(別紙7)
学位論文(Thesis)の要約
Navchaa Gombodorj
Inhibition of Ubiquitin-conjugating enzyme E2 may activate the degradation of hypoxia-inducible factors and thus overcome cellular resistance to radiation in colorectal cancer
(UBE2阻害剤による低酸素誘導因子の分解は大腸癌の放射線耐性の克服に寄与する阻害剤による低酸素誘導因子の分解は大腸癌の放射線耐性の克服に寄与する阻害剤による低酸素誘導因子の分解は大腸癌の放射線耐性の克服に寄与する阻害剤による低酸素誘導因子の分解は大腸癌の放射線耐性の克服に寄与する)
指導教員 中野隆史 教授 平成25年入学
臓器病態制御系腫瘍放射線学
発表予定論文
Inhibition of Ubiquitin-conjugating enzyme E2 may activate the degradation of hypoxia-inducible factors and thus overcome cellular resistance to radiation in colorectal cancer
(BMC cancer 投稿中)
Navchaa Gombodorj, Takehiko Yokobori, Shinji Yoshiyama, Reika Kawabata-Iwakawa, Susumu Rokudai, I kuko Horikoshi, Masahiko Nishiyama and Takashi Nakano
平成28年8月11日(投稿受付)
群馬大学大学院医学系研究科
平成25年入学
医学系研究科 病態腫瘍制御学 腫瘍放射線学分野 Navchaa Gombodorj
A. Introduction
Significant areas of rapid growth tumors are located at a distance from supporting blood vessels, which results in the formation of a microenvironment, such as low oxygen and nutrients [1,2]. Hypoxia-Inducible Factor 1-Alpha Subunit (HIF-1α) and Hypoxia-Inducible Factor 2-Alpha subunit (HIF-2α), known as EPAS1, are essential factors in the upkeep of cellular oxygen homeostasis and hypoxia adaptation [1, 3-5]. High expression of HIF-1α and -2α was associated not only with radiation resistance but also poor prognosis in CRC [6-10].
Recently it was reported that targeting against ubiquitin-conjugating enzyme E2 (UBE2) family prevents HIF-1α and -2α degradation by the proteasome systems [11-14]. The purpose of this study was to elucidate whether UBE2 inhibitor NSC697923 can actively overcome radiation resistance in CRC cells by regulating HIF-1α and -2α in vitro and in vivo using radiation-sensitive HCT116 and radiation-resistance SW480 cells. We also examined other probable action determinants of NSC697923, using a next-generation sequence (NGS) analysis system.
B. Methods and Methods
Cell lines and reagents::::The human CRC cell lines HCT116, SW480, HCC56, CCK-81, WiDr, LoVo, and DLD-1 were cultured in RPMI1640, Ham's, E-MEM, or DMEM mediums (Wako, Osaka, Japan) supplemented with 10% FBS and 1% penicillin and streptomycin antibiotics, and then incubated at 37°C and 5% CO2. UBE2 inhibitor NSC697923 and CDDP were purchased from SIGMA-ALDRICH Japan (Tokyo, Japan) and Wako laboratory chemicals (Osaka, Japan).
Cytotoxic analysis:Cell viability was analyzed using the Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan).
Radiation
X-ray machine (FAXITRON, RX-650) with 100 kV, Al 0.3 mm filter and the X-ray machine (TITAN-225S, Shimadzu) with 200 kV, 14.6 mA with Al 0.5 mm and Cu 0.5 mm filtration were used for the treatment.
Protein extraction and Western blotting::::The proteins were detected using anti-HIF-1α rabbit monoclonal antibody, anti-HIF-2α rabbit monoclonal antibody (1:1000 in vitro and 1:500 in vivo) (Cell Signaling Technology), and anti-HSC70 mouse monoclonal antibody (1:3000 in vitro and in vivo) (Santa Cruz Biotechnology). HSC70 expression was used as a loading control.
Nude mouse experimental system (mouse xenograft model)::::Eight-week-old male BALB/c nu/nu nude mice received subcutaneous injections of 1 x 107 CRC cells. Tumor volume was calculated using this formula: Volume = S2×L/2, where S is the short length of the tumor in mm and L is the greatest length of the tumor in mm. Mice tissue samples were harvested on days 1, 4, 8 and 12 of the experiment.
RNA-sequencing:One microgram of total RNA was used to generate sequencing libraries of the barcoded fragment using the TruSeq Stranded mRNA Sample Prep Kit (Illumina) following the manufacturer’s instructions. Libraries were subjected to paired-end sequencing of 45bp reads on the NextSeq500 System (Illumina) using NextSeq500 High Output v2 Kit (Illumina). Statistical analysis::::For continuous variables, the data are expressed as the mean ± standard deviation.
Cell viability and differences between treatment groups and in vitro assay data were analyzed using MS Excel. A probability (P) value of less than 0.05 was considered statistically significant. All statistical analyses were performed using JMP software (SAS Institute, Cary, NC, USA).
C. Results
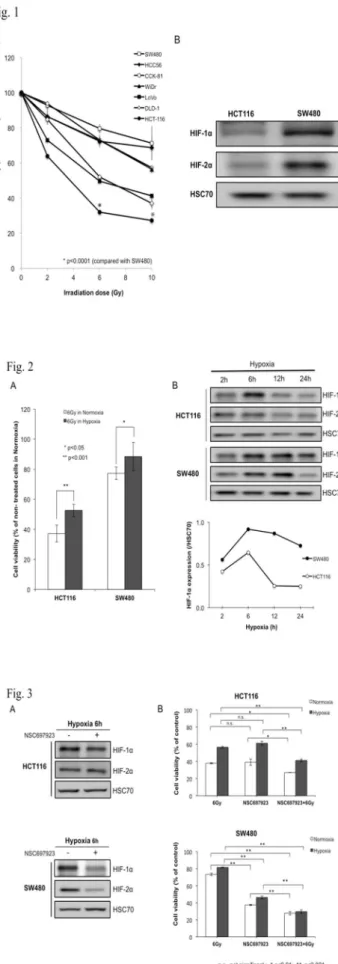
In vitro radiation sensitivity in CRC cell lines in normoxic environment. Cell viability in radiation-sensitive HCT116 cells was significantly suppressed compared with radiation-resistant SW480 cells at all treatment doses (p < 0.0001) (Fig.
1a). Western-blot analysis showed that baseline-expression levels of both HIF-1α and -2α were higher in SW480 than those in HCT116, which was likely related to the cellular resistant levels to radiation (Fig. 1b).
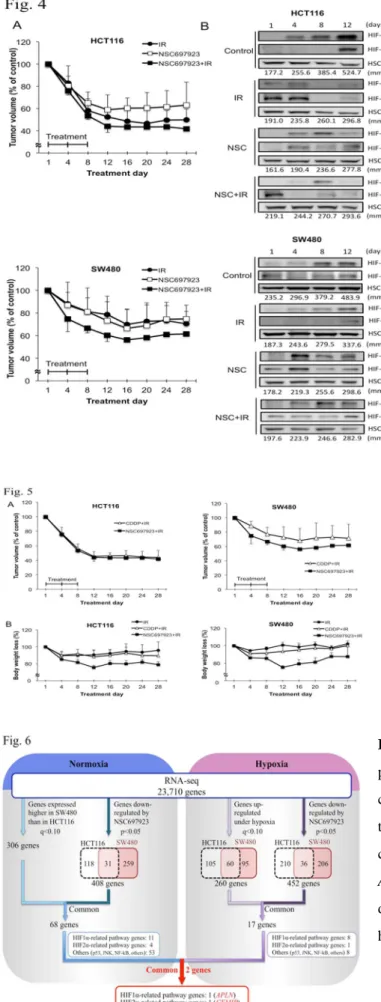
Accumulation of hypoxia inducible factors under hypoxia and radiation resistance. Hypoxia caused a statistically significant increase of cellular resistance to radiation in both cell lines as compared with normoxia (Fig. 2a). Cell viability increased from 37.2% to 52.7% in HCT116 cells (p < 0.001) and from 77.3% to 88.5% in SW480 cells (p <
0.05) under hypoxic condition (Fig. 2a). The induced-HIF-1α and -2α were more stable in radiation-resistant SW480 cells than in HCT116 cells (Fig. 2b).
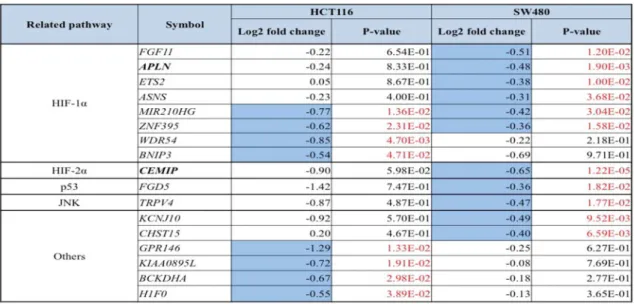
UBE2 inhibitor can repress HIF-1α and -2α in radiation-resistant SW480 even in hypoxic condition. NSC697923 significantly repressed both HIF-1α and -2α induced by hypoxia in SW480 cells, although those remarkable suppressions were not being observed in HCT116 cells (Fig. 3a). Associated with the repressive effect on HIF-1α and -2α, NSC697923 induced significant cytotoxic activity, especially against SW480 cells, even when used by itself, and the combination with IR yielded the most remarkable cell growth inhibition in both HCT116 and SW480 cells even in hypoxic condition (Fig. 3b).
NSC697923 combination with IR in mouse xenograft model. The combination of NSC697923 with IR was the most active against both tumor xenografts, HCT116 and SW480, among all treatments investigated (Fig. 4a). Expression of HIF-1α and -2α in tumors increased, along with an increase of tumor growth in control group (Fig. 4b). In HCT116 xenograft system, the expression was repressed by all treatments investigated, likely due to the tumor growth inhibition activity (Fig. 4b). IR combined with NSC697923 was most active against both radiation-sensitive and -resistant tumor xenografts, and it had a therapeutic advantage in combination with IR even compared with a well-known IR-modulator, CDDP (Fig. 5a). In SW480 tumors, the combination treatment of IR with NSC697923 inhibited tumor growth more than the IR and CDDP combination, with tolerable toxicity (Fig. 5b).
NSC697923 regulated several radiation-resistant pathways including the HIF-1α and -2α in SW480 cells. RNA sequencing of radiation-sensitive HCT116 and radiation-resistant SW480 cells was performed after incubation under normoxia or hypoxia, with or without NSC697923 treatment. We then chose genes expressed at higher levels in radiation-resistant SW480 than in radiation-sensitive HCT116 cells under normoxic condition. We also chose genes more up-regulated under hypoxic conditions, and further down-regulated after NSC697923 treatment under both normoxic and hypoxic conditions in both cell lines, matched for any anticancer activity induced by the drug (Fig. 6). We chose 68 genes (Table 1) in normoxic, and 17 genes (Table 2) in hypoxic conditions as possible drug targets of NSC697923.
Among the genes, we reached to Apelin (APLN) and cell migration inducing protein, hyaluronan binding (CEMIP), which have recently attracted attention as factors in HIF-1 and -2 pathways.
D. Discussion
In this study, we found that NSC697923 can overcome radiation resistance via suppression of HIF-1α and -2α expression
in in vitro and in vivo analysis. For in vivo analysis, the combination of NSC697923 with IR was more effective than that with an established-IR modulator, CDDP. Next-generation RNA sequencing showed that NSC697923 treatment could suppress the HIF family pathway genes, including APLN and CEMIP, in both normoxic and hypoxic conditions.
NSC697923 induced cancer cell death by targeting the JNK pathway, DNA damage signaling, NF-κB signaling, the Mitogen-Activated Protein Kinase (MAPK) pathway and p53-mediated apoptosis [15-17]: p53 is known as an important tumor suppressor in regulating cellular apoptosis, survival and HIF stabilization [18, 19]. In our study, HIF family baseline expression in SW480 is higher than that of HCT116 in normoxic condition. It is possible that survival of SW480 cell line harboring p53 mutation depends on the inactivation of p53 and HIF-family, compared with p53 wild type HCT116. From these previous reports, it has been suggested that NSC697923 monotherapy might have anti-tumor effects via induction of p53 apoptosis.
Previous findings showed that CEMIP is a downstream effector of HIF-2α mediated CRC aggressiveness, and that it directly facilitates colon tumor growth and high expression correlated with poor outcome in the advanced stages of CRC [20, 21], and also that APLN protected cells from apoptosis and is a potent activator of tumor angiogenesis [22, 23].
Therefore, tumor suppression mechanisms of NSC697923 treatment in normoxic and hypoxic conditions might depend on APLN and CEMIP as some of promising downstream targets for HIF family in CRC.
Preclinical studies to find ways to overcome radiation resistance are an important and worthy step in cancer therapeutics. The UBE2 inhibitor NSC697923 has inhibitory activity against radiation resistance through suppression of HIF-1α and -2α. NSC697923 might be particularly effective against the radiation resistance by targeting HIF downstream pathway genes, including APLN and CEMIP. Considering new molecular cancer therapies, NSC697923 may be a promising IR modulator against refractory CRC.
E. Summary
Background: Hypoxia is one of the crucial causes of radiation resistance. Recently, NSC697923, an ubiquitin-conjugating enzyme E2 (UBE2) inhibitor, was suggested to an agent to activate the degradation of hypoxia-inducible factor 1 alpha subunit (HIF-1α), a key factor in hypoxic reaction. We attempted to clarify whether NSC697923 could overcome radiation resistance.
Methods: From 7 colorectal cancer (CRC) cell lines, we selected HCT116 and SW480 cells as the most radiation-sensitive and -resistant lines to use in the following experiments. Radiation resistance was evaluated in the 2 cell lines treated with or without NSC697923 and radiation under normoxic and hypoxic conditions in vitro and in vivo.
We then checked the expression of HIF-1α and -2α in these 2 cell lines. To clarify the mechanism for cytotoxic activity of NSC697923, we examined the NSC697923 regulated-genes using the next-generation RNA sequencing.
Results: Baseline expression levels of HIF-1α and -2α were higher in SW480 than in HCT116 cells. In both cell lines, their expression levels significantly increased under hypoxia with an increase of cellular radiation resistance. The 2 proteins were induced and reached a peak after 6 hours under hypoxia in the 2 cell lines, but decreases varied: rapid in radiation-sensitive HCT116, gradual in radiation-resistant SW480. NSC697923 augmented the radiation effect: Their combination yielded the most promising cytotoxic activity in vitro, especially in radiation-resistant SW480 cells,
regardless of the oxygen supply conditions. Western-blot analyses demonstrated that cytotoxicity induced by the treatment was associated with the repression of HIF-1α and -2α in the cells. In vivo xenograft models confirmed the putative therapeutic activity of NSC697923, especially in SW480: The combination with irradiation (IR) inhibited tumor growth more than the IR combination with an established modulator, CDDP. Although the observed antitumor activity was not directly connected to the regulation of HIF-1α and -2α in the tumor xenografts, next-generation RNA sequencing revealed that NSC697923 distinctively regulated the expression of CEMIP and APLN genes, which have recently attracted attention as an important combination of HIF-1 and -2 pathways.
Conclusion: NSC697923 thus might effectively regulate HIF families, and so be a promising partner with radiation to overcome the resistance.
F. References
1. Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006; 70 (5):1469-1480.
2. Ioannou M, Paraskeva E, Baxevanidou K, Simos G, Papamichali R, Papacharalambous C, Samara M, Koukoulis G.
HIF-1alpha in colorectal carcinoma: review of the literature. J BUON. 2015; 20 (3):680-689.
3. Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008; 8 (12):967-975.
4. Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012; 33 (4):207-214.
5. Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012; 12 (1):9-22.
6. PO DEL, Jorge CC, Oliveira DT, Pereira MC. Hypoxic condition and prognosis in oral squamous cell carcinoma.
Anticancer Res. 2014; 34 (2):605-612.
7. Yoshimura H, Dhar DK, Kohno H, Kubota H, Fujii T, Ueda S, Kinugasa S, Tachibana M, Nagasue N. Prognostic impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal cancer patients: correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res. 2004; 10 (24):8554-8560.
8. Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases.
Cancer Res. 1999; 59 (22):5830-5835.
9. Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003; 29 (4):297-307.
10. Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001; 85 (6):881-890.
11. Strickson S, Campbell DG, Emmerich CH, Knebel A, Plater L, Ritorto MS, Shpiro N, Cohen P. The anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system. Biochem J. 2013; 451 (3):427-437.
12. Roos FC, Evans AJ, Brenner W, Wondergem B, Klomp J, Heir P, Roche O, Thomas C, Schimmel H, Furge KA et al.
Deregulation of E2-EPF ubiquitin carrier protein in papillary renal cell carcinoma. Am J Pathol. 2011; 178
(2):853-860.
13. Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene. 2008; 27 (40):5354-5358.
14. Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000; 19 (48):5435-5443.
15. Cheng J, Fan YH, Xu X, Zhang H, Dou J, Tang Y, Zhong X, Rojas Y, Yu Y, Zhao Y et al. A small-molecule inhibitor of UBE2N induces neuroblastoma cell death via activation of p53 and JNK pathways. Cell Death Dis.
2014; 5:e1079.
16. Pulvino M, Liang Y, Oleksyn D, DeRan M, Van Pelt E, Shapiro J, Sanz I, Chen L, Zhao J. Inhibition of proliferation and survival of diffuse large B-cell lymphoma cells by a small-molecule inhibitor of the ubiquitin-conjugating enzyme Ubc13-Uev1A. Blood. 2012; 120 (8):1668-1677.
17. Hodge CD, Edwards RA, Markin CJ, McDonald D, Pulvino M, Huen MS, Zhao J, Spyracopoulos L, Hendzel MJ, Glover JN. Covalent Inhibition of Ubc13 Affects Ubiquitin Signaling and Reveals Active Site Elements Important for Targeting. ACS Chem Biol. 2015; 10 (7):1718-1728.
18. Li XL, Zhou J, Chen ZR, Chng WJ. P53 mutations in colorectal cancer - molecular pathogenesis and pharmacological reactivation. World J Gastroenterol. 2015; 21 (1):84-93.
19. Datta A, Dey S, Das P, Alam SK, Roychoudhury S. Transcriptome profiling identifies genes and pathways deregulated upon floxuridine treatment in colorectal cancer cells harboring GOF mutant p53. Genom Data. 2016;
8:47-51.
20. Evensen NA, Li Y, Kuscu C, Liu J, Cathcart J, Banach A, Zhang Q, Li E, Joshi S, Yang J et al. Hypoxia promotes colon cancer dissemination through up-regulation of cell migration-inducing protein (CEMIP). Oncotarget. 2015; 6 (24):20723-20739.
21. Fink SP, Myeroff LL, Kariv R, Platzer P, Xin B, Mikkola D, Lawrence E, Morris N, Nosrati A, Willson JK et al.
Induction of KIAA1199/CEMIP is associated with colon cancer phenotype and poor patient survival. Oncotarget.
2015; 6 (31):30500-30515.
22. Picault FX, Chaves-Almagro C, Projetti F, Prats H, Masri B, Audigier Y. Tumour co-expression of apelin and its receptor is the basis of an autocrine loop involved in the growth of colon adenocarcinomas. Eur J Cancer. 2014; 50 (3):663-674.
23. Sorli SC, Le Gonidec S, Knibiehler B, Audigier Y. Apelin is a potent activator of tumour neoangiogenesis.
Oncogene. 2007; 26 (55):7692-7699.
G. Figures, Tables, and Figure legends
Fig. 1 Radiation sensitivity in CRC cell lines under normoxia environment. a Cell viability in various CRC cell lines. These cells were irradiated at 2, 6, and 10 Gy. Radiation sensitivity in HCT116 was higher than that in SW480 (p < 0.0001). From these data, SW480 and HCT116 were identified as radiation-resistant and radiation-sensitive CRC cell lines, respectively. b Western blotting of HIF-1α and -2α baseline expression in SW480 and HCT116 cells under normoxia. The baseline expression of HIF-1α and -2α was higher in radiation-resistant SW480 cells than in radiation-sensitive HCT116 cells. HSC70 was used as a loading control.
Fig. 2 HIF-1α accumulation by hypoxia induced radiation resistance in SW480 and HCT116 cells. a Cell viability in SW480 and HCT116 cells treated with 6 Gy IR under normoxic and hypoxic environments. Hypoxia induced radiation resistance in both cell lines. b Time course of the expression of HIF-1α and -2α under hypoxia. Western blotting was used to evaluate expression in SW480 and HCT116 cells. HIF-1α and -2α expressions were at the highest level 6 hours from initial exposure to hypoxia in both cell lines. These proteins were normalized to the level of HSC70. Intensity was measured using Image J software.
Fig. 3 UBE2 inhibitor NSC697923 can repress HIF-1α and -2α levels in radiation-resistant SW480 in hypoxia environment. a HIF-1α and -2α expression in SW480 and HCT116 cells treated with the IC50 concentration of NSC697923 under 6 h hypoxia.
Western blotting was used to evaluate these expressions in both cells.
NSC697923 significantly repressed both HIF-1α and -2α induced by hypoxia in SW480 cells, but not in HCT116 cells. These proteins were normalized to the level of HSC70. b IR combination with NSC697923 can suppress cell viability more than IR alone in SW480 and HCT116 cells in hypoxic environment (HCT116; 5.61 µM and SW480; 1.87 µM).
Fig. 4 The combination effect of NSC697923 with IR induced tumor growth inhibition in mouse xenograft models. a Tumor volume in mouse xenograft models of SW480 and HCT116 cells treated with IR and/or NSC697923. The combination was effective against both tumor xenografts of HCT116 and SW480, especially against radiation-resistant SW480 xenografts. Tumor volume was normalized to non-treated control. IR was done on day 1 and intra-peritoneal injection of NSC697923 was done on days 1, 4 and 8 of treatment schedule. b Time course of HIF-1α and -2α expression and tumor volume in the xenografted tumors. Western blotting was used to evaluate HIF-1α and -2α expression. HIF-1α and -2α suppression in the combination of NSC697923 and IR were not prominent even at day 12. These proteins were normalized to the level of HSC70.
The tumor volume (mm3) was calculated using the formula:
S2×L/2.
Fig. 5 NSC697923 overcomes radiation resistance in SW480 more than IR-modulator CDDP does. a Tumor volume in mouse xenograft models of SW480 and HCT116 cells treated with IR with CDDP or NSC697923. Tumor volume was normalized to non-treated control. b Percentage of body weight loss in xenografted mice treated with IR alone, and IR with CDDP or NSC697923. Body weight was normalized to non-treated control.
Fig. 6 Analytical method of NSG RNA sequencing to identify promising targets of NSC697923 in normoxic and hypoxic conditions. We chose as 68 (Table 1) and 17 (Table 2) genes as targets downregulated by NSC697923 in normoxic and hypoxic conditions, respectively. From these targets, we found to 2 genes, APLN and CEMIP, which were respectively classified as downstream pathway genes of HIF-1α and -2α in both normoxic and hypoxic conditions.
Tables
Table 1. Significantly suppressed gene list after NSC697923 treatment in normoxia. We chose 68 genes in normoxic condition as possible drug targets of NSC697923 from the results of the next-generation RNA sequencing.
Table 2. Significantly suppressed gene list after NSC697923 treatment in hypoxia. We chose 17 genes in hypoxic condition as possible drug targets of NSC697923 from the results of the next-generation RNA sequencing.