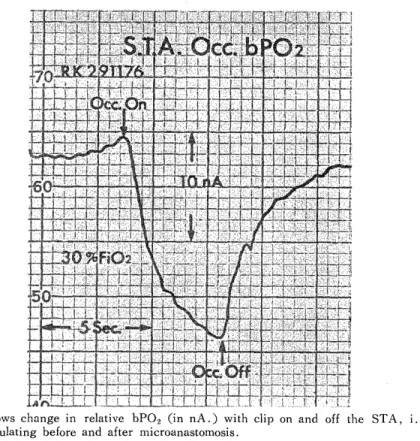
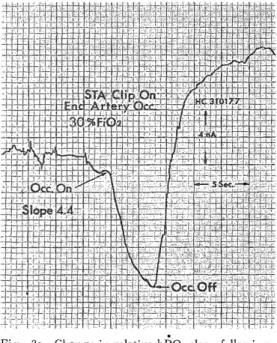
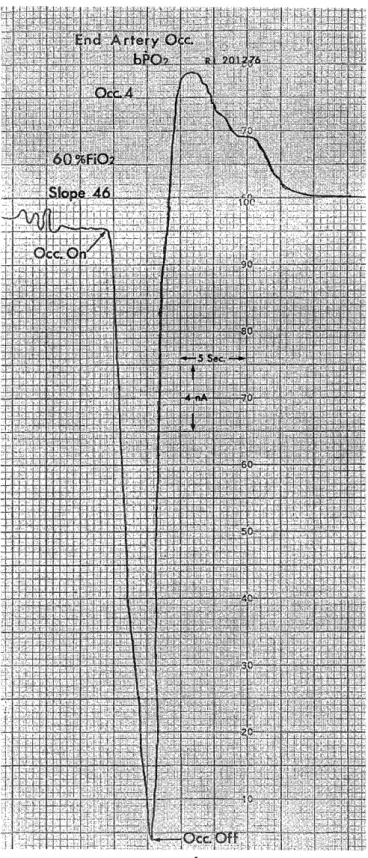
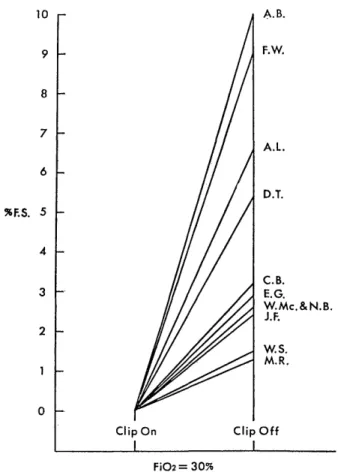
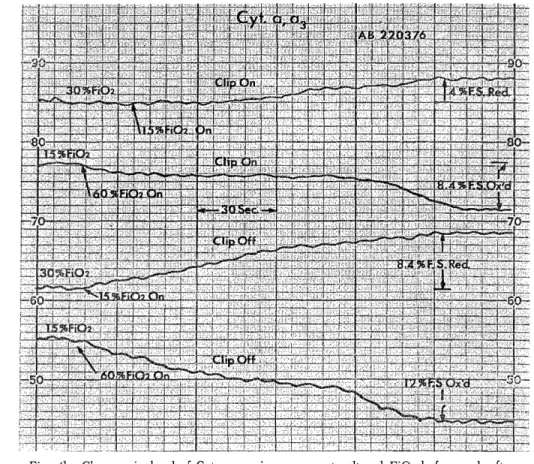
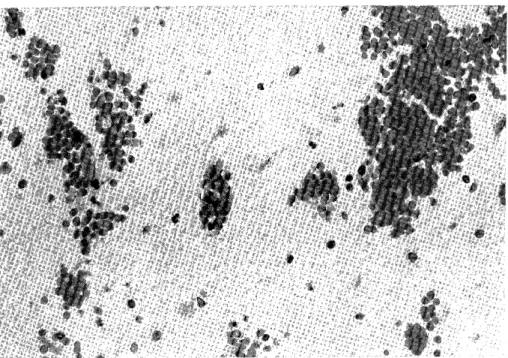
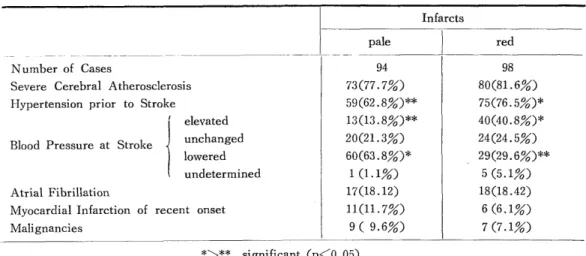
H e m o r r h a g i c C e r e b r a l I n f a r c t i o n P r o c e e d i n g s o f t h e 4 t h a n n u a l m e e t i n g o f T h e J a p a n e s e I s c h e m i c C e r e b r o v a s c u l a r D i s e a s e C o n f e r e n c e , N a g a s a k i , M a r c
49
0
0
全文
図
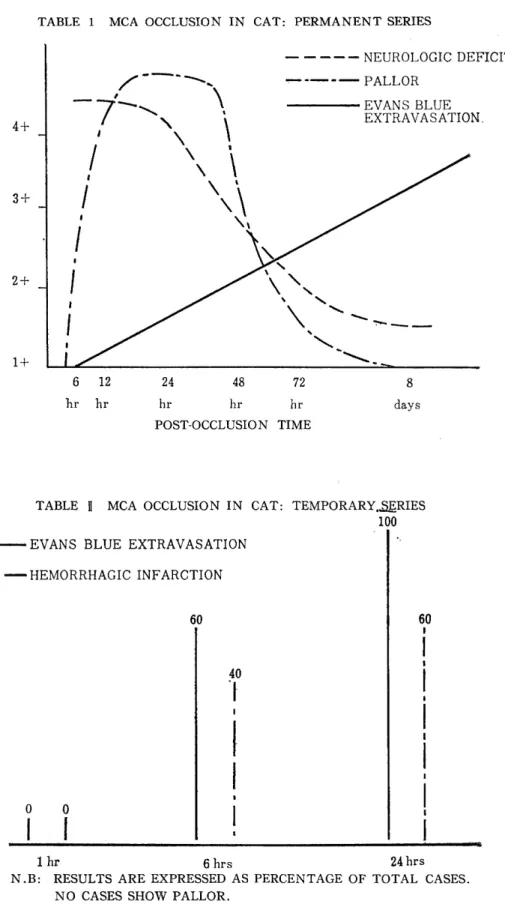
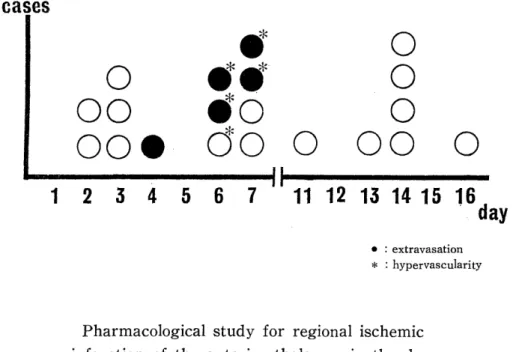
+7
Outline
関連したドキュメント
[r]
ー コネクテッド・ドライブ・サービス ー Apple CarPlay プレパレーション * 2 BMW サービス・インクルーシブ・プラス(
のようにすべきだと考えていますか。 やっと開通します。長野、太田地区方面
Q is contained in the graph of a
※ MSCI/S&P GICSとは、スタン ダード&プアーズとMSCI Inc.が共 同で作成した世界産業分類基準 (Global Industry Classification
[r]
[r]
[r]