Kobe Shoin Women’s University Repository
Title
Comparison of Mycolic Acid Subclass and Molecular
Species
Composition
among
Non-chromogenic
Rapidly Growing Mycobacteria
非発色性迅速発育抗酸菌群のミコール酸 Subclass と分子種
組成の比較
Author(s)
馬場 恒子(Tuneko Baba)
Citation
生活科学論叢(Review of Living Science)
,
No.20:55-67
Issue Date
1988
Resource Type
Bulletin Paper / 紀要論文
Resource Version
URL
Right
Comparison
of Mycolic
Acid
Subclass
and
Molecular
Species
Composition
among
Non-chromogenic
Rapidly
Growing
Mycobacteria
Tuneko Baba SUMMARYThe detailed analysis of mycolic acid composition of several strains of M . smegmatis, M. fortuitum, M. chitae and M. chelonae was performed using thin-layer chromatography (TLC), gas chromatography (GC) and gas chromatography/mass spectrometry (GC/MS) . They all belong to non-chromogenic rapidly growing mycobacteria . However, two of them are pathogenic to human or animals but others are normal flora of the skin and not pathogenic. The present paper describes on the chemotaxonomical comparison of mycolic acid composition between pathogenic and non-pathogenic mycobacterial species among non-chromogenic rapidly growing group. It was revealed that the mycolic acid subclass composition was common among each species of non-chromogenic rapidly growing mycobacteria while molecular species composition of mycolic acids differed distinctively by the species of mycobacteria. The differrence of mycolic acid composition between path-ogenic and non-pathpath-ogenic species was not significant in the present study . The importance of the relationship between structures of mycolic acid contained in glycolipids such as trehalose dimycolate and bioresponse modifying activity or pathogenicity was discussed .
INTRODUCTION
Mycolic acids are defined as high-moleculer weight fatty acids with fl-hydroxy and a-alkyl branches. They are present as the characteristic component of the cell wall lipids in mycobacterial3'2) and related bacteria, such as nocardiae,") rhodococcis) and corynebacteria.6).7) The structural analysis of individual mycolic acids was not so easy because they were decomposed by high temperatures during the analysis . In recent years, Yano, et al. have developed an analytical method for the determination of individual molecular species of mycolic acids using gas chromatography (GC) and gas
- 55 —
phy/mass spectrometry (CG/MS) of trimethylsilylated (TMS) derivatives of mycolic acid esters.8)-12) Analysis of mycolic acid composition has revealed that the structure and compo-sition of mycolic acids are characteristic to each bacterial species. Nocardiae, corynebacter-ia and rhodococci have one subclass of mycolic acid with several unsaturated bonds. On the other hand, mycobacteria has several subclasses of mycolic acids, which consist of longer alkyl chain, two or one double bonds and various functional groups. Therefore, determina-tion of mycolic acid composidetermina-tion seems to be very useful for the chemotaxonomy.
I have previously reported mycolic acid composition from eight strains of Mycobacter-ium smegmatis.") It has two major subclasses, designated as a -mycolates (M1) and a'-mycolates (M1'), and one minor subclass, hydroxy mycolates (M4) derived from epoxy mycolates. GC/MS showed that a- and a'-mycolates are C72 "C80 dienoic acids and C60 -C66 monoenoic acids, respectively. M.smegmatis is the most representative non-chromogenic species among rapidly growing mycobacteria. It is particularly interested in viewpoint of chemotaxonomy to compare the mycolic acid composition between M. smegmatis and other non-chromogenic rapidly growing mycobacteria because M. smegmatis is non-pathogenic but others are pathogenic.") The present paper describes on the chemotaxonomical comparison of mycolic acid compositions among various species of non-chromogenic rapidly growing mycobacteria.
MATERIALS and METHODS
M.smegmatis was grown in a medium containing 1% glucose, 1% peptone and 0.5% yeast extract with pH 7.4. Other species were cultured in a medium with 1% glucose, 0.5% peptone and 0.2% yeast extract (pH 7.0). Cells inoculated with shaking at 37t until the late logarithmic stage of the growth. They were harvested by centrifugation and washed with distilled water. The packed cells were hydrolyzed with alkaline (10% KOH/80% methanol) under reflux for 5 hr. The fatty acids were methylated with benzene/methanol/H2SO4 (10: 20:1,v/v) and extracted with n-hexane. The fatty acid methyl esters were separated into subclasses by TLC (n-hexane/diethylether, 4:1,v/v). The subclasses were then visualized with iodine vapor and recovered from the gel with chloroform. The methyl esters of mycolic acids were trimethylsilylated with N,O-bis,trimethylsilyltrifluoroacetamide/ pyridine (2:1,v/v) at 80t for 30 min. Gas chromatography of TMS derivatives of mycolic acid methyl esters was carried out with a Shimadzu GC-6A apparatus.GC/MS and mass chromatography (MC) were
— 56 —
performed on a Hitachd M-80B Double Focussing apparatus as described in the previous papers.'2^3).15)
RESULTS
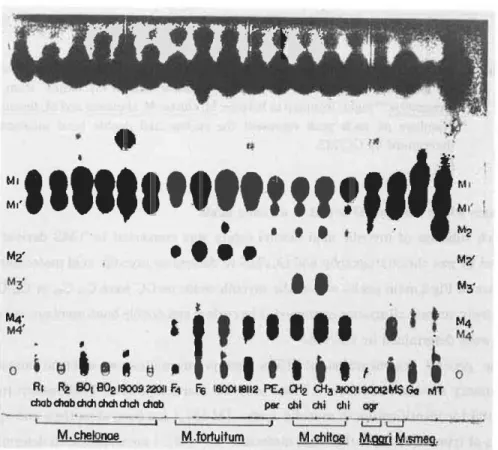
Figure 1 shows the thin-layer chromatographic profiles of total fatty acid methyl esters
..L
(I'M
I 14114
P- 4 .t1111
••1 III*
111
111 /111.11illtvihi
•6421+42'
~Ss*
0.42,
fv13.M4'
if01008m4
- f 10I A •
I/ I/
I t
RI R2 130i 802 19009 2201i F4 F6 6001 18112 PEI CH2 CH3 30041 900!2 M$ Go MT
chab chab chch chat chch chab par chi chi chi agr
I J
M. chelonoe M.fortuitum M.chnoe gad Msmeg. Fig. 1 Thin-layer chromatograms of total fatty acid methyl esters from
rapidly growing mycobacteria (M, chelonae, M. fortuitum, M. chitae,
M. agri and M. smegmatis). M1 and M1', a- and a'- mycolic; M2',
epoxy mycolic; M4 and M4', hydroxy mycolic methyl esters.
from several species of non-chromogenic rapidly growing mycobacteria. M. fortuitum has a.-,epoxy and hydroxy mycolic acids. M. chitae and M. smegmatis have three subclasses, such as a-, a'- and hydroxy mycolates. On the other hand, M.chelonae has only two
Fig. 2 Representative gas chromatograms of TMS methyl a-and a-mycolates of
ly growing mycobactria. Left and middle:a-and a-mycolates from M.
smegmatis,") right: from top to bottom, M. chitae, M. chelonae and M. fortuitum.
Numbers on each peak represent the carbon and double bond numbers as
determined by GC/MS.
subclasses which consists of a-and a-mycolic acids.
Each subclass of mycolic acid methyl esters was converted to TMS derivatives and
analyzed by gas chromatography and GC/MS to determine mycolic acid molecular species.
As shown in Fig.2,main peaks of a -anda-mycolic acids on GC were C74-C79
or C62-C68
acids,
respectively, among all species examined. The carbon and double bond numbers of each peak
on GC were determined by GC/MS.
The general fragmentaion of TMS methyl mycolates on electron impact mass
spectrometry showed in Scheme 1. These ions are characteristic to TMS methyl mycolates
and useful for identification of mycolic acids. [M-15] + ion (loss of methyl) and [M-90]
ion (loss of trimethylsilyl) rather than molecular ion [M] + are available in determining the
carbon and double bond numbers of whole moleculars. The numbers of carbons and double
bonds of the fl-unit and the a-uint could be similarly determined from the mass numbers of
the characteristic fragment ions, such as [A] + (TMS-alkoxy ion due to C,C3 cleavage),
[A-90] + (loss of trimethylsilanol from
[A] +), [B]
(TMS-oxymethylene carboxylic
acid ester from C3-C4 cleavage) and [B-29] + (loss of CHO from [B] +).
In the case of a-mycolates from M. fortuitum (Fig.3), the original structure was
determined as from C72:2
to C79:2
from the fragment ions [M] +, [M-15] + and [M-90] -E.
— 58 —
Rights were not granted to include this image in electronic media.
Please refer to the printed journal.
Fragment ions [A] + and [A-90] + varied in the individual peaks of GC, indicating that the
straight chain (8-uint) varied from C48:2
to C55:2,
while the a-unit was C24:0
among all molecular
[M-15]
[M-901
\ -(CH3)
/ -{(CH3)3SiOH)}
-
_m_
_______),
1(----p
unit---*1*--a
unit--.
1(--_[A]--)I
1CH3CmH2n-CHICH-COOCH3
(C
H2)21
-{(CH3)Si0H}0
I
1 CH3
[A-901
Si(CH3)3
,,.___. [B] ___...
1 -(CHO)
[B-29]
SCHEME. 1 General structure and mass fragmentation
pattern
of
trimethylsilyl ether derivatives of methyl mycolats.
species because the fragment ions [B]+ and [B-29]
+ were distinctly 483 and 454, respectively.
Moreover, mass chromatography of TMS methyl a -mycolates of M . fortuitum (Fig. 4-A)
showed that the odd numbered acids overlapped with one less (even)
carbon-numbered species in a single peak on GC. Judging from the relationship between log
retention times and carbon numbers, the odd carbon-numbered a-mycolate appeared to have
one methyl branch in the even carbon-numbered alkyl chain unit . The proportion of odd
carbon-numbered mycolates became larger as the total carbon chain length became longer .
On the other hand, the odd carbon-numbered a-mycolates from M. chitae (Fig. 4-B) were
relatively higher than the even carbon-numbered ones similarly as those from M. smegmatis
(Fig. 4-D).13)
Mass chromatogram of a-mycolates from M. chelonae was more complex (Fig.
4-C) and C75 acid consisted of both one methyl branched acids and acids with odd
carbon-numbered (C75) straight skeleton.
1166
Fig. 3 Mass specra of TMS methyl a-mycolates of M. fortuitum: from top
to bottom, C72:2,
C74:2,
C76:2
and C78:2+79:2.
— 60 —
Rights were not granted to include this
image in electronic media. Please refer
to the printed journal.
Mass spectra of the individual TMS methyl a'-mycolates on GC were recorded . The a-unit was commonly C24:0 among all species examined. The carbon numbers of main peaks were all even and odd carbon-numbered acids were hardly observed in mass chromatogram . Only M. fortuitum had two double bonds in a-mycolates, while other species had one double bond. The molecular species composition of a'-mycolates was calculated from the area percentages on gas chromatograms or mass chromatograms. These results were summar-ized in Table 1.
Fig.5 showed the GC profile and mass spectra of TMS derivatives of hydroxy mycolates from M. fortuitum. A very intense and characteristic mass ion was observed at m/z = 355, including O-TMS group , due to the cleavage between co-19 and co-20 positions . These mass
7
B
Fig. 4 Mass chromatograms of TMS methyl cr-mycolates of (A) M . tuitum, (B) M. chitae, (C) M. chelonae and (D) M. smegmatis .")
[M-15] + ion corresponding to each carbon-numbered TMS methyl a-mycolates were monitored.
— 61 —
Rights were not granted to include this image in electronic media.
Please refer to the printed journal.
Table. 1 Molecular species composition of a'- mycolic acids in
rapidly growing mycobacteria
w ca)
ti
m
g
a) a crs 0 -4-, a) Carbon No. 58 1.3 60 23.2 2.0 62 56.1 49.2 11.1 64 18.4 44.9 83.9 3.4 66 1.0 3.9 5.0 25.2 68 63.5 70 7.9 a-unit 24 (22) 24 24 24 /3-unit 36-42 36-42 38-42 40-46 Av. Carbon No. 61.5 63.0 63.9 67.5
Double Bond No. 1 1 1 2
fragmentation patterns were the same as hydroxy mycolates from M. smegmatis.13)
The biological properties and mycolic acid subclass composition of non-chromogenic rapidly growing mycobacteria examined in this investigation were summarized in Table 2.
DISCUSSION
The previous paper described the detailed mycolic acid compositions from eight strains of M. smegmatis13) and this paper reported those from other species of non-chromogenic rapidly growing mycobacteria, such as M. fortuitum, M. chitae and M. chelonae.
The complexity of mycobacterial mycolates have been recognized to be particularly useful for the chemotaxonomy, because molecular mass, degree of unsaturation (double bond or cyclopropane ring) and existence of functional groups (methyl, keto, epoxy, hydroxy, carboxy and so on ) are characteristic in each bacterial species.'"15)
The present study showed that a-mycolates from all species examined were diunsaturat-ed acids containing 72-80 carbon atoms. The carbon number of a-unit of all mycolic acid subclasses in this group was saturated C24 acid, commonly, but a -unit of mycolic acids from
Fig. 5 Gas chromatogram (lower) and mass spectra (upper) of TMS methyl hydroxy mycolates (M4) from M. fortuitum. Numbers on each peak of
gas chromatogram represent the carbon numbers as determined by
GC/MS.
chromogenic rapidly growing mycobacteria consisted of C24:0, C22:0 or a mixture of the both. In non-chromogenic rapidly growing mycobacteria I detected two double bonds in cr-mycolate in general, but did not cyclopropane rings instead. However , a -mycolates from chromogenic rapidly growing mycobacteria had two cyclopropane rings or both a cyclo-propane ring and a double bond.
a-Mycolic acids are present in all mycobacterial species commonly, but or-mycolic acids are specific for rapidly growing mycobacteria and absent in slowly growing mycobacteria , such as M. tuberculosis, M. bovis and M. intracellurare.
Hydroxy or epoxy mycolic acids were found on TLC in spite of as a minor subclass in M. smegmatis but as a subclass in M. fortuitum and M. chitae abundantly . However, it was
63 —
Rights were not granted to include this image in electronic
media. Please refer to the printed journal.
Table. 2 Properties and mycolic acid subclass of rapidly growing mycobacteria co a Pigment production — Growth in <5 days Pathogenicity in human or animal Mycolic acid subclass
M1, a— C. N. (D. B.) 77( 2 ) 77,79(2) 74 , 75 ( 2) 76 — 79 ( 2 ) M1', a'— C. N. (D. B.) 62( 1) 62,64(1) 64( 1) 68( 2 ) M2 keto— C. N. (D. B.) M2' epoxy— C. N. (D. B.) 77,79( 1) 77,79(1) 76-79( 1 ) M3, dicarboxy— C. N. (D. B.) — C. N.: Carbon number, D. B. :Double bond, M2' :Including M4
noted that M. chelonae did not have either hydroxy or epoxy mycolates.
From the results of these studies, it was evident that the mycolic acid subclass tion was common among each species of non-chromogenic rapidly growing mycobacterda
while molecular species composition of mycolic acids was characteristic to each species of mycobacteria. Then the analysis of mycolic acid composition is very useful for the
classification or identification of mycobacteria. However, the difference of mycolic acid
composition between pathogenic and non-pathogenic species was not significant in the
present study. It may be difficult to find an index of pathogenicity among non-chromogenic
rapidly growing mycobacterda because M. fortuitum and M.chelonae are not so strongly pathogenic to human as M. tuberculosis.
Trehalose dimycolate (cord factor) has been first recognized to be toxic glycolipid produced by the pathogenic mycobacteria (M. tuberculosis) and to be related to the
ogenicity of mycobacteria.'6) M. tuberculosis was thought to resist against phagocytosis of
macrophages due to the rigid structure of cell walls containing trehalose dimycolates and
alabinogalactan mycolates as shown in Fig 6.17) In recent years, however, it has been found
widely among not only the saprophytic species of mycobacteria but also the soil source
Actinomycetes,'''-22' such as nocardiae or rhodococci. Moreover, trehalose dimycolates from
a
Fig. 6 A model of structure of cell wall containing mycolic acids in mycobacteria described by Minnikin.")
both non-pathogenic and pathogenic species were revealed to possess strong biomodifying activities although the degree of the toxicity differed greatly.")-") Therefore, it may be especially interesting to investigate on the relationship between structures of mycolic acid or trehalose dimycolate and bioresponse modifying activity or pathogenicity.
ACKNOWLEDGMENTS
I would like to thank Prof. I. Yano for helpful advises and discussions and Dr. K. Kaneda for GC/MS analysis.
65
Rights were not granted to include this image in electronic media.
Please refer to the printed journal.
REFERENCES
1) Stodola,F. H., Lesuk, A. and Anderson, R. J. : J. Biol. Chem. 126, 505-513 (1938) 2) Asselineau, J. and Lederer,E. :Nature (London) 166, 782-783 (1950)
3) Bordet, C. and Mitchel, G. : Bull. Soc. Chim. Biol. 51, 527-547 (1969)
4) Alshamaony, L., Goodfellow, M. and Minnikin, D. E. : J. Gen. Microbiol. 92, 188-199 (1976)
5) Goodfellow, M. and Anderson, G. : J. Gen. Microbiol. 100, 99-122 (1977) 6) Assenlineau, J. : Biochem. Biophys. Acta 54, 359-361 (1964)
7) Collins, M.D., Goodfellow, M. and Minnikin, D. E. :J.Gen. Microbiol. 128, 129-149 (1982) 8) Yano, I., Kageyama, K. , Ohno, Y., Masui, M., Kusunose, E., Kusunose, M. and Akimori,
N. : Biomed. Mass Spectrom. 5, 14-24 (1978)
9) Toriyama, S., Yano, I., Masui, M., Kusunose, M. and Kusunose, E. : FEBS Lett. 95, 111-115 (1978)
10) Toriyama, S., Yano, I., Masui, M., Kusunose, E., Kusunose, M. and Akimori, N. : J. Biochem. 88, 211-221 (1980)
11) Tomiyasu, I. : J. Bacteriol. 151,828-837 (1982)
12) Kaneda, K., Naito, S., Imaizumi, S., Yano, I., Mizuno, S., Tomiyasu. I., Baba, T., Kusunose, E. and Kusunose, M. : J. Clin. Microbiol. 24, 1060-1070 (1986)
13) Baba. T., Kaneda, K., Kusunose, E., Kusunose, M. and Yano, I. : Lipids 23, 1132-1138
(1988)
14) Wayne, L.G. and Kubica, G. P. : in Bergys manual of systematic bacteriology vo1.2, pp1435-1457 The williams & Wilkinsons, Baltimore (1986)
15) Kaneda,K., Imaizumi,S., Mizuno, S., Baba, T., Tsukamura, M. and Yano, I. : J. Gen. Microbiol. 134, 2213-2229 (1988)
16) Kato, M. and Imaeda, J. : Infect. Immun. 9, 8-14 (1974)
17) Minnikin, D. E. : in The Biology of the Mycobacteria vol. 1, pp95-184 Academic Press London (1982)
18) Lederer, E. : Chem. Phys. Lipids 1, 294-315 (1967)
19) Senn, M. T., Ioneda, T., Puddles, J. and Lederer, E. : Eur. J. Biochem. 1, 353-356 (1967) 20) Ioneda, T., Lederer, E. and Rozanio, J. : Chem. Phys. Lipids 4, 375-392 (1970)
21) Yano, I., Furukawa, Y. and Kusunose, M. : J. Gen. Appl. Microbiol. 17, 329-334 (1971) 22) Ioneda, T., Larz, M. and Puddles, J. : Biochem. Biophys. Res. Commun. 13, 110-114 (1976)
66
23) Yano, I.. Tomiyasu, I., Kitabatake, S. and Kaneda, K, Acta Lepro . 95, 341-349 (1984) 24) Kaneda, K., Sum Y., Miran°, F., Kato, Y. and Yano, I. : Infect. Immun . 54, 860-875 (1986) 25) Yano, I,, Tomiyasu, I., Kaneda, K., Kato, Y., Simi, Y., Kurano, S., Sugimoto , N. and
Sawai, H.: J. Pharmaco, Dynam. 10, 113-123 (1987)