2.7.4. 臨床的安全性
2.7.4. 臨床的安全性
2.7.4 - p. 1 Feb 24 2015 17:16:00
「2.7.4.臨床的安全性」の略号一覧
略号(略称) 定義・省略されていない名称
AE Adverse Event(有害事象)
AIDS Acquired Immune Deficiency Syndrome(後天性免疫不全症候群)
AOM Acute Otitis Media(急性中耳炎)
B-CAP likely Bacterial Community Acquired Pneumonia(細菌性と考えられる市中
肺炎)
C-AOM Clinically confirmed Acute Otitis Media(臨床的急性中耳炎)
CAP Community Acquired Pneumonia(市中肺炎)
C-CAP CAP with alveolar consolidation or pleural effusion on chest X-ray(胸部 X 線 検査で肺胞浸潤または胸水が認められた市中肺炎)
CI Confidence Interval(信頼区間)
CIOMS Council for the International Organization of Medical Sciences (国際医科学団体協議会)
COMPAS Clinical Otitis Media and Pneumonia Study
DT or Di Diphtheria Toxoid(ジフテリアトキソイド)
eCRF Electronic Case Report File(電子症例報告書)
EMA European Medicines Agency(欧州医薬品庁)
EPI Expanded Program on Immunization(拡大予防接種計画)
FinIP Finnish Invasive Pneumococcal Disease Vaccine Effectiveness Study
GSK GlaxoSmithKline(グラクソ・スミスクライン)
HIV Human Immunodeficiency Virus(ヒト免疫不全ウイルス)
IPD Invasive Pneumococcal Disease(侵襲性肺炎球菌感染症)
LRTI Lower Respiratory Tract Infection(下気道感染)
MedDRA Medical Dictionary for Regulatory Activities(国際医薬用語集)
MenC Neisseria meningitidis polysaccharide C(C 群髄膜炎菌ポリサッカライド)
MRSA Methicillin-resistant Staphylococcus aureus(メチシリン耐性黄色ブドウ球
菌)
NTHi Non-typeable Haemophilus influenzae(無莢膜型インフルエンザ菌)
PD Protein D(プロテイン D)
PICU Pediatric Intensive Care Unit(小児集中治療室)
POET Pneumococcal Otitis Efficacy Trial
PSUR Periodic Safety Update Report(定期的安全性最新報告)
PT Preferred Term(基本語)
RR Relative Risk(相対リスク)
SAE Serious Adverse Event(重篤な有害事象)
SCID Severe Combined Immune Deficiency(重症複合型免疫不全症)
SD Standard Deviation(標準偏差)
SOC System Organ Class(器官別大分類)
THL The Finnish National Institute for Health and Welfare(フィンランド国立健
康福祉研究所)
TT Tetanus Toxoid(破傷風トキソイド)
TVC Total Vaccinated Cohort(全ワクチン接種集団)
URTI Upper Respiratory Tract Infection(上気道感染)
WHO World Health Organization(世界保健機関)
2.7.4. 臨床的安全性
2.7.4 - p. 2 Feb 24 2015 17:16:00
「2.7.4.臨床的安全性」のワクチン一覧
略号(略称) 内容
10Pn-PD-DiT Decavalent (10-valent) pneumococcal protein D, diphtheria toxoid and tetanus toxoid conjugate (vaccine). Serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F. プロテインD、ジフテリアトキソ イドおよび破傷風トキソイド結合 型10 価肺炎球菌ワクチン 血清型1、4、5、6B、7F、9V、 14、18C、19F および 23F 11Pn-PD Undecavalent (11-valent)
pneumococcal protein D conjugate (vaccine). Serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F. プロテインD 結合型 11 価肺炎球 菌ワクチン 血清型1、3、4、5、6B、7F、9V、 14、18C、19F および 23F 11Pn-PD&Di Undecavalent (11-valent)
pneumococcal protein D and
diphtheria toxoid conjugate (vaccine). Serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F. プロテインD およびジフテリアト キソイド結合型11 価肺炎球菌ワク チン 血清型1、3、4、5、6B、7F、9V、 14、18C、19F および 23F 11Pn-PD-DiT Undecavalent (11-valent)
pneumococcal protein D, diphtheria toxoid and tetanus toxoid conjugate (vaccine). Serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F. プロテインD、ジフテリアトキソ イドおよび破傷風トキソイド結合 型11 価肺炎球菌ワクチン 血清型1、3、4、5、6B、7F、9V、 14、18C、19F および 23F 23vPS 23-valent plain polysaccharide vaccine
(Pneumovax™ 23) 23 価肺炎球菌ポリサッカライドワクチン
DTP Diphtheria, tetanus, pertussis (vaccine) ジフテリア、破傷風および百日咳
混合ワクチン DTPa Diphtheria, tetanus, acellular pertussis
(vaccine) (Infanrix™) ジフテリア、破傷風および無細胞百日咳混合ワクチン
DTPa-HBV-IPV Diphtheria, tetanus, acellular pertussis-hepatitis B virus-inactivated poliovirus combined vaccine (Infanrix penta™ or Pediarix™)
ジフテリア、破傷風、無細胞百日 咳、B 型肝炎ウイルスおよび不活 化ポリオウイルス混合ワクチン DTPa-HBV-IPV/Hib Diphtheria, tetanus, acellular
pertussis-hepatitis B virus-inactivated poliovirus - Haemophilus influenzae type b combined vaccine (Infanrix hexa™)
ジフテリア、破傷風、無細胞百日 咳、B 型肝炎ウイルス、不活化ポ リオウイルスおよびインフルエン
ザ菌b 型混合ワクチン
DTPa-IPV/Hib Diphtheria, tetanus, acellular pertussis-inactivated poliovirus - Haemophilus
influenzae type b combined vaccine
(Infanrix™-IPV/Hib, Pediacel™ or Pentavac™) ジフテリア、破傷風、無細胞百日 咳、不活化ポリオウイルスおよび インフルエンザ菌b 型混合ワクチ ン
DTPw Diphtheria, tetanus, whole-cell
pertussis (vaccine) (Tritanrix™)
ジフテリア、破傷風および全菌体 百日咳ワクチン
2.7.4. 臨床的安全性
2.7.4 - p. 3 Feb 24 2015 17:16:00
略号(略称) 内容 DTPw-HBV/Hib Diphtheria, tetanus, whole-cell
pertussishepatitis B virus
-Haemophilus influenzae type b vaccine
(Tritanrix™ HepB/Hib or Zilbrix™ Hib)
ジフテリア、破傷風、全菌体百日 咳、B 型肝炎ウイルスおよびイン
フルエンザ菌b 型混合ワクチン
HAV Hepatitis A virus (vaccine) (Havrix™) A 型肝炎ウイルスワクチン
HBV Hepatitis B virus (vaccine)
(Engerix™) B 型肝炎ウイルスワクチン
Hib Haemophilus influenzae type b vaccine
(Hiberix™ or ActHIB™ ) インフルエンザ菌b 型ワクチン
Hib-MenC Haemophilus influenzae type b-Neisseria meningitidis group C-tetanus
toxoid conjugate vaccine (Menitorix™)
インフルエンザ菌b 型および破傷
風トキソイド結合型C 群髄膜炎菌
混合ワクチン
HRV Human rotavirus vaccine (Rotarix™) ヒトロタウイルスワクチン
IPV Inactivated poliovirus vaccine
(Poliorix™) 不活化ポリオワクチン
MenACWY-TT Neisseria meningitidis group A, C,
W135and Y tetanus toxoid conjugate vaccine
A 群、C 群、W135群およびY 群髄
膜炎菌、破傷風トキソイド結合型 ワクチン
MenC-CRM Meningococcal C-diphtheria CRM197
conjugate vaccine (Meningitec™) CRM197
結合型C 群髄膜炎菌ワクチ
ン
MenC-TT Meningococcal C-tetanus toxoid
conjugate vaccine (NeisVac-C™)
破傷風トキソイド結合型C 群髄膜
炎菌ワクチン
MMR Measles, mumps and rubella vaccine
(Priorix™ or M-M-RVAXPRO™)
麻疹、ムンプスおよび風疹混合ワ クチン
MMRV Measles, mumps, rubella and varicella vaccine (Priorix- Tetra™)
麻疹、ムンプス、風疹および水痘 混合ワクチン
OPV Oral poliovirus vaccine (Polio
Sabin™) 経口ポリオワクチン
RV Rotavirus vaccine (RotaTeq™) ロタウイルスワクチン
2.7.4. 臨床的安全性
2.7.4 - p. 4 Feb 24 2015 17:16:00
「2.7.4.臨床的安全性」の商標登録製品名 GlaxoSmithKline 社の
商標登録製品名
一般的記述
Engerix™ Hepatitis B virus (vaccine) B 型肝炎ウイルスワクチン
Havrix™ Hepatitis A virus (vaccine) A 型肝炎ウイルスワクチン
Hiberix™ Haemophilus influenzae type b vaccine インフルエンザ菌 b 型ワクチン
Infanrix™ Diphtheria, tetanus, acellular pertussis
(vaccine) ジフテリア、破傷風および無細胞百日咳混合ワクチン
Infanrix hexa™ Diphtheria, tetanus, acellular pertussis-hepatitis B virus-inactivated poliovirus - Haemophilus influenzae type b combined vaccine
ジフテリア、破傷風、無細胞百日 咳、B 型肝炎ウイルス、不活化ポ リオウイルスおよびインフルエン
ザ菌b 型混合ワクチン
Infanrix™-IPV/Hib Diphtheria, tetanus, acellular pertussis-inactivated poliovirus - Haemophilus
influenzae type b combined vaccine
ジフテリア、破傷風、無細胞百日 咳、不活化ポリオウイルスおよび
インフルエンザ菌b 型混合ワクチ
ン Infanrix penta™ or
Pediarix™ Diphtheria, tetanus, acellular pertussis-hepatitis B virus-inactivated poliovirus combined vaccine
ジフテリア、破傷風、無細胞百日 咳、B 型肝炎ウイルスおよび不活 化ポリオウイルス混合ワクチン Menitorix™ Haemophilus influenzae type
b-Neisseria meningitidis group C-tetanus
toxoid conjugate vaccine
インフルエンザ菌b 型および破傷
風トキソイド結合型C 群髄膜炎菌
ワクチン
Poliorix™ Inactivated poliovirus vaccine 不活化ポリオワクチン
Polio Sabin™ Oral poliovirus vaccine 経口ポリオワクチン
Priorix™ Measles, mumps and rubella vaccine 麻疹、ムンプスおよび風疹混合ワ
クチン Priorix-Tetra™ Measles, mumps, rubella and varicella
vaccine 麻疹、ムンプス、風疹および水痘混合ワクチン
Rotarix™ Human rotavirus vaccine ヒトロタウイルスワクチン
Synflorix™ 10-valent pneumococcal non-typeable
Haemophilus influenzae type b protein
D conjugate vaccine
タンパク結合型10 価肺炎球菌ワク
チン(10Pn-PD-DiT ワクチン)
Tritanrix™ Diphtheria, tetanus, whole-cell
pertussis (vaccine) ジフテリア、破傷風および全菌体百日咳混合ワクチン
Tritanrix™ HepB/Hib Diphtheria, tetanus, whole-cell pertussishepatitis B virus
-Haemophilus influenzae type b vaccine
ジフテリア、破傷風、全菌体百日
咳、B 型肝炎ウイルスおよびイン
フルエンザ菌b 型混合ワクチン
Zilbrix™ Hib Diphtheria, tetanus, whole-cell pertussishepatitis B virus
-Haemophilus influenzae type b vaccine
ジフテリア、破傷風、全菌体百日 咳、B 型肝炎ウイルスおよびイン フルエンザ菌b 型混合ワクチン 2.7.4. 臨床的安全性 2.7.4 - p. 5 Feb 24 2015 17:16:01
他社の商標登録製品名 一般的記述
ActHIB™ Sanofi Pasteur MSD’s Haemophilus
influenzae type b vaccine Sanofi Pasteur MSD 社のインフルエンザ菌b 型ワクチン
Meningitec™ Pfizer’s Meningococcal C-diphtheria CRM197conjugate vaccine
Pfizer 社の CRM197結合型C 群髄膜 炎菌ワクチン
M-M-RVAXPRO™ Sanofi Pasteur MSD’s measles,
mumps and rubella vaccine Sanofi Pasteur MSD 社の麻疹、ムンプスおよび風疹混合ワクチン NeisVac-C™ Baxter International’s Meningococcal
C-tetanus toxoid conjugate vaccine Baxter International 社の破傷風トキソイド結合型C 群髄膜炎菌ワクチ ン
Pediacel™ or Pentavac™
Sanofi Pasteur MSD’s diphtheria, tetanus, acellular pertussis-inactivated poliovirus - Haemophilus influenzae type b combined vaccine
Sanofi Pasteur MSD 社のジフテリ ア、破傷風、無細胞百日咳、不活 化ポリオウイルスおよびインフル
エンザ菌b 型混合ワクチン
Pneumovax™ 23 Merck’s 23-valent plain
polysaccharide vaccine Merck 社の 23 価肺炎球菌ポリサッカライドワクチン Prevenar™ Pfizer’s 7-valent pneumococcal
conjugate vaccine with diphtheria CRM197as protein carrier
Pfizer 社の CRM197結合型7 価肺炎 球菌ポリサッカライドワクチン Prevenar 13™ Pfizer’s 13-valent pneumococcal
conjugate vaccine with diphtheria CRM197as protein carrier
Pfizer 社の CRM197結合型13 価肺 炎球菌ポリサッカライドワクチン RotaTeq™ Sanofi Pasteur MSD’s human-bovine
rotavirus reassortants vaccine Sanofi Pasteur MSD 社のヒト-ウシロタウイルス再集合体ワクチン 2.7.4. 臨床的安全性
2.7.4 - p. 6 Feb 24 2015 17:16:01
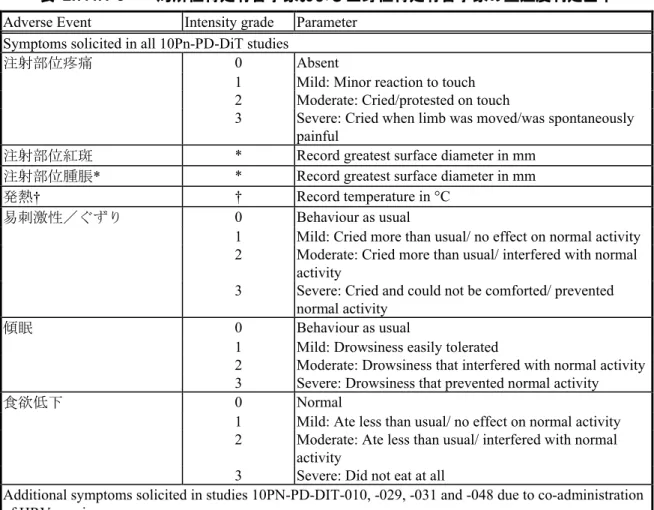
2.7.4.1. 医薬品への曝露 2.7.4.1.1. 総括的安全性評価計画および安全性試験の記述 10Pn-PD-DiT ワクチンの安全性は、生後 7 ヵ月未満の乳児を対象に実施した初回免疫試験、 生後11 ヵ月あるいは生後 2 年目で追加免疫接種を実施した追加免疫試験、生後 7 ヵ月~5 歳の乳幼児を対象に実施したキャッチアップ免疫試験および生後3~4 歳時に追加接種を実 施した試験を用いて評価した。大部分の試験で、10Pn-PD-DiT ワクチンは DTPa 混合ワクチ ンと同時接種した。その他の同時接種ワクチンとしては、DTPw 混合ワクチン、Hib ワクチ ン(DTP 混合ワクチンとしてあるいは単独ワクチン)、HBV ワクチン(DTP 混合ワクチン として)、IPV/OPV ワクチン(DTP 混合ワクチンとしてあるいは単独ワクチン)、HRV ワ クチン、MenC ワクチンおよび MMRV ワクチンを設定した。これらの臨床試験での安全性 および副反応の評価は、主要目的あるいは副次目的として設定した。特定有害事象(局所性 および全身性)および重篤な有害事象を含む特定外有害事象などのすべての安全性の解析は 試験ごとに行った。安全性の記述および比較統計解析の詳細は2.7.4.1.1.4 に示した。10Pn-PD-DiT ワクチンの市販後の安全性成績の概要は 2.7.4.6 に示した。 2.7.4.1.1.1. 臨床開発計画 10Pn-PD-DiT ワクチンの安全性は、初回免疫、追加免疫、キャッチアップ免疫および追加 接種を実施した11 本の評価試験と 26 本の参考試験を用いて評価した。これら 37 試験の概 要を表 2.7.4.1-1、表 2.7.4.1-2、表 2.7.4.1-3 および表 2.7.4.1-4 に示した。これらの試験のう ち非盲検試験は22 試験、単盲検試験は 6 試験、および二重盲検試験あるいは観察者盲検試 験は9 試験であった。データ固定までに収集されたデータは各試験の総括報告書に記載した。 10PN-PD-DIT-043 試験および 10PN-PD-DIT-061 試験を除き、初回免疫期、追加免疫期およ びキャッチアップ免疫期の評価を行った35 試験については、2012 年 12 月 10 日をデータロ ックポイントとして安全性の併合解析を行った。 10PN-PD-DIT-001 試験(ロット間の同質性評価)、10PN-PD-DIT-043 試験(クラスターラ ンダム化を用いた試験)、10PN-PD-DIT-048 試験(第Ⅲ相臨床試験使用ロットと市販ロット とのブリッジング)および10PN-PD-DIT-053 試験(クラスターランダム化を用いた試験) では、二重盲検法とした。その他、多くの臨床試験では、試験ワクチンの外観や包装形態の 相違、接種群間の接種スケジュールが異なるため、単盲検法または非盲検法とした。観察者 盲検とした試験は、10PN-PD-DIT-005 試験、10PN-PD-DIT-009 試験、10PN-PD-DIT-012 試験、 10PN-PD-DIT-018 試験および 10PN-PD-DIT-028 試験の 5 試験、単盲検試験は、10PN-PD-DIT-001 試験(免疫学的非劣性の検証)、10PN-PD-DIT-003 試験、10PN-PD-DIT-007 試験、 10PN-PD-DIT-027 試験、10PN-PD-DIT-036 試験(免疫学的非劣性の検証)、10PN-PD-DIT-037 試験および 10PNPD-DIT-063 試験の 7 試験、10PN-PD-DIT-058 試験を含むその他の臨床 試験は非盲検とした。
10PN-PD-DIT-007 010 011 試験、10PN-PD-DIT-012 試験、10PN-PD-DIT-014 試験、10PN-PD-DIT-017 試験、10PN-PD-DIT-018 試験および 10PN-PD-DIT-022 試験の 8 試験では、主要目的として推測統計解析または記述統計解析によ
2.7.4. 臨床的安全性
2.7.4 - p. 7 Feb 24 2015 17:16:01
り発熱反応を評価した。発熱反応の診断は客観的な指標となる直腸温により判断し、被験者 の親または保護者がワクチン接種後4 日間(10PN-PD-DIT-022 試験では 15 日間)に測定を 行った。本診断方法は、非盲検法や単盲検法などの試験デザインによるバイアスを最小限に する。 10PN-PD-DIT-005 試験および 10PN-PD-DIT-009 試験では、主要目的としてワクチン接種 後4 日間のグレード 3 の特定および特定外有害事象の発現を評価した。本試験は安全性の評 価に関して客観性を保つため、観察者盲検法により実施した。その他、10PN-PD-DIT-063 試 験、10PN-PD-DIT-066 試験および 10PN-PD-DIT-069 試験の 3 試験も同様に、主要目的とし てグレード3 の特定および特定外有害事象の発現を評価し、10PN-PD-DIT-063 試験は単盲検 法、10PN-PD-DIT-066 試験および 10PN-PD-DIT-069 試験は非盲検法により実施した。 10PN-PD-DIT-015 試験および 10PN-PD-DIT-016 試験は、主要目的として早産児に初回免 疫(3 回接種)および追加免疫した時の安全性を評価し、非盲検法により行った。10PN-PD-DIT-043 試験を除く他の臨床試験では、安全性を副次目的として評価した。なお、10PN-PD-DIT-008 試験、10PN-PD-DIT-041 Y1 試験および 10PN-PD-DIT-041 Y2 試験では 10Pn-PD-DiT ワクチンの接種を実施しなかった。そのため、これらの試験は安全性評価には用いず有効性 (免疫原性)の評価のみに使用した。 10Pn-PD-DiT ワクチンの安全性を評価した計 35 試験で、10Pn-PD-DiT ワクチンを 1 回以 上接種した被験者は、初回免疫試験で22,429 例、追加免疫試験で 19,466 例、キャッチアッ プ免疫試験で1,579 例(初回接種年齢ごとの症例数は、生後 1 年目が 391 例、生後 2 年目が 666 例、その他が 522 例)および 10Pn-PD-DiT ワクチンの追加接種を実施した試験で 357 例 であった。 また、生後2 歳未満の乳幼児を対象に実施した 11 価ワクチンの 5 本の臨床試験(評価試 験)で得られた安全性成績は、本申請資料概要別冊1 に示した(2.7.4.1.1.6 および 2.7.4.2.1.9)。 2.7.4. 臨床的安全性 2.7.4 - p. 8 Feb 24 2015 17:16:01
表 2.7.4.1-1 初回免疫を評価した試験(全ワクチン接種集団) Study
Number (country)
Design
Main Study Objectives
Population Age (at time of first dose) Vaccine schedule Study vaccine + co-administered vaccines Number of vaccinated subjects Per group Total
10PN-PD-DIT-001* (Finland, France, Poland)
Double-blind (for lot-to-lot consistency), single-blind (for non-inferiority), randomized, controlled
> Primary objective: ・Lot-to-lot consistency
・Immunological non-inferiority of 10Pn-PD-DiT versus Prevenar for at least 7 of the pneumococcal serotypes
> Key secondary objectives:
・Non-inferiority versus Prevenar in terms of post-vaccination febrile reactions with rectal temperature > 39C
・Assessment of reactogenicity of 10Pn-PD-DiT when co-administered with DTPa-combined vaccines
・Assessment of immunogenicity of DTPa-combined vaccines when co-administered with 10Pn-PD-DiT Healthy infants 6 to 12 weeks 2-3-4 months 10Pn-PD-DiT (3 lots) + Infanrix hexa§ Prevenar + Infanrix hexa§ § except for dose 2 in France (co-ad with Infanrix IPV/Hib) 1235 415 1650 10PN-PD-DIT-002*1 (Denmark, Norway, Slovakia, Sweden) Open, randomized > Primary objective:
・Assessment of the post-dose 2 immune response elicited by 10Pn-PD-DiT administered according to a 2-4-11 months vaccination schedule co-administered with DTPa-combined vaccine
> Key secondary objectives:
・Assessment of post-dose 3 immune response to 10Pn-PD-DiT administered according to a 2-3-4-11 months vaccination schedule
・Assessment of persistence of pneumococcal antibodies prior to the booster dose at 11 months of age
・Assessment of immune response elicited by a booster dose at 11 months of age following 2 or 3 dose primary vaccination
・Assessment of safety and reactogenicity of 10Pn-PD-DiT
Healthy infants 8 to 16 weeks 2-4 months (2 dose) 2-3-4 months (3 dose) 2 doses: 10Pn-PD-DiT + Infanrix hexa or Infanrix IPV/Hib at 2-4 months 3 doses: 10Pn-PD-DiT + Infanrix hexa or Infanrix IPV/Hib at 2-4 months 175 176 351 2.7.4. 臨床的安全性 2.7.4 - p. 9 Feb 24 2015 17:16:02
Study Number (country)
Design
Main Study Objectives
Population Age (at time of first dose) Vaccine schedule Study vaccine + co-administered vaccines Number of vaccinated subjects Per group Total
10PN-PD-DIT-003* (Germany)
Single-blind, randomized, controlled
> Primary objective:
・Assessment of the immunogenicity of 10Pn-PD-DiT > Key secondary objectives:
・Assessment of safety and reactogenicity of 10Pn-PD-DiT
・Assessment of immunogenicity of Infanrix hexa when co-administered with 10Pn-PD-DiT
Healthy infants 8 to 16 weeks 2-3-4 months 10Pn-PD-DiT + Infanrix hexa Prevenar + Infanrix hexa 70 64 134 10PN-PD-DIT-013*2 (Finland) Open, controlled > Primary objective:
・Assessment of immunogenicity of 10Pn-PD-DiT when given as a catch-up vaccination in children older than 7 months
> Key secondary objectives:
・Assessment of safety and reactogenicity of 10Pn-PD-DiT when given as a catch-up vaccination
・Assessment of immunogenicity, safety and reactogenicity of 3-dose primary course of Infanrix IPV/Hib co-administered with 10Pn-PD-DiT
Healthy infants < 6 months 3, 4, 5 months 3 primary doses of 10Pn-PD-DiT+ Infanrix IPV/Hib 150 150 10PN-PD-DIT-028*3 (Panama, Argentina, Columbia)
Double blind(observer-blind), randomized, controlled
> Primary objective:
・Efficacy of a 3-dose primary course followed by a booster dose in second year of life with 10Pn-PD-DiT against likely bacterial community acquired pneumonia (B-CAP)
> Key secondary objectives:
・Efficacy of 10Pn-PD-DiT against clinically confirmed Acute Otitis Media (C-AOM) cases in the 7,000 children enrolled in Panama
・Efficacy of 10Pn-PD-DiT against CAP with alveolar consolidation or pleural effusion on chest X-ray (C-CAP)
・Efficacy of 10Pn-PD-DiT in preventing bacteriologically confirmed AOM cases caused by H. influenzae and vaccine serotypes
・Efficacy of 10Pn-PD-DiT in preventing invasive disease
・Impact of 10Pn-PD-DiT on nasopharyngeal carriage of S. pneumoniae and H. influenzae ・Immunogenicity, safety and reactogenicity of 10Pn-PD-DiT
Healthy infants 2-4-6 months 10Pn-PD-DiT + Infanrix hexa HBV + Infanrix IPV/Hib 11798 11799 23597 2.7.4. 臨床的安全性 2.7.4 - p. 10 Feb 24 2015 17:16:02
Study Number (country)
Design
Main Study Objectives
Population Age (at time of first dose) Vaccine schedule Study vaccine + co-administered vaccines Number of vaccinated subjects Per group Total
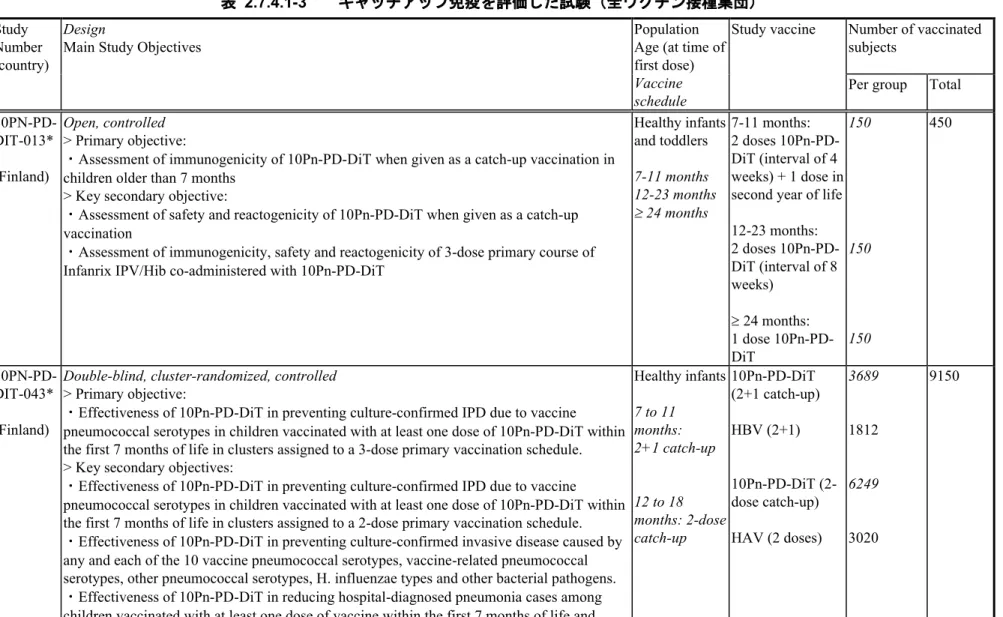
10PN-PD-DIT-043*4 (Finland)
Double-blind, cluster-randomized, controlled
> Primary objective:
・Effectiveness of 10Pn-PD-DiT in preventing culture-confirmed IPD due to vaccine pneumococcal serotypes in children vaccinated with at least one dose of 10Pn-PD-DiT within the first 7 months of life in clusters assigned to a 3-dose primary vaccination schedule. > Key secondary objectives:
・Effectiveness of 10Pn-PD-DiT in preventing culture-confirmed IPD due to vaccine pneumococcal serotypes in children vaccinated with at least one dose of 10Pn-PD-DiT within the first 7 months of life in clusters assigned to a 2-dose primary vaccination schedule. ・Effectiveness of 10Pn-PD-DiT in preventing culture-confirmed invasive disease caused by any and each of the 10 vaccine pneumococcal serotypes, vaccine-related pneumococcal serotypes, other pneumococcal serotypes, H. influenzae types and other bacterial pathogens. ・Effectiveness of 10Pn-PD-DiT in reducing hospital-diagnosed pneumonia cases among children vaccinated with at least one dose of vaccine within the first 7 months of life and within or beyond the first 7 months of life.
・Impact of 10Pn-PD-DiT on occurrence of tympanostomy tube placements in children vaccinated with at least one dose of vaccine within the first 7 months of life and within or beyond the first 7 months of life.
・Impact of vaccination with 10Pn-PD-DiT on the incidence of lower respiratory tract infections (LRTI) and upper respiratory tract infections (URTI), including AOM, in the Turku area. Healthy infants 6 weeks to 6 months: 2 or 3 dose primary 10Pn-PD-DiT (3+1) HBV (3+1) 10Pn-PD-DiT (2+1) HBV (2+1) 8427 4473 9112 4399 (26411) 4 10PN-PD-DIT-053*5 (Finland)
Double-blind, cluster-randomized, controlled
> Primary objective:As in study 10PN-PD-DIT-043
> Key secondary objectives:As in study 10PN-PD-DIT-043 with the following
・Impact of vaccination with 10Pn-PD-DiT on the nasopharyngeal carriage of S. pneumoniae, H. influenzae and/or other bacterial pathogens in children starting vaccination below 18 months of age. Healthy infants 6 weeks 6 weeks to 6 months: 10Pn-PD-DiT (10Pn3+1) HBV (Ctrl3+1) 10Pn-PD-DiT 1849 1069 1316 5093 2.7.4. 臨床的安全性 2.7.4 - p. 11 Feb 24 2015 17:16:02
Study Number (country)
Design
Main Study Objectives
Population Age (at time of first dose) Vaccine schedule Study vaccine + co-administered vaccines Number of vaccinated subjects Per group Total ・Impact of vaccination with 10Pn-PD-DiT on the incidence of acute otitis media with level 1
of diagnostic certainty (as defined in protocol), in children starting vaccination below 18 months of age.
・Assessment, one month post-primary and post-booster vaccination course, the
immunogenicity of DTPa vaccine when co-administered with GSK Biologicals’ 10Pn-PD-DiT. ・Assessment of the antibody persistence 12-14 months following the completion of the three-dose primary vaccination course with GSK Biologicals’ 10Pn-PD-DiT when co-administered with DTPa vaccine.
・Assessment of the antibody persistence 12-14 months following the completion of the three-dose primary vaccination course with DTPa vaccine when co-administered with GSK
Biologicals’ 10Pn-PD-DiT.
・Assessment of the safety and reactogenicity of GSK Biologicals’ 10Pn-PD-DiT when co-administered with DTPa vaccine.
2 or 3 dose primary (10Pn2+1) HBV (Ctrl2+1) 859 10PN-PD-DIT-058* (Japan)
Open, randomized, controlled
> Primary objective:
・Comparison of the immunogenicity of GSK Biologicals 10Pn-PD-DiT in healthy Japanese children, one month post-dose 3, to the immune response of 10Pn-PD-DiT as observed in the pivotal non-inferiority study 10PN-PD-DIT-001 in Europe.
> Key secondary objectives:
・Comparison of the immunogenicity of GSK Biologicals’ 10Pn-PD-DiT in healthy Japanese children, one month post-dose 3, to the historical immunogenicity data obtained with the 11Pn-PD vaccine formulation in study Undeca-Pn-010 (POET [347414/010]).
・Comparison of the immunogenicity of GSK Biologicals’ 10Pn-PD-DiT in healthy Japanese children, one month post-dose 3, to the immune response of 10Pn-PD-DiT as observed in study 10PN-PD-DIT-028 (COMPAS [109563]).
・Assessment, of immunogenicity of GSK Biologicals’ 10Pn-PD-DiT when co-administered with DTPa vaccine, one month post-primary and post-booster vaccination course.
・Assessment of the immunogenicity of DTPa vaccine when co-administered with GSK Biologicals’ 10Pn-PD-DiT one month post-primary and post-booster vaccination course.
Healthy children 3 months 3-4-5 months 10Pn-PD-DiT + DTPa DTPa 237 123 360 2.7.4. 臨床的安全性 2.7.4 - p. 12 Feb 24 2015 17:16:03
Study Number (country)
Design
Main Study Objectives
Population Age (at time of first dose) Vaccine schedule Study vaccine + co-administered vaccines Number of vaccinated subjects Per group Total ・Assessment of the antibody persistence 12-14 months following the completion of the
three-dose primary vaccination course with GSK Biologicals’ 10Pn-PD-DiT when co-administered with DTPa vaccine.
・Assessment of the antibody persistence 12-14 months following the completion of the three-dose primary vaccination course with DTPa vaccine when co-administered with GSK
Biologicals’ 10Pn-PD-DiT.
・Assessment of the safety and reactogenicity of GSK Biologicals’ 10Pn-PD-DiT when co-administered with DTPa vaccine.
10PN-PD-DIT-005 (Chile)
Observed-blind, randomized, controlled
> Primary objective:
・Assessment of reactogenicity of 10Pn-PD-DiT when co-administered with Infanrix hexa in terms of occurrence of grade 3 adverse events (solicited and unsolicited)
> Key secondary objectives:
・Assessment of safety, reactogenicity and immunogenicity of 10Pn-PD-DiT when co-administered with Infanrix hexa
・Assessment of immune response to PD following primary course of 10Pn-PD-DiT when co-administered with Infanrix hexa
Healthy infants 6 to 12 weeks 2-4-6 months 10Pn-PD-DiT + Infanrix hexa Havrix + Infanrix hexa 119 121 240 10PN-PD-DIT-010 (Czech Republic)
Open, randomized, controlled
> Primary objective:
・Determination of the percentage reduction in febrile reactions (rectal temperature 38.0C) when prophylactic antipyretic treatment is administered compared to no prophylactic
antipyretic treatment after primary vaccination with 10Pn-PD-DiT > Key secondary objectives:
・Assessment of safety and reactogenicity of the 10Pn-PD-DiT
・Assessment of immunogenicity of 10Pn-PD-DiT when co-administered with Infanrix hexa vaccine
・Assessment of immunogenicity of the Rotarix vaccine co-administered at 3-4 months of age with 10Pn-PD-DiT
・Comparison of the immune response induced by 10Pn-PD-DiT to the historical
Healthy infants 9 to 16 weeks 3-4-5 months 10Pn-PD-DiT + Infanrix hexa + Rotarix with prophylactic antipyretic medication 10Pn-PD-DiT + Infanrix hexa + Rotarix without prophylactic antipyretic 226 233 459 2.7.4. 臨床的安全性 2.7.4 - p. 13 Feb 24 2015 17:16:03
Study Number (country)
Design
Main Study Objectives
Population Age (at time of first dose) Vaccine schedule Study vaccine + co-administered vaccines Number of vaccinated subjects Per group Total immunogenicity data obtained with the 11Pn-PD vaccine formulation in study Undeca-Pn-010
(POET), 1 month after primary vaccination
medication 10PN-PD-DIT-011 6 (Germany, Poland, Spain)
Open, randomized, controlled
> Primary objective:
・Non-inferiority of 10Pn-PD-DiT versus Prevenar, both co-administered with DTPa-combined and Hib-MenC vaccines, in terms of post-vaccination febrile reactions with rectal temperature >39.0C
> Key secondary objectives:
・Assessment of immunogenicity, safety and reactogenicity of 10Pn-PD-DiT
・Assessment of immunogenicity post dose 2 of Hib-MenC conjugate when co-administered with 10Pn-PD-DiT or Prevenar and DTPa-HBV-IPV
・Assessment of immunogenicity post dose 3 of Hib-MenC and DTPa-HBV-IPV vaccines when co-administered with 10Pn-PD-DiT or Prevenar
Healthy infants 6 to 16 weeks 2-4-6 months § § Meningitec and NeisVac-C: were administered at 2 and 4 months 10Pn-PD-DiT + Infanrix hexa + Meningitec 10Pn-PD-DiT + Infanrix hexa + NeisVac-C 10Pn-PD-DiT + DTPa-HBV-IPV + Hib-MenC Prevenar + DTPa- HBV-IPV + Hib-MenC 385 387 386 390 1548 10PN-PD-DIT-012 (Philippines Poland)
Double blind(observer-blind), randomized, controlled
> Primary objective:
・Non-inferiority of 3-dose priming with 10Pn-PD-DiT versus Prevenar in terms of incidence of post-vaccination fever > 39C when co-administered with DTPw-HBV/Hib and OPV or IPV > Key secondary objective:
・Assessment of immunogenicity and reactogenicity of 10Pn-PD-DiT when co-administered with DTPw-HBV/Hib and OPV or IPV vaccines
Healthy infants 6 to 12 weeks 2-4-6 months (Poland) or 6-10-14 weeks (Philippines) 10Pn-PD-DiT + DTPw-HBV/Hib + OPV or IPV Prevenar + DTPw-HBV/Hib + OPV or IPV 603 203 806 10PN-PD-DIT-015 Open, controlled > Primary objective: Preterm or full term infants 10Pn-PD-DiT + Infanrix hexa 137 (preterm) 286 2.7.4. 臨床的安全性 2.7.4 - p. 14 Feb 24 2015 17:16:03
Study Number (country)
Design
Main Study Objectives
Population Age (at time of first dose) Vaccine schedule Study vaccine + co-administered vaccines Number of vaccinated subjects Per group Total (Greece,
Spain)
・Assessment of safety and reactogenicity of 10Pn-PD-DiT when co-administered with Infanrix hexa in preterm infants
> Key secondary objective:
・Assessment of immunogenicity of 10Pn-PD-DiT when co-administered with Infanrix hexa in preterm infants 8 to 16 weeks 2-4-6 months 10Pn-PD-DiT + Infanrix hexa 149 (full term) 10PN-PD-DIT-027 PRI 7 (Nether-lands)
Single-blind, randomized, controlled
> Primary objective:
・Non-inferiority of 10Pn-PD-DiT co-administered with DTPa-IPV/Hib (Pediacel) versus 10Pn-PD-DiT co-administration with DTPa-HBV-IPV/Hib (Infanrix hexa), in terms of immune response to the 10 vaccine pneumococcal serotypes and to protein D
> Key secondary objective:
・Non-inferiority of Pediacel when administered with 10Pn-PD-DiT versus the co-administration with Prevenar in terms of immune response to DTPa-IPV/Hib antigens
・Assessment of the impact of vaccination with 10Pn-PD-DiT on the nasopharyngeal carriage of H. influenzae.
・Assessment of safety and reactogenicity of 10Pn-PD-DiT when co-administered with Infanrix hexa or Pediacel
・Assessment of immunogenicity of a booster dose of 10Pn-PD-DiT co-administered with a booster dose of Infanrix hexa or Pediacel at 11-12 months of age.
・Assessment of persistence of antibodies 12 months after completion of the four-dose vaccination course with 10Pn-PD-DiT co-administered with Infanrix hexa or Pediacel
Healthy infants 6 to 12 weeks 2-3-4-11 months 10Pn-PD-DiT + Infanrix hexa 10Pn-PD-DiT+ Pediacel Prevenar + Pediacel 260 260 260 780 10PN-PD-DIT-029 (Mexico) Open > Primary objective:
・Comparison of immunogenicity of 3-dose primary course with 10Pn-PD-DiT when co-administered with Infanrix hexa and Rotarix to immune response in study 10PN-PD-DIT-001 > Key secondary objective:
・Assessment of safety and reactogenicity of 3-dose priming with 10Pn-PD-DiT when co-administered with Infanrix hexa and Rotarix
Healthy infants 6 to 12 weeks 2-4-6 months 10Pn-PD-DiT + Infanrix hexa + Rotarix 230 230 2.7.4. 臨床的安全性 2.7.4 - p. 15 Feb 24 2015 17:16:03
Study Number (country)
Design
Main Study Objectives
Population Age (at time of first dose) Vaccine schedule Study vaccine + co-administered vaccines Number of vaccinated subjects Per group Total
10PN-PD-DIT-031 (Taiwan)
Open
> Primary objective:
・Assessment of immunogenicity of 3-dose priming with 10Pn-PD-DiT when co-administered with Infanrix hexa and Rotarix
> Key secondary objective:
・Assessment of safety and reactogenicity of 3-dose priming with 10Pn-PD-DiT when co-administered with Infanrix hexa and Rotarix
Healthy infants 6 to 8 weeks 1.5-3-6 months 10Pn-PD-DiT + Infanrix hexa + Rotarix 230 230 10PN-PD-DIT-032 (Mali, Nigeria)
Open, randomized, controlled
> Primary objective:
・Assessment of immunogenicity of 10Pn-PD-DiT when co-administered with DTPw-HBV/Hib (Zilbrix Hib) + OPV one month post dose 3
> Key secondary objective:
・Assessment of safety and reactogenicity of 3-dose primary vaccination with 10Pn-PD-DiT when co-administered with Zilbrix Hib + OPV in Sub-Saharan Africa
・Assessment of immunogenicity of DTPw-HBV/Hib vaccine
Healthy infants 6 to 10 weeks 6-10-14 weeks 10Pn-PD-DiT+ Zilbrix Hib + OPV Zilbrix Hib +OPV 239 118 357 10PN-PD-DIT-034 8 (South Africa)
Open, partially randomized, controlled
> Primary objective:
・Immune response to 10Pn-PD-DiT one month following a 3-dose (6, 10 and 14 weeks of age) primary vaccination course in HIV infected infants, HIV exposed uninfected infants and HIV unexposed uninfected infants.
> Key secondary objectives:
・Safety and reactogenicity of 10Pn-PD-DiT, immunogenicity after two doses, persistence of antibodies after 6 months following primary vaccination, immunogenicity of a booster dose, persistence of antibodies 14 months after persistence, immunogenicity of three doses and booster dose of DTPw-HBV/Hib
HIV-, HIV+/-and HIV+/+ infants 6 to 10 weeks 6-(10)-14 weeks HIV+/+ (3+1): 10Pn-PD-DiT + DTPw-HBV/Hib + HRV HIV+/- (3+1): 10Pn-PD-DiT + DTPw-HBV/Hib + HRV HIV- (3+1): 10Pn-PD-DiT + DTPw-87 97 100 100 300 (184) 8 2.7.4. 臨床的安全性 2.7.4 - p. 16 Feb 24 2015 17:16:04
Study Number (country)
Design
Main Study Objectives
Population Age (at time of first dose) Vaccine schedule Study vaccine + co-administered vaccines Number of vaccinated subjects Per group Total HBV/Hib + HRV HIV- (EPI): 10Pn-PD-DiT + DTPw-HBV/Hib + HRV HIV- (2+1): 10Pn-PD-DiT + DTPw-HBV/Hib + HRV 100 10PN-PD-DIT-036 (Korea)
Single-blind, randomized, controlled
> Primary objective:
・Non-inferiority of 10Pn-PD-DiT versus Prevenar when both co-administered with Hiberix, in terms of immune response to at least 7 of the pneumococcal serotypes contained in 10Pn-PD-DiT in Korean children.
> Key secondary objectives:
・Assessment of safety and reactogenicity of 3-dose primary vaccination with 10Pn-PD-DiT when co-administered with Hiberix in Korea
Healthy infants 6 to 12 weeks 2-4-6 months 10Pn-PD-DiT + Hiberix Prevenar + Hiberix 374 129 503 10PN-PD-DIT-037 (India)
Single-blind, randomized, controlled
> Primary objective:
・Assessment of immunogenicity of 10Pn-PD-DiT in India one month post-dose 3 > Key secondary objectives:
・Assessment of safety and reactogenicity of 10Pn-PD-DiT in India
・Assessment of immunogenicity of DTPw-HBV/Hib vaccine co-administered with 10Pn-PD-DiT Healthy infants 6 to 10 weeks 6-10-14 weeks 10Pn-PD-DiT + DTPw-HBV/Hib Hiberix + DTPw-HBV 240 120 360 2.7.4. 臨床的安全性 2.7.4 - p. 17 Feb 24 2015 17:16:04
Study Number (country)
Design
Main Study Objectives
Population Age (at time of first dose) Vaccine schedule Study vaccine + co-administered vaccines Number of vaccinated subjects Per group Total 10PN-PD-DIT-048 (Malaysia, Singapore) Double-blind randomized > Primary objective:
・Comparison of the immunogenicity of the commercial lot to the phase III clinical lot of 10Pn-PD-DiT, one month post-dose 3.
> Key secondary objectives:
・Assessment of safety and reactogenicity of 10Pn-PD-DiT (clinical and commercial lots) and of GSK Biologicals’ DTPa-combined and HRV vaccines.
・Assessment of immunogenicity of GSK Biologicals’ DTPa-combined vaccines, one month post-dose 3.
・Assessment of immunogenicity of GSK Biologicals’ HRV vaccine, three months post-dose 2. Healthy infants 6 to 12 weeks 2-3-5 months 10Pn-PD-DiT (clinical lot) + Infanrix hexa or Infanrix IPV/Hib + Rotarix (2 doses) 10Pn-PD-DiT (commercial lot) + Infanrix hexa or Infanrix IPV/Hib + Rotarix (2 doses) 233 233 466 10PN-PD-DIT-066 9 (Vietnam)
Open, randomized, controlled
> Primary objective:
・Assessment of safety and reactogenicity of 10Pn-PD-DiT co-administered with Infanrix hexa at 2-3-4 months of age, in terms of grade 3 solicited and unsolicited adverse events.
> Secondary objectives:
・Assessment of safety and reactogenicity of 10Pn-PD-DiT co-administered with Infanrix hexa at 2-3-4 months of age Healthy infants 6 to 12 weeks 2-3-4 months 10Pn-PD-DiT + Infanrix hexa Infanrix hexa 199 99 298
TOTAL number of subjects in primary vaccination study groups included in pooled safety analyses 38198
10TOTAL number of subjects in Total vaccinated cohort primed with 10Pn-PD-DiT included in pooled safety analyses of SAEs 22429
* Pivotal studies
Numbers in italics correspond to the subjects vaccinated with 10Pn-PD-DiT vaccine (Synflorix) during the primary vaccination phase and are used to calculate TOTAL number of subjects in Total vaccinated cohort primed with 10Pn-PD-DiT.
1 Study 10PN-PD-DIT-002 evaluated both primary and booster vaccination: numbers of subjects are the numbers evaluated for the primary vaccination phase.
2 Study 10PN-PD-DIT-013 evaluated primary, booster and catch-up vaccination: numbers of subjects is the number evaluated in the primary vaccination group below 6 months of age. 3 Study 10PN-PD-DIT-028 evaluated both primary and booster vaccination: numbers of subjects are the numbers evaluated for the primary vaccination phase.
2.7.4. 臨床的安全性
2.7.4 - p. 18
4 Study 10PN-PD-DIT-043 evaluated primary, booster and catch-up vaccination: numbers of subjects are the numbers evaluated for the primary vaccination phase (excluding the nested 10PN-PD-DIT-053 study which is shown separately). This study was not included in the pooled analysis of safety as a national surveillance system was used to monitor safety, but a summary of SAEs is shown in Sections 2.7.4.2.1.2.5, 2.7.4.2.1.3.1.5 and 2.7.4.2.1.3.2.5.
5 Study 10PN-PD-DIT-053 evaluated primary, booster and catch-up vaccination: numbers of subjects are the numbers evaluated for the primary vaccination phase.
6 For Study 10PN-PD-DIT-011, 1572 subjects were actually vaccinated but 24 of them in study centre 28251 were excluded from all analyses due to GCP compliance issues and consequence of an audit. 7 Study 10PN-PD-DIT-027 evaluated both primary and booster vaccination: numbers of subjects are the numbers evaluated for the primary vaccination phase.
8 Study 10PN-PD-DIT-034 evaluated both primary and booster vaccination: numbers of subjects are the numbers evaluated for the primary vaccination phase. HIV- = HIV uninfected children born from an HIV negative mother; HIV+/- = HIV uninfected children born from an HIV positive mother; HIV+/+ = HIV infected children born from an HIV positive mother. Only HIV- subjects were included in the pooled analysis of safety.
9 Study 10PN-PD-DIT-066 evaluated safety and reactogenicity post-primary vaccination only (no ELISA and no OPA data were generated), and is therefore only included in the evaluation for safety. 10 The TVC for analyses of solicited and unsolicited symptoms varies depending on study specificities.
2.7.4. 臨床的安全性
2.7.4 - p. 19
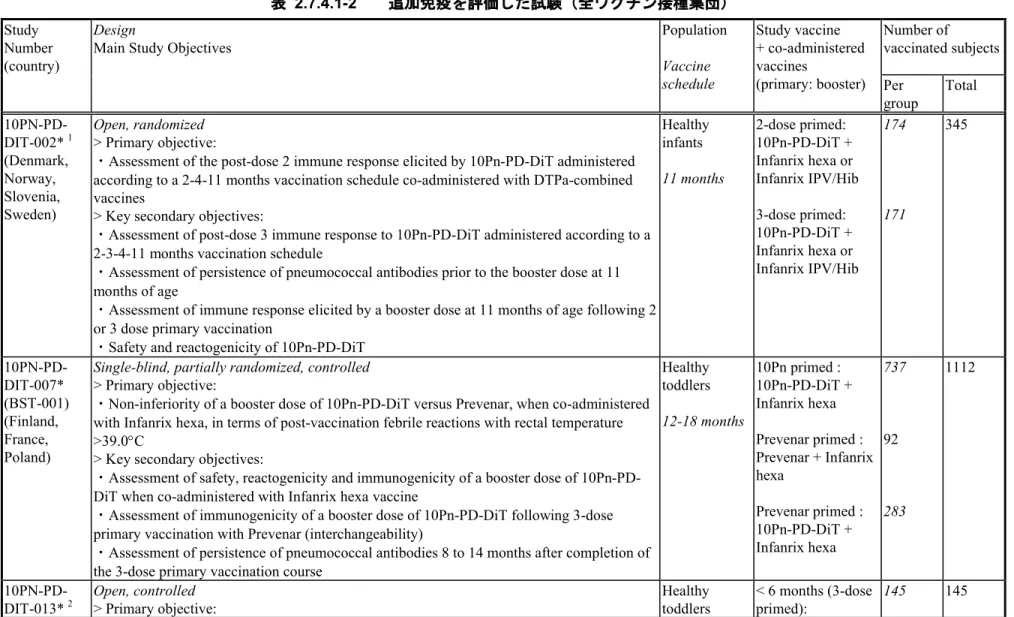
表 2.7.4.1-2 追加免疫を評価した試験(全ワクチン接種集団) Study
Number (country)
Design
Main Study Objectives
Population Vaccine schedule Study vaccine + co-administered vaccines (primary: booster) Number of vaccinated subjects Per group Total 10PN-PD-DIT-002*1 (Denmark, Norway, Slovenia, Sweden) Open, randomized > Primary objective:
・Assessment of the post-dose 2 immune response elicited by 10Pn-PD-DiT administered according to a 2-4-11 months vaccination schedule co-administered with DTPa-combined vaccines
> Key secondary objectives:
・Assessment of post-dose 3 immune response to 10Pn-PD-DiT administered according to a 2-3-4-11 months vaccination schedule
・Assessment of persistence of pneumococcal antibodies prior to the booster dose at 11 months of age
・Assessment of immune response elicited by a booster dose at 11 months of age following 2 or 3 dose primary vaccination
・Safety and reactogenicity of 10Pn-PD-DiT
Healthy infants 11 months 2-dose primed: 10Pn-PD-DiT + Infanrix hexa or Infanrix IPV/Hib 3-dose primed: 10Pn-PD-DiT + Infanrix hexa or Infanrix IPV/Hib 174 171 345 10PN-PD-DIT-007* (BST-001) (Finland, France, Poland)
Single-blind, partially randomized, controlled
> Primary objective:
・Non-inferiority of a booster dose of 10Pn-PD-DiT versus Prevenar, when co-administered with Infanrix hexa, in terms of post-vaccination febrile reactions with rectal temperature >39.0C
> Key secondary objectives:
・Assessment of safety, reactogenicity and immunogenicity of a booster dose of 10Pn-PD-DiT when co-administered with Infanrix hexa vaccine
・Assessment of immunogenicity of a booster dose of 10Pn-PD-DiT following 3-dose primary vaccination with Prevenar (interchangeability)
・Assessment of persistence of pneumococcal antibodies 8 to 14 months after completion of the 3-dose primary vaccination course
Healthy toddlers 12-18 months 10Pn primed : 10Pn-PD-DiT + Infanrix hexa Prevenar primed : Prevenar + Infanrix hexa Prevenar primed : 10Pn-PD-DiT + Infanrix hexa 737 92 283 1112 10PN-PD-DIT-013* 2 Open, controlled > Primary objective: Healthy toddlers < 6 months (3-dose primed): 145 145 2.7.4. 臨床的安全性 2.7.4 - p. 20 Feb 24 2015 17:16:05
Study Number (country)
Design
Main Study Objectives
Population Vaccine schedule Study vaccine + co-administered vaccines (primary: booster) Number of vaccinated subjects Per group Total
(Finland) ・Assessment of immunogenicity of 10Pn-PD-DiT when given as a catch-up vaccination in children older than 7 months
> Key secondary objective:
・Assessment of safety and reactogenicity of 10Pn-PD-DiT when given as a catch-up vaccination
・Assessment of immunogenicity, safety and reactogenicity of 3-dose primary course of Infanrix IPV/Hib co-administered with 10Pn-PD-DiT
12-15 months 10Pn-PD-DiT+ Infanrix IPV/Hib 10PN-PD-DIT-028*3 (Panama, Argentina, Columbia)
Double blind(observer-blind), randomized, controlled
> Primary objective:
・Efficacy of a 3-dose primary course followed by a booster dose in second year of life with 10Pn-PD-DiT against likely bacterial community acquired pneumonia (B-CAP)
> Key secondary objectives:
・Efficacy of 10Pn-PD-DiT against clinically confirmed Acute Otitis Media (C-AOM) cases in the 7,000 children enrolled in Panama.
・Efficacy of 10Pn-PD-DiT against CAP with alveolar consolidation or pleural effusion on chest X-ray
・Efficacy of 10Pn-PD-DiT in preventing bacteriologically confirmed AOM cases caused by H. influenzae and vaccine serotypes
・Efficacy of 10Pn-PD-DiT in preventing invasive disease
・Impact of 10Pn-PD-DiT on nasopharyngeal carriage of S. pneumoniae and H. influenzae ・Immunogenicity, safety and reactogenicity of 10Pn-PD-DiT
Healthy infants 15 to 18 months 10Pn-PD-DiT + Infanrix IPV/Hib HAV + Infanrix IPV/Hib 9938 9906 19844 10PN-PD-DIT-043*4 (Finland)
Double-blind, cluster-randomized, controlled
> Primary objective:
・Effectiveness of 10Pn-PD-DiT in preventing culture-confirmed IPD due to vaccine pneumococcal serotypes in children vaccinated with at least one dose of 10Pn-PD-DiT within the first 7 months of life in clusters assigned to a 3-dose primary vaccination schedule. > Key secondary objectives:
・Effectiveness of 10Pn-PD-DiT in preventing culture-confirmed IPD due to vaccine pneumococcal serotypes in children vaccinated with at least one dose of 10Pn-PD-DiT within
Healthy infants 18 months 2 or 3 dose primary + booster 10Pn-PD-DiT (3+1) HBV (3+1) 10Pn-PD-DiT (2+1) HBV (2+1) 8427 4473 9112 4399 (26411) 4 2.7.4. 臨床的安全性 2.7.4 - p. 21 Feb 24 2015 17:16:05
Study Number (country)
Design
Main Study Objectives
Population Vaccine schedule Study vaccine + co-administered vaccines (primary: booster) Number of vaccinated subjects Per group Total
the first 7 months of life in clusters assigned to a 2-dose primary vaccination schedule. ・Effectiveness of 10Pn-PD-DiT in preventing culture-confirmed invasive disease caused by any and each of the 10 vaccine pneumococcal serotypes, vaccine-related pneumococcal serotypes, other pneumococcal serotypes, H. influenzae types and other bacterial pathogens. ・Effectiveness of 10Pn-PD-DiT in reducing hospital-diagnosed pneumonia cases among children vaccinated with at least one dose of vaccine within the first 7 months of life and within or beyond the first 7 months of life.
・Impact of 10Pn-PD-DiT on occurrence of tympanostomy tube placements in children vaccinated with at least one dose of vaccine within the first 7 months of life and within or beyond the first 7 months of life.
・Impact of vaccination with 10Pn-PD-DiT on the incidence of lower respiratory tract infections (LRTI) and upper respiratory tract infections (URTI), including AOM, in the Turku area.
10PN-PD-DIT-053*5 (Finland)
Double-blind, cluster-randomized, controlled
> Primary objective: As in study 10PN-PD-DIT-043
> Key secondary objectives: As in study 10PN-PD-DIT-043 with the following ・Impact of vaccination with 10Pn-PD-DiT on the nasopharyngeal carriage of S.
pneumoniae, H. influenzae and/or other bacterial pathogens in children starting vaccination below 18 months of age.
・Impact of vaccination with 10Pn-PD-DiT on the incidence of acute otitis media with level 1 of diagnostic certainty (as defined in protocol), in children starting vaccination below 18 months of age.
・Assessment, one month post-primary and post-booster vaccination course, the
immunogenicity of DTPa vaccine when co-administered with GSK Biologicals’ 10Pn-PD-DiT.
・Assessment of the antibody persistence 12-14 months following the completion of the three-dose primary vaccination course with GSK Biologicals’ 10Pn-PD-DiT when co-administered with DTPa vaccine.
・Assessment of the antibody persistence 12-14 months following the completion of the
Healthy infants 12-18 months 6 months: 2 or 3 dose primary + booster 10Pn-PD-DiT (3+1) HBV (3+1) 10Pn-PD-DiT (2+1) HBV (2+1) 1786 1043 1275 837 4941 2.7.4. 臨床的安全性 2.7.4 - p. 22 Feb 24 2015 17:16:05
Study Number (country)
Design
Main Study Objectives
Population Vaccine schedule Study vaccine + co-administered vaccines (primary: booster) Number of vaccinated subjects Per group Total
three-dose primary vaccination course with DTPa vaccine when co-administered with GSK Biologicals’ 10Pn-PD-DiT.
・Assessment of the safety and reactogenicity of GSK Biologicals’ 10Pn-PD-DiT when co-administered with DTPa vaccine.
10PN-PD-DIT-058* (Japan)
Open, randomized, controlled
> Primary objective:
・Comparison of the immunogenicity of GSK Biologicals. 10Pn-PD-DiT in healthy Japanese children, one month post-dose 3, to the immune response of 10Pn-PD-DiT as observed in the pivotal non-inferiority study 10PN-PD-DIT-001 in Europe.
> Key secondary objectives:
・Comparison of the immunogenicity of GSK Biologicals’ 10Pn-PD-DiT in healthy Japanese children, one month post-dose 3, to the historical immunogenicity data obtained with the 11Pn-PD vaccine formulation in study Undeca-Pn-010 (POET [347414/010]).
・Comparison of the immunogenicity of GSK Biologicals’ 10Pn-PD-DiT in healthy Japanese children, one month post-dose 3, to the immune response of 10Pn-PD-DiT as observed in study 10PN-PD-DIT-028 (COMPAS [109563]).
・Assessment, one month post-primary and post-booster vaccination course, the immunogenicity of GSK Biologicals’ 10Pn-PD-DiT when co-administered with DTPa vaccine.
・Assessment, one month post-primary and post-booster vaccination course, the
immunogenicity of DTPa vaccine when co-administered with GSK Biologicals’ 10Pn-PD-DiT.
・Assessment of the antibody persistence 12-14 months following the completion of the three-dose primary vaccination course with GSK Biologicals’ 10Pn-PD-DiT when co-administered with DTPa vaccine.
・Assessment of the antibody persistence 12-14 months following the completion of the three-dose primary vaccination course with DTPa vaccine when co-administered with GSK Biologicals’ 10Pn-PD-DiT.
・Assessment of the safety and reactogenicity of GSK Biologicals’ 10Pn-PD-DiT when
co-Healthy children 17 to 19 months 10Pn-PD-DiT + DTPa DTPa 228 120 348 2.7.4. 臨床的安全性 2.7.4 - p. 23 Feb 24 2015 17:16:06
Study Number (country)
Design
Main Study Objectives
Population Vaccine schedule Study vaccine + co-administered vaccines (primary: booster) Number of vaccinated subjects Per group Total
administered with DTPa vaccine.
10PN-PD-DIT-062*6 BST:037 (India)
Partially randomized, open
> Primary objective:
・Immune responses following vaccination with a booster dose of 10Pn-PD-DiT
administered at either 9-12 or 15-18 months of age in children previously vaccinated with 10Pn-PD-DiT in study 10PN-PD-DIT-037
> Secondary objective:
・Antibody persistence following primary vaccination with the 10Pn-PD-DiT.
・Immunogenicity of 10Pn-PD-DiT following a 2-dose primary vaccination and following booster vaccination administered as a catch-up immunization according to a 2+1 vaccination schedule in unprimed children during the second year of life.
・Safety and reactogenicity of 10Pn-PD-DiT when administered as a catch-up immunization according to a 2+1 vaccination schedule in unprimed children during the second year of life. ・Safety and reactogenicity of a booster dose of 10Pn-PD-DiT when administered at either 9-12 or 15-18 months of age in children primed in study 10PN-PD-DIT-037 Antibody
persistence following booster vaccination with 10Pn-PD-DiT up to approximately 24 months of age in the primed groups.
Healthy children 9 to 18 months or 15 to 18 months (booster) 10Pn-PD-DiT (Pn-Pn9 group: booster) (Pn-Pn15 group: booster) 100 95 195 10PN-PD-DIT-004 7 (Germany) Open > Primary objective:
・Assessment of immunological memory following priming with either 3μg 18C-TT 11Pn-PD-DiT, 11Pn-PD or Prevenar through administration of a single dose of unconjugated 23-valent pneumococcal polysaccharide vaccine
・Assessment of immune response after a booster dose of 10Pn-PD-DiT following primary vaccination with one of 8 different 11Pn-PD-DiT vaccine formulations
> Key secondary objectives:
・Assessment of safety and reactogenicity of 10Pn-PD-DiT co-administered with Infanrix hexa as a booster dose
・Assessment of persistence of antibodies induced by the different pneumococcal conjugate
Healthy toddlers 11-18 months 11Pn-PD, 11Pn-PD-DiT or Prevenar primed: Pneumovax + Infanrix hexa 11Pn-PD-DiT primed: 10Pn-PD-DiT + Infanrix hexa
150 397 547 2.7.4. 臨床的安全性 2.7.4 - p. 24 Feb 24 2015 17:16:06
Study Number (country)
Design
Main Study Objectives
Population Vaccine schedule Study vaccine + co-administered vaccines (primary: booster) Number of vaccinated subjects Per group Total
vaccines 7-14 months after priming 10PN-PD-DIT-009 8 (BST: 005) (Chile) Observer-blind, controlled > Primary objective:
・Assessment of reactogenicity of study vaccines in terms of occurrence of grade 3 adverse events
> Key secondary objectives:
・Assessment of safety, reactogenicity and immunogenicity of 10Pn-PD-DiT as booster dose or as 2-dose catch-up immunisation
・Assessment of persistence of pneumococcal antibodies induced by 10Pn-PD-DiT 12 to 15 months after completion of the 3-dose primary vaccination course
Healthy toddlers 20-23 months 10Pn primed: visit 1: Havrix + Infanrix hexa – visit 2: 10Pn-PD-DiT 83†† 83 10PN-PD-DIT-014 9 (BST: 010) (Czech Rep) Open, controlled > Primary objective:
・Determination of the percentage reduction in febrile reactions (rectal temperature 38.0C) when prophylactic antipyretic treatment is administered compared to no prophylactic
antipyretic treatment after booster vaccination with 10Pn-PD-DiT co-administered with Infanrix hexa
> Key secondary objectives:
・Assessment of safety and reactogenicity of a booster dose of 10Pn-PD-DiT and Infanrix hexa when co-administered with or without antipyretic treatment
・Assessment of immunogenicity of a booster dose of 10Pn-PD-DiT one month post booster dose
・Assessment of impact of a booster dose of 10Pn-PD-DiT in reducing nasopharyngeal carriage of S. pneumoniae and H. influenzae
・Assessment of persistence of antibodies induced by 10Pn-PD-DiT 7-10 months post-primary vaccination and 12-15 months post-booster vaccination
Healthy toddlers
12-15 months
Primed antipyretic: 10Pn-PD-DiT + Infanrix hexa with or without prophylactic antipyretic medication Primed non-antipyretic: 10Pn-PD-DiT + Infanrix hexa without prophylactic antipyretic medication Unprimed: MenACWY-TT + Infanrix hexa 205 209 336 750 2.7.4. 臨床的安全性 2.7.4 - p. 25 Feb 24 2015 17:16:06
Study Number (country)
Design
Main Study Objectives
Population Vaccine schedule Study vaccine + co-administered vaccines (primary: booster) Number of vaccinated subjects Per group Total 10PN-PD-DIT-016 (BST: 015) (Greece, Spain) Open, controlled > Primary objective:
・Assessment of safety and reactogenicity of a booster dose of 10Pn-PD-DiT co-administered with Infanrix-IPV/Hib in preterm infants
> Key secondary objective:
・Assessment of immunogenicity of a booster dose of 10Pn-PD-DiT when co-administered with Infanrix-IPV/Hib in preterm infants
・Assessment of antibody persistence 10 to 12 months after the completion of the 3-dose primary vaccination course with 10Pn-PD-DiT co-administered with Infanrix-IPV/Hib in preterm infants Preterm or full term toddlers 16-18 months 10Pn-PD-DiT + Infanrix-IPV/Hib 116 (preterm) 129 (full term) 245 10PN-PD-DIT-017 (BST: 011) (Germany, Poland, Spain) Open, controlled > Primary objective:
・Non-inferiority of a booster dose of 10Pn-PD-DiT versus Prevenar in terms of incidence of post-booster febrile reactions with temperature > 39C when co-administered with DTPa-combined and Hib-MenC vaccines
> Key secondary objectives:
・Assessment of safety and reactogenicity of a booster dose of 10Pn-PD-DiT when co-administered with DTPa-combined, Hib-MenC and MenC booster vaccines
・Assessment of immunogenicity of a booster dose of 10Pn-PD-DiT
・Assessment of antibody persistence of a booster dose of 10Pn-PD-DiT or Prevenar and Hib-MenC or MenC Healthy toddlers 11-18 months 10Pn-PD-DiT + Infanrix hexa + Meningitec 10Pn-PD-DiT + Infanrix hexa + NeisVac-C 10Pn-PD-DiT + Infanrix penta + Hib-MenC Prevenar + Infanrix penta + Hib-MenC 359 363 358 357 1437 10PN-PD-DIT-018 (BST: 012) (Philippines, Poland) Observer-blind, controlled > Primary objective:
・Non-inferiority of a booster dose of 10Pn-PD-DiT versus Prevenar in terms of incidence of post-booster immunisation fever > 39C when co-administered with DTPw-HBV/Hib and OPV or IPV vaccines.
> Key secondary objective:
Healthy infants 12-18 months 10Pn primed: 10Pn-PD-DiT + DTPw-HBV/Hib + OPV or IPV Prevenar primed: 565 191 756 2.7.4. 臨床的安全性 2.7.4 - p. 26 Feb 24 2015 17:16:06
Study Number (country)
Design
Main Study Objectives
Population Vaccine schedule Study vaccine + co-administered vaccines (primary: booster) Number of vaccinated subjects Per group Total
・Assessment of safety, reactogenicity and immunogenicity of a booster dose of 10Pn-PD-DiT when co-administered with DTPw-HBV/Hib and OPV or IPV vaccines
・Assessment of persistence of antibodies 7-12 months after completion of the 3-dose primary course Prevenar + DTPw-HBV/Hib+ OPV or IPV 10PN-PD-DIT-022 10 (BST-001) (Finland)
Open, randomized, controlled
> Primary objective:
・Incidence of post-vaccination rectal temperature >39.0°C following a booster dose of the 10Pn-PD-DiT when co-administered with a first dose of MMRV vaccine
> Key secondary objectives:
・Assessment of safety, reactogenicity and immunogenicity of a booster dose 10Pn-PD-DiT co-administered with MMRV vaccine
・Assessment of persistence of antibodies 8 - 10 months after completion of the 3-dose primary course
・Assessment of safety, reactogenicity and immunogenicity of MMRV when co-administered with 10Pn-PD-DiT
Healthy toddlers 12-14 months, 14-16 months 10Pn primed : visit 1: 10Pn-PD-DiT + MMRV ; visit 2 : MMRV + Infanrix hexa 10Pn primed : visit 1: MMRV + Infanrix hexa; visit 2: 10Pn-PD-DiT + MMRV 10Pn primed : 10Pn-PD-DiT + Infanrix hexa 110 100 † 114 324 10PN-PD-DIT-027 BST11 (Netherlands)
Single-blind, randomized, controlled
> Primary objective:
・Non-inferiority of 10Pn-PD-DiT co-administered with DTPa-IPV/Hib (Pediacel) versus 10Pn-PD-DiT co-administrated with DTPa-HBV-IPV/Hib (Infanrix hexa), in terms of immune response to the 10 pneumococcal vaccine serotypes and to protein D
> Key secondary objective:
・Non-inferiority of Pediacel when administered with 10Pn-PD-DiT versus the co-administration with Prevenar, in terms of immune response to DTPa-IPV/Hib antigens ・Assessment of the impact of vaccination with 10Pn-PD-DiT on the nasopharyngeal carriage of H. influenzae. Healthy infants 11 to 12 months 10Pn-PD-DiT+ Infanrix hexa 10Pn-PD-DiT+ Pediacel Prevenar + Pediacel 257 259 258 774 2.7.4. 臨床的安全性 2.7.4 - p. 27 Feb 24 2015 17:16:07
Study Number (country)
Design
Main Study Objectives
Population Vaccine schedule Study vaccine + co-administered vaccines (primary: booster) Number of vaccinated subjects Per group Total
・Assessment of safety and reactogenicity of 10Pn-PD-DiT when co-administered with Infanrix hexa or Pediacel
・Assessment of immunogenicity of a booster dose of 10Pn-PD-DiT co-administered with a booster dose of Infanrix hexa or Pediacel at 11-12 months of age.
・Assessment of persistence of antibodies 12 months after completion of the four-dose vaccination course with 10Pn-PD-DiT co-administered with Infanrix hexa or Pediacel
10PN-PD-DIT-034 12 (South Africa)
Open, partially randomized, controlled
> Primary objective:
・Immune response to 10Pn-PD-DiT one month following a 3-dose (6, 10 and 14 weeks of age) primary vaccination course in HIV infected infants, HIV exposed uninfected infants and HIV unexposed uninfected infants.
> Key secondary objectives:
・Safety and reactogenicity of 10Pn-PD-DiT, immunogenicity after two doses, persistence of antibodies after 6 months following primary vaccination, immunogenicity of a booster dose, persistence of antibodies 14 months after persistence, immunogenicity of three doses and booster dose of DTPw-HBV/Hib
HIV-, HIV+/-and HIV+/+ infants 9 to 10 months HIV+/+ (3+1): 10Pn-PD-DiT HIV+/- (3+1): 10Pn-PD-DiT HIV- (3+1): 10Pn-PD-DiT HIV- (2+1): 10Pn-PD-DiT 80 92 98 98 196 (172) 12 10PN-PD-DIT-049 (BST: 048) (Singapore) Open, controlled > Primary objective:
・Assessment of antibody persistence induced by 10Pn-PD-DiT (commercial lot versus phase III clinical lot), when co-administered with DTPa-IPV/Hib 13-16 months after completion of the 3-dose primary vaccination course in study 10PN-PD-DIT-048 (111654). > Key secondary objectives:
・Assessment of safety, reactogenicity and immunogenicity of a booster dose of 10Pn-PD-DiT (commercial lot), when co-administered with DTPa-IPV/Hib at 18-21 months of age in children primed at 2, 3 and 5 months of age in study 10PN-PD-DIT-048 (111654).
Healthy toddlers 18 to 21 months 10Pn-PD-DiT + DTPa-IPV/Hib (primed with clinical lot) 10Pn-PD-DiT + DTPa-IPV/Hib (primed with commercial lot) 118 120 238 10PN-PD-DIT-063 (BST: 036) Single-blind, controlled > Primary objective:
・Assessment of the reactogenicity of a booster dose of 10Pn-PD-DiT in terms of the
Healthy toddlers 10Pn-PD-DiT + Hiberix 335 448 ††† 2.7.4. 臨床的安全性 2.7.4 - p. 28 Feb 24 2015 17:16:07
Study Number (country)
Design
Main Study Objectives
Population Vaccine schedule Study vaccine + co-administered vaccines (primary: booster) Number of vaccinated subjects Per group Total
(Korea) occurrence of adverse events with intensity grade 3, when co-administered with Hiberix at 12-18 months of age in children primed with the same vaccines at 2, 4 and 6 months of age in study 10PN-PD-DIT-036 (110808).
> Key secondary objectives:
・Assessment of the safety and reactogenicity of a booster dose of 10Pn-PD-DiT when co-administered with Hiberix at 12-18 months of age in children primed with the same vaccines at 2, 4 and 6 months of age in study 10PN-PD-DIT-036 (110808).
・Assessment of the immunogenicity of a booster dose of 10Pn-PD-DiT when
co-administered with Hiberix at 12-18 months of age in children primed with the same vaccines at 2, 4 and 6 months of age in study 10PN-PD-DIT-036 (110808).
12 to 18 months Prevenar + Hiberix 113 10PN-PD-DIT-069 13 (BST: 032) (Mali) Open > Primary objective:
・Assessment of safety and reactogenicity of 10Pn-PD-DiT in terms of occurrence of adverse events with grade 3 intensity after booster vaccination.
> Key secondary objectives:
・Assessment of safety and reactogenicity of 10Pn-PD-DiT after administration of any vaccine dose.
・Assessment, one month post-booster dose, of the immunogenicity of 10Pn-PD-DiT ・Assessment of immunogenicity of a 2-dose catch-up vaccination with 10Pn-PD-DiT in the second year of life.
・Assessment of antibody persistence 11 to 18 months after completion of the primary vaccination course with 10Pn-PD-DiT in study 10PN-PD-DIT-032.
Healthy children 15 to 21 months 10Pn primed: 10Pn-PD-DiT 141 141
TOTAL number of subjects in booster vaccination study groups included in pooled safety analyses 32719
14TOTAL number of subjects in booster Total vaccinated cohort receiving 10Pn-PD-DiT included in pooled safety analyses of SAEs 19466
* Pivotal studies
Numbers in italics correspond to the subjects vaccinated with 10Pn-PD-DiT vaccine (Synflorix) during the booster vaccination phase and are used to calculate TOTAL number of subjects in booster Total vaccinated cohort receiving Synflorix.
1 Study 10PN-PD-DIT-002 evaluated both primary and booster vaccination: numbers of subjects corresponds to subjects who received booster vaccination.
2 Study 10PN-PD-DIT-013 evaluated primary, booster and catch-up vaccination: numbers of subjects corresponds to subjects who received booster vaccination following primary vaccination in the first 6 months of life.
2.7.4. 臨床的安全性
2.7.4 - p. 29
3 Study 10PN-PD-DIT-028 evaluated both primary and booster vaccination: numbers of subjects corresponds to subjects who received booster vaccination.
4 Study 10PN-PD-DIT-043 evaluated primary, booster and catch-up vaccination: numbers of subjects are the numbers evaluated for the primary vaccination phase (excluding the nested 10PN-PD-DIT-053 study which is shown separately) as booster specific numbers were not recorded in this study. This study was not included in the pooled analysis of safety as a national surveillance system was used to monitor safety, but a summary of SAEs is shown in Sections 2.7.4.2.1.2.5, 2.7.4.2.1.3.1.5 and 2.7.4.2.1.3.2.5.
5 Study 10PN-PD-DIT-053 evaluated primary, booster and catch-up vaccination: numbers of subjects corresponds to subjects who received booster vaccination. 6 Study 10PN-PD-DIT-062 evaluated both booster and catch-up vaccination: numbers of subjects corresponds to subjects who received booster vaccination.
7 Study 10PN-PD-DIT-004 evaluated a Synflorix booster dose after primary course of 8 different 11-valent vaccine formulations and is therefore only included in the evaluation of safety. Subjects receiving pneumovax are not included in the TVC.
8 Study 10PN-PD-DIT-009 evaluated booster and catch-up vaccination: numbers of subjects corresponds to subjects who received booster vaccination. †† In addition to these 83 subjects, 1 subject was enrolled but only received vaccines at visit 1 (no Synflorix) as he withdrew after visit 1.
9 Study 10PN-PD-DIT-014 evaluated booster vaccination. Numbers of subjects corresponds to subjects who received booster vaccination covering PIV(M1) and PIV(M12) timepoints. The age-matched unprimed group did not receive Synflorix; therefore only safety data from this group is presented.
10 Study 10PN-PD-DIT-022 included two groups that received two doses of vaccines and one group that received one dose of vaccines: numbers of subjects corresponds to subjects who received doses that included Synflorix.
† In addition to these 100 subjects, 1 subject was enrolled but only received vaccines at visit 1 (no Synflorix) as he withdrawn after the first visit.
11 Study 10PN-PD-DIT-027 evaluated both primary and booster vaccination: numbers of subjects corresponds to subjects who received booster vaccination covering PIV(M10) and PIV(M21) timepoints. 12 Study 10PN-PD-DIT-034 evaluated both primary and booster vaccination: numbers of subjects corresponds to subjects who received booster vaccination with Synflorix. HIV- = HIV uninfected children born from an HIV negative mother; HIV+/- = HIV uninfected children born from an HIV positive mother; HIV+/+ = HIV infected children born from an HIV positive mother. Only HIV- subjects were included in the pooled analysis of safety.
††† In addition to these 448 subjects, 2 subjects were enrolled in the 10Pn group but were not vaccinated.
13 Study 10PN-PD-DIT-069 evaluated booster and catch-up vaccination: numbers of subjects corresponds to subjects who received booster vaccination. 14 The TVC for analyses of solicited and unsolicited symptoms varies depending on study specificities.
2.7.4. 臨床的安全性
2.7.4 - p. 30