Acccepted:2015.3.12
Corresponding author: Masanobu Mino (mino@kpu.ac.jp)
Improving the Chrysanthemum stunt viroid (CSVd) resistance of chrysanthemum
Hiromi Takino
1)・ Misako Furuya
1)・ Sumiko Yamamoto
1)・ Atsuko Sakuma
2)・ Masato Tsuro
2)・
Tatsuya Yanagimoto
1)・Yoshikazu Tanaka
3)・ Masanobu Mino
1)*
1)Graduate School of Life and Environmental Sciences, Kyoto Prefectural University
(Hangi-cho, Shimogamo, Sakyo-ku, Kyoto, 606-8522, Japan)
2)Faculty of Agriculture, Meijo University(Shiogamaguchi, Tenpaku-ku, Nagoya, 468-8502, Japan) 3)The Wakasa Wan Energy Research Center(Nagatani, Tsuruga, 914-0135, Japan)
* Corresponding author
Summary: Viroids, the smallest RNA pathogen, infect different kinds of horticultural and agricultural crop, and
lead to marked quality deterioration in the case of symptomatic infection. It is apparent that their autonomous replication and systemic movement in plants fully depend on the host machinery, since the pathogens lack their own protein-coding genes, whereas how viroids cause disease remains elusive. A model of short interfering RNA (siRNA) derived from viroid RNA that disrupts host gene expression, followed by an abnormal morphological appearance, is more acceptable than those previously proposed. On the other hand, the application of siRNA to block viroid replication is a plausible strategy for the molecular breeding of viroid-resistant plants. Recent advancements in the knowledge of viroid biology, as well as our approach to molecular breeding for
Chrysanthemum stunt viroid (CSVd)-resistant chrysanthemum, are brieÀ y reported.
Keywords: Viroid, siRNA, Molecular breeding, Chrysanthemum
Introduction
Viroids are plant-pathogenic, single-stranded, covalently closed, circular RNA molecules that range in size from 250 to 400 nucleotides, without any apparent open reading frame coding for proteins (Di Serio et al., 2014). Because of their simple genomic composition, it is believed that replications and movements of viroids in plants definitely depend on the host machinery (Flores et al., 2008). More than thirty species belong-ing to two families, Pospivioidae and Avsunviroidae, have been identi¿ ed, and most of them are members of Pospiviodae (NCBI taxonomy browser for viroid: http://www.ncbi.nlm.nih.gov./Tax-onomy/Brower/ wwwtax.cgi?p=7&id=12884). In spite of their naked RNA molecule without a protein coat, viroids generally show physical robustness, e.g., the infectious capacity of Tomato
chlorotic dwarf viroid (TCDVd) was eventually lost after
boil-ing infected plant extracts for more than 40 min (Matsushita, 2011). They can enter plants through mechanical injury when contaminated pruning scissors or other tools are used. Thus, careful ¿ eld management of disease and establishing viroid-re-sistant varieties are required. CSVd, a member of Pospivioidae, causing the stunted growth of many varieties of chrysanthemum (Dendranthema x morifolium Ramat.), was initially identified in 1945 in the USA and is now distributed globally (Matsushi-ta, 2011). Since chrysanthemum is ranked as the second most economically important cut À ower in the world, much effort is
needed to establish resistant cultivars. In the present article, we briefly summarize the current biological knowledge of viroids and introduce our research approach for the molecular breeding of CSVd-resistant chrysanthemum.
Symptoms and proposed mechanisms
of viroid-host interactions
Symptoms of plants infected with viroids are macroscopically similar to those of plants infected with many plant viruses, i.e., stunting, epinasty, chlorotic or necrotic spots, malformation of À ower shape, À ower color bleaching, poor root growth, and loss of plants (Kovalskaya and Hammond, 2014). Thus, it can be speculated that the basic mechanisms involved in the patho-genicity of both viruses and viroids are similar. However, plant viruses have more than 5 viral origin proteins that induce patho-logical effects, whereas viroids have no coding protein (Gómez
et al., 2008). Accordingly, their replication and interactions with
host factors have been intensively studied. Viroids of
Pospiviroi-dae and AvsunvioiPospiviroi-dae replicate in the nucleus and chloroplasts
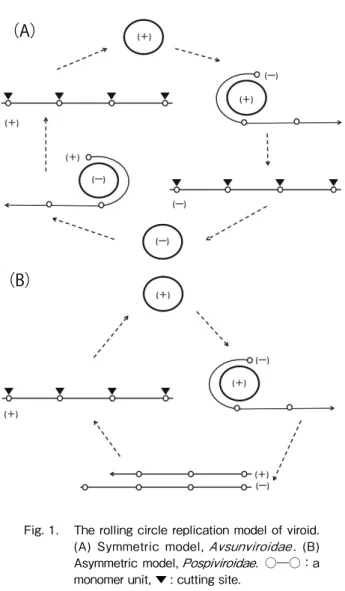
of the host cell, respectively, through an RNA-based rolling circle mechanism, but the former is in an asymmetric manner and the latter is in a symmetric manner (Nohales et al., 2012). The infected circular (+) monomer is replicated into linear (-) concatemeric strands which are then cut and ligated, eventually producing a (-) monomer in a symmetric model. Using this (-) monomer as a template, the same steps are repeated to produce (+) monomer (Fig. 1A). On the other hand, in an asymmetric
総 説
model, linear (-) concatemeric strands replicated from a circu-lar (+) monomer are used to produce a linear (+) concatemeric strand which is directly cut and ligated as a (+) monomer (Fig. 1B). A member of Pospivirodase replicates in the nucleus us-ing a host DNA-dependent RNA polymerase II (Diner, 1986), suggesting that disruption of the host transcription system by viroid RNA multiplication is involved in the pathogenic symp-toms of infected plants. Proteomic analysis of Citrus exocortis
viroid (CEVd)-infected tomato plants has shown that
differen-tial expressions of defense proteins, such as an endochitinase, and pathogenesis-related proteins, such as β-glucanase, were induced, whereas constant expressions were maintained in ribo-somal protein and translational factors, such as elongation and translation initiation factors (Lisón et al., 2013). The binding of viroid RNA to host factors has been reported in some mem-bers of Pospiviroidae, e.g., phloem lectin protein PP2 binds to Hop stunt viroid (HSVd) (Gómez and Pallás, 2004), a bro-modomain-containing Virp1 protein that specifically interacts with Potato spindle tuber viroid (PSTVd) (+) RNA (Martínez
et al., 2003; Kalantidis et al., 2007). On the other hand, using
the northwestern hybridization technique, Dubé et al. (2009)
showed that a Peach latent viroid (PMLVd) of Avsunviroidae binds to elongation factor α-1 (eEF1A), which is involved in diverse cellular mechanisms including translation, cytoskeleton formation, and protein export. The structural domain of viroid RNA binding to the host factors mimics those of cellular func-tional RNA in plants, and it is reasonable to speculate that viroid RNA changes host enzymes and other components indispensable for normal biochemical and physiological cellular processes. Although accumulated evidence has allowed speculations or discussions of viroid pathogenesis, clear evidence connecting the presence of viroid RNA and macroscopic symptoms in in-fected plants is limited. In this context, another possibility that post-transcriptional gene silencing in infected plants is related to viroid pathogenicity was proposed (Wang et al., 2004).
Small interfering RNAs (siRNA) inducs gene silencing
A general scheme of siRNA generation and the process of gene silencing ar e summarized in Fig. 2. It is speculated that siRNA was primitively developed as an immune response to invasion by foreign nucleic acids. siRNA is 21-30 base pairs of double-strand RNA (dsRNA) which is produced by ribonuclease Dicer protein (also known as Dicer like-protein (DCL) in plants) from long dsRNA that is not usually found in cells. This primary siRNA is used as a template, amplifying the secondary siRNA with the aid of RNA-dependent RNA polymerase (RDR). This ampli¿ cation mechanism allows abundant siRNA generation, eventually in-ducing a more intense silencing of the target gene. Either strand of siRNA is incorporated as a guide strand into the RNA-induced silencing complex (RISC) with Argonaute proteins (AGOs). The guide strand in RISC pairs with a complementary sequence of target mRNA and cleaves it through the action of AGOs, even-tually inducing post-transcriptional gene silencing. Furthermore, siRNA is also used to direct the de novo DNA methylation of the original genes that produce RNA, and this transcriptional gene(A)
Fig. 1. The rolling circle replication model of viroid. (A) Symmetric model, Avsunviroidae . (B) Asymmetric model, Pospiviroidae. ○ ―○ :a monomer unit, ▼ : cutting site.
(B)
Fig. 2. Transcriptional and post-transcriptional gene silencing caused by siRNA. The scheme was drawn based on descriptions of Wang et al. (2004), Martinez et al. (2014), and
silencing is suggested to be a defense mechanism against virus infection and/or transposons.
Since their intramolecular base paired secondary structure and dsRNA formation during replication give DCL a chance to recognize these molecules as target substrates, viroids generate siRNA of their own sequence. Early evidence that viroids in-duce gene silencing was reported by Wassenegger et al. (1994), whereby a PSTVd sequence incorporated into a transgenic to-bacco genome was methylated by the over-expression of PSTVd RNA. Using PSTVd-infected tomato plants, Itaya et al. (2001) reported that there was quantitative relationship among the level of siRNA, level of PSTVd accumulation, and development of symptoms. Wang et al. (2004) showed that PSTVd and cucum-ber mosaic virus satellite pathogenecities were mediated by RNA silencing; a pathogenic symptom of tobacco caused by Y satellite (parasite RNA harbored in virus particles, containing a high degree of secondary structural similarity to viroids) of cucumber mosaic virus was markedly reduced by suppressing RNA silencing using the potent suppressor Hc-Pro; expression of hairpin RNA derived from PSTVd in tomato led to symptoms similar to those of PSTVd infection. Gómez et al. (2008), using a grafting technique with Nicotiana benthamiana, showed that RDR 6, which plays a role in secondary siRNA initiation, was required for the development of HSVd symptoms. In this case, the scion of rdr 6 lacking RDR6 activity or wild-type was graft-ed onto HSVd-infectgraft-ed stock, and the amount of HSVd RNA and symptom development were evaluated. Although HSVd RNA accumulated in both scions, no symptom was observed in the rdr6 scion, whereas marked symptoms were detected in the wild-type scion. Tsuro et al. (2013) detected À uctuations of CSVd siRNA in infected chrysanthemums cultured from 5 to 28ºC. Under low temperature conditions (5ºC), stunted growth, the most prominent symptom of CSVd infection, was relieved, and siRNA was lowered to undetectable level. However, an in-termediate temperature (22ºC) increased the maximum siRNA level and induced markedly stunted growth compared to the uninfected control. These results suggest that viroid RNA can cause the silencing of endogenous genes normally expressed in the host plant. Besides the induction of the post-transcriptional gene silencing, viroid RNA may interact with host enzymes in-volved in the RNA-directed DNA methylation pathway, which is important in transcriptional gene silencing (Navarro et al., 2009). More recently, direct evidence of speci¿ c mRNA cleav-age targeted by viroid siRNA was reported; mRNA encoding chloroplastic heat-shock protein 90 (cHSP90) in Prunus persica was targeted and degraded by siRNA of Peach latent mosaic
vi-roid (PLMVd), eventually causing albinism, a typical symptom
of PLMVd infection, since cHSP90 is involved in chloroplast biogenesis and plastid-to-nucleus signal transduction (Navarro
et al., 2012). Taken together, the evidence suggests that viroids
generate siRNA of their own sequence and this transcriptionally and/or post-transcriptionally suppresses the expression of endog-enous genes that are indispensable for the normal growth and development of plants.
Our approach toward CSVd-resistant breeding of
chrysanthemum
Conventional plant breeding methods to improve CSVd re-sistance using the useful genetic resources is the most valuable approach (Nabeshima et al., 2012, 2014), whereas the molecular approach is also effective, since the genome of the pathogen only consists of RNA. Accordingly, an application of ribozymes that speci¿ cally cleaved the CSVd RNA sequence was our initial approach (Ando et al., 2006). However, since ribozymes require both high temperature and high salt conditions to maximize their efficiency to cleave target RNA, ribozyme activities in planta are not suf¿ cient to reduce CSVd RNA, even though high-lev-el expression of ribozyme genes can be achieved in transgenic chrysanthemum. Thus, we considered whether siRNA of CSVd targets and cleaves its own sequence.
Plant viruses develop defense mechanisms to counter host resistance by producing the p19 protein that directly binds to siRNA targeting the viral RNA genome (Várallyay et al., 2014). Although viroids have no such proteins, several lines of evidence suggest that viroid RNA is free from silencing mechanisms due to their (1) highly ordered secondary structure, (2) association with host factors, (3) subcellular localization, and (4) unknown novel suppressor (Kovalskaya and Hammond, 2014). Howev-er, some evidence for siRNA mediating viroid resistance was obtained: artificially designed hairpin RNA (hpRNA)-derived siRNA of PSTVd appeared to effectively target the mature PST-Vd RNA, eventually preventing PSTPST-Vd replication in tomato (Schwind et al., 2009); the accumulation of PSTVd in the scion of N. benthamiana was attenuated when grafted to transgenic N.
benthamiana stock expressing hpRNA of PSTVd (Kasai et al.,
2013). These results support using the siRNA technique for the molecular breeding of CSVd-resistant chrysanthemum cultivars. To test if CSVd-siRNA targets and cleaves CSVd RNA, Yan-agimoto (2010) constructed the luciferase (luc) reporter gene in which the head to head dimer of the CSVd sequence was insert-ed between luc ORF and the nopaline synthase (NOS) terminator in the Ti-plasmid vector (pIL-CSVd) (Fig. 3A), and carried out a transit expression assay by agroin¿ ltraion in the leaves of Vd-infected or healthy chrysanthemum plants. In the case of CS-Vd-siRNA cleaving the CSVd sequence of the reporter mRNA, it was expected that activities of luc tend to be lower than in healthy plants. As shown in Fig. 3B, this was true, while no such decline was observed in the luc reporter gene lacking CSVd se-quence (pIL), suggesting that naturally generated siRNA from
CSVd RNA targets and cleaves its own RNA sequence. Finally, we have developed Ti-plasmid constructs expressing hpRNA of some parts of the CSVd genome and developed a transgenic chrysanthemum plant expressing siRNA of CSVd. The analysis of the CSVd resistance of these plants is ongoing.
References
Ando, Y., K. Oda, M. Iwabuchi and M. Mino (2006) Assessment of ribozyme-mediated gene suppression using a luciferase re-porter gene containing the sequence of Chrysanthemum stunt viroid as a target. Mol. Breeding 18: 209-218.
Diener, T. O. (1986) Viroid processing: a model involving the central conserved region and hairpin I. PNAS 83: 58-62. Di Serio, F, R. Flores, J. Th. J. Verhoeven, S. F. Li, V. Pallás, J. W.
Randles, T. Sano, G. Vidalakis and R. A. Owens (2014) Cur-rent status of viroid taxonomy. Arch. Virol. 159: 3467-3478. Dubé, A., M. Bisaillon and J. P. Perreault (2009) Identi¿ cation of
proteins from Prunus persica that interact with Peach latent mosaic viroid. J. Virol. 83: 12057-12067.
Flores, R., A. Carbonell, S. Gago, A. E. Martínez de Alba, S. Delgado, M. E. Rodio and F. Di Serio (2008) Vioroid-host in-teraction: A molecular dialogue between two uneven partners.
in Biology of Plant-Microbe Interactions, Vol.6. (Ed.) Lorito,
M., S. L. Woo, F. Scala. ISBN: 978-0-9654625-5-6.
Gómez, G. and V. Pallás (2004) A long-distance translocatable phloem protein from cumcumber forms a ribonuleoprotein complex in vivo with Hop stunt viroid RNA. J. Virol. 78: 10104-10110.
Gómez, G., G. Martínez and V. Pallás (2008) Viroid-induced symptoms in Nicotiana benthamiana plants are dependent on RDR6 activity. Plant Phyisol. 148: 414-423.
Itaya, A., A. Folimonov, Y. Matsuda, R. S. Nelson and B. Ding (2001) Potato spindle tuber viroid as inducer of RNA silenc-ing in infected tomato. Mol. Plant Microbe Interact 14: 1332-1334.
Kalantidis, K., M. A. Denti, S. Tzortzakaki, E. Marinou, M. Ta-bler and M. Tsagris (2007) Virp1 is a host protein with a ma-jor role in potato spindle tuber viroid infection in Nicotiana plants. J. Virol. 81: 12872-12880.
Kasai, A., T. Sano and T. Harada (2013) Scion on a stock pro-ducing siRNAs of Potato spindle tuber viroid (PSTVd) atten-uates accumulation of the viroid. PLoS ONE 8: e57736. Kovalskaya, N. and R. W. Hammond (2014) Molecular
biolo-gy of viroid-host interactions and disease control strategies. Plant Science 228: 48-60.
Lisón, P., S. Tárraga, P. López-Gresa, A. Saurí, C. Torres, L. Campos, J. M. Bellés, V. Conejero and I. Rodrigo (2013) A noncoding plant pathogen provokes both transcriptional and posttranscriptional alterations in tomato. Proteomics 13: 833-844.
Martínez de Alba, A. E., R. Sägesser, M. Tabler and M. Tsagris (2003) A bromodomain-containing protein from tomato binds speci¿ cally potato spindle tuber viroid RNA in vitro and in vivo. J. Virol. 77: 9685-9694.
Martinez, G., M. Castellano, M. Tortosa, V. Pallas and G. Gomez (2014) A pathogenic non-coding RNA induces changes in dynamic DNA methylation of ribosomal RNA genes in host plants. Nucleic Acids Res. 42: 1553-1562.
Matsushita, Y. (2011) Distribution of viroid variants and their in-fectivity in horticultural plants in Japan. Bull. Natl. Inst. Flor. Sci. 11: 9-48.
Nabeshima, T., M. Hosokawa, S. Yano, K. Ohisihi and M. Doi (2012) Screening of chrysanthemum cultivars with resistance to chrysanthemum stunt viroid. J. Japan. Hort. Sci. 81: 285-294.
Nabeshima, T., M. Hosokawa, S. Yano, K. Ohishi and M. Doi (2014) Evaluation of chrysanthemum stunt viroid (CSVd) infection in newly-expanded leaves from CSVd-inoculated shoot apical meristems as a method of screening for CS-Vd-resistant chrysanthemum cultivars. J. Hort. Sci. Biotech. 89: 29-34.
Navarro, B., V. Pantaleo, A. Gisel, S. Moxon, T. Dalmay, G. Bisztray, F. Di Serio and J. Burgyán (2009) Deep sequencing of viroid-derived small RNAs from grapevine provides new
Fig. 3. (A) CSVd-luc reporter gene construct. The gene cassette was inserted into the site between RB and LB of pBI121 (Ti-plasmid). (B) Activities of luc in the area of the chrysanthemum leaf where the reporter gene is expressed transiently after agroinfi ltraiton. ns and * mean no signifi cant and a significant (P<0.05) difference detected between the means of healthy and CSVd-infected plants, respectively, based on a two-sample t -test. □ : Healthy plant, : CSVd-infected plant.
insights on the role of RNA silencing in plant-viroid interac-tion. PLoS ONE 4: e7686.
Navarro, B., A. Gisel, M. E. Rodio, S. Delgado, R. Flores and F. Di Serio (2012) Small RNAs containing the pathogenic deter-minant of a chloroplast-replicating viroid guide the degrada-tion of a host mRNA as predicted by RNA silencing. Plant J. 70: 991-1003.
Nohales, M. Á., R. Flores and J. A. Daròs (2012) Viroid RNA redirects host DNA ligase 1 to act as an RNA ligase. PNAS 109: 13805-13810.
Schwind, N., M. Zwiebel, A. Itaya, B. Ding, M. B. Wang, G. Krczal and M. Wassengger (2009) RNAi-mediated resistance to potato spindle tuber viroid in transgenic tomato expressin a viroid hairpin RNA construct. Mol. Plant. Patho. 10: 459-469. Tsuro, M., A. Sakuma and M. Mino (2013) In vitro evaluation
of small RNA accumulation in Chrysanthemum mediated by Chrysanthemum Stunt Viroid. Environ. Control Biol. 51: 95-98.
Várallyay, É., E. Oláh and Z. Havelda (2014) Independent par-allel functions of p19 plant viral suppressor of RNA silencing require for effective suppressor activity. Nucleic Acids Res. 42: 599-608.
Wang, M. B., X. Y. Bian, L. M. Wu, L. X. Liu, N. A. Smith, D. Iseegger, R. M. Wu, C. Masuta, V. B. Vance, J. M. Watson, A. Rezaian, E. S. Dennis and P. M. Waterhouse (2004) On the role of RNA silencing in the pathogenicity and evolution of viroids and viral satellites. PNAS 101: 3275-328.
Wassenegger, M., S. Heimes, L. Riedel and H. L. Sänger (1994) RNA-directed de novo methylation of genomic sequences in plants. Cell 76: 567-576.
Yanagimoto, T. (2010) Development of luc reporter system de-tecting CSVd infection. Master thesis, Graduate School of Life and Environmental Sciences, Kyoto Prefectural Univer-sity.
キクのわい化ウイロイド(CSVd)病抵抗性の向上にむけての研究
瀧野博己
1)・古谷美佐子
1)・山本すみ子
1)・佐久間敦子
2)・津呂正人
2)・柳本達也
1)・田中良和
3)・
三野真布
1) 1) 京都府立大学生命環境科学研究科(〒 606-8522 京都市左京区下鴨半木町 1-5) 2) 名城大学農学部(〒 468-8502 名古屋市天白区塩釜口 1-501) 3) 若狭湾エネルギー研究センター(〒 914-0135 敦賀市長谷 64-52-1) 要旨:ウイロイドは最小の RNA 病原体で,園芸作物ならびに農作物に著しい被害をもたらす.ウイロイドはタンパク質をコー ドする遺伝子を持たず,感染した植物体内での複製と移動は宿主細胞の諸因子に依存する.しかし,ウイロイドにより病徴が現 れる機構は不明な点が多い.近年,ウイロイドに由来する short interfering RNA (siRNA) が宿主遺伝子の発現を撹乱し,これが病 徴発現につながるとする説が有力になりつつある.ウイロイドの生物学についての最近の知見を簡潔にまとめると共に,キクわ い化ウイロイド(CSVd)病抵抗性育種に向けての我々の取り組みについて報告する.キーワード:ウイロイド,siRNA,分子育種,キク
作物研究 60 号(2015) 連絡責任者:三野真布(mino@kpu.ac.jp)