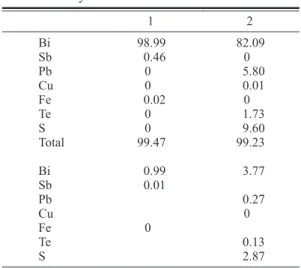
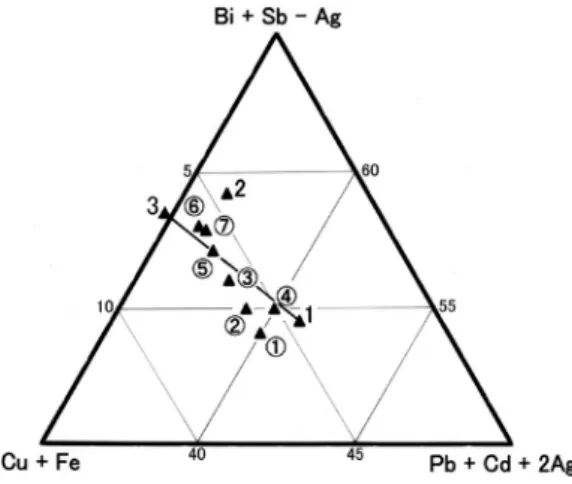
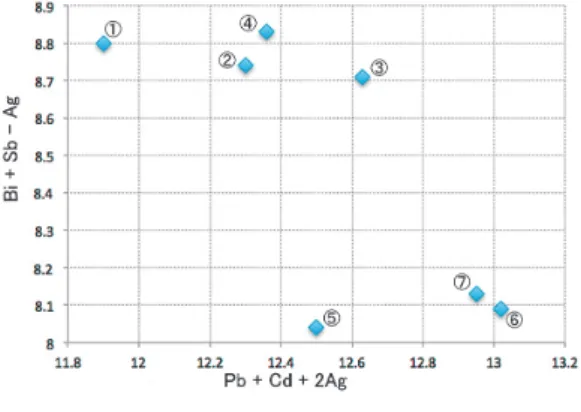
Eclarite and other Bi-minerals from the Jishakuyama ore deposit of the Akagane mine, Iwate Prefecture, Japan
Seiji Harada
1, Yasumitsu Suzuki
2, Ritsuro Miyawaki
3, Koichi Momma
3, Masako Shigeoka
3and Satoshi Matsubara
31
8–12 Nanatsuike-cho, Koriyama, Fukushima 963–8831, Japan
2
4–3–15 Miyata-cho, Hitachi, Ibaraki 317–0055, Japan
3