Introduction
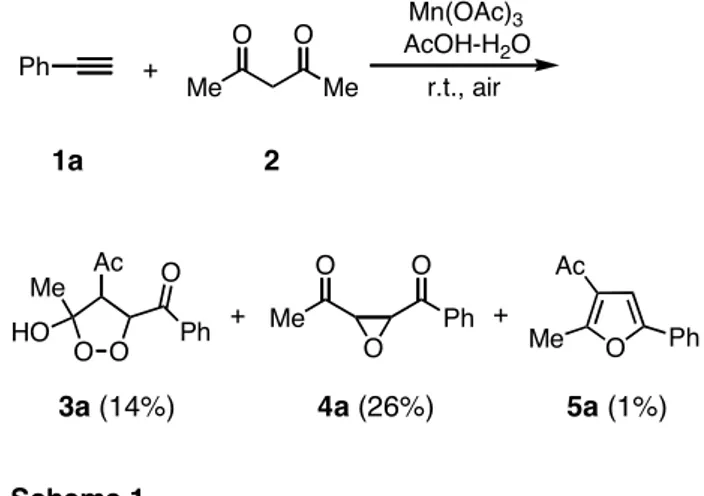
A number of cyclic peroxides have been isolated from natural sources and some of them exhibit significant bi- ological and cytotoxic properties.1 Recently, we devel- oped the manganese(III)-catalyzed aerobic peroxidation using 1,3-dicarbonyl compounds with alkenes to produce 1,2-dioxanes.2 In connection with our study, we have preliminary reported the reaction of phenylacetylene (1a) with 2,4-pentanedione (2) in the presence of manga- nese(III) acetate at room temperature in air.3 In the reac- tion, contrary to our expectation, 1,2-dioxolane 3a, ox- irane 4a, and furan 5a were barely isolated instead of 1,2- dioxane (Scheme 1). However, the product distribution and the mechanism for the formation of these products were not clear. Therefore, in order to optimize the reac- tion and elucidate the mechanism for the formation of these products, the reaction was scrutinized under various reaction conditions.
Results and Discussion
Manganese(III)-based oxidation of phenylacetylene with 2,4-pentanedione. According to the previous re- port,3 a mixture of phenylacetylene (1a) (1 mmol), 2,4- pentanedione (2) (5 mmol), and manganese(III) acetate (2.5 mmol) was stirred in glacial acetic acid at room tem- perature in air for 24 h until 1a was completely consumed.
After work-up, solidified 1,2-dioxolane 3a was filtered off in a 19% yield and oxirane 4a was isolated in a 5%
*Corresponding author. Tel.: +81-96-342-3374; fax: +81-96- 342-3374; e-mail: nishino@sci.kumamoto-u.ac.jp
yield from the filtrate by thin-layer chromatography (sili- ca gel) (Table 1, entry 1). Furan 5a was not detected at all under all the reaction conditions. The structural assign- ment of 3a and 4a was based on their 1H NMR, 13C NMR, and IR spectra, as well as their elemental analyses, and these data were agreed with those previously reported.3 Prolongation of the reaction times led to decrease the product yield (entry 2). To promote the reaction, dry air was bubbled through the reaction mixture, but the yield did not increase (entries 3 and 4). When the reaction was carried out at the molar ratio of 1:10:10 (1a:2:manganese(III) acetate), dioxolane 3a was obtained in a 45% yield along with 4a in a 5% yield (entry 9). In addition, 3-acetyl-4-hydroxy-3-hexene-2,5-dione (6) was also obtained in all the reactions.4 Hexenedione 6 could be formed by the radical dimerization of two diace- tylmethyl radicals followed by further oxidation.4b In general, only a catalytic amount of manganese(III) acetate is enough in the aerobic peroxidation using 1,3-dicarbonyl compounds with alkenes,2 whereas the present reaction needed a large amount of 2 and manganese(III) acetate to increase the yield of 3a. Probably, it would be attributed to the relatively low reactivity of the carbon-carbon triple bond toward the manganese(III)-enolate complex (see mechanism).5
Table 1. Reaction of phenylacetylene (1a) with 2,4- pentanedione (2) in the presence of manganese(III) acetatea
Time Product
(Yield, %) Entry 1a:2:Mn(OAc)3
h 3ab 4ab 6c
1 1:5:2.5 24 19 5 2
2 1:5:2.5 36 16 4 2
3d 1:5:2.5 12 4 4 3
4 d 1:5:2.5 24 3 3 2
5 e 1:5:2.5 24 6 1 trace
6 1:5:5 24 20 7 3
7 1:7.5:7.5 24 25 7 3
8 1:10:7.5 24 41 4 2
9 1:10:10 24 45 5 2
10 1:10:12.5 24 39 9 2
11 1:12.5:10 24 37 6 2
12 1:12.5:12.5 24 37 4 1
a The reaction was carried out at 23 °C in glacial acetic acid (30 mL) in air.
b The yield was based on the amount of 1a used.
c The yield was based on the amount of 2 used.
d The reaction was carried out at 23 °C in glacial acetic acid (30 mL) under a dry air stream.
e Water (1 mL) was added.
Formation of 1,2-Dioxolanes Using Mn(III)-Based Reaction of Various Acetylenes with 2,4-Pentanedione and Related Reaction
Takuma Tsubusaki and Hiroshi Nishino*
Department of Chemistry, Graduate School of Science and Technology, Kumamoto University, Kurokami 2-39-1, Kumamoto 860-8555, Japan
Ph Me Me
O O
O O
Ac
O
r.t., air +
Ph Me
HO
O
Ph O Me
O
O Ph Me
Ac + Mn(OAc)3 AcOH-H2O
1a 2
3a (14%) 4a (26%) 5a (1%)
Scheme 1 +
Reaction of supposed intermediate. In previous study,3 it was reported that the yield of the products increased by adding a small amount of water to the reaction mixture.
That is, the oxygen atom of the benzoyl group of 3a and 4a must be derived from the added water or hydrate water of manganese(III) acetate, since the reaction would pro- ceed via the formation of vinyl cation intermediate VC (Scheme 2). However, the reproducibility of the reaction was not observed (Table 1, entry 5).
Hence, the manganese(III)-catalyzed oxidation of the plausible intermediate 7 derived from the vinyl cation VC was examined in order to reveal whether the vinyl cation could be formed under the reaction conditions. 3-Acetyl- 1-phenylpentane-1,4-dione (7), of which the enol form seemed to be the intermediate derived from the vinyl cation, was easily prepared in a 80% yield by the reaction of 2 with 2-bromoacetophenone in ethanol in the presence of sodium ethoxide. Although the reaction of 7 was car- ried out under similar aerobic conditions, the desired 3a and 4a were not formed, but compounds 8, 9, and 10 were isolated in low yields (Scheme 3). This indicates that 1,4- pentanedione 7 was not the real intermediate during the aerobic oxidation. Therefore, the possibility for the for- mation of vinyl cation VC could be ruled out, and the oxygen atom of the benzoyl group would not be derived from water. Accordingly, we needed to propose an alter-
native mechanism for the formation of 3a (vide infra).
Mechanism for the formation of dioxolane. The forma- tion of 1,2-dioxolane 3a can be accounted for according to the mechanism outlined in Scheme 4. In the case of the manganese(III) acetate system, the ligand-exchange reac- tion of acetate ligands on manganese(III) acetate with 2,4- pentanedione must occur at the first stage to form manga- nese(III)-enolate complex A. Phenylacetylene (1a) attacks the manganese(III)-enolate complex A to give σ-like vinyl radicals B or C. In general, trans addition (anti addition) should take place in the carbon-carbon triple bond as the same manner as the double bond. Therefore, the introduced diacetylmethyl group should be trans to- ward the σ-radical lobe, that is, the diacetylmethyl group must be located cis to the phenyl group such as B. Be- cause the efficient stereoelectronic effect between the σ- radical lobe and the covalent bond of the diacetylmethyl group should easily undergo and the starting 1a must be reversibly reproduced since antiperiplanar relationship between the σ-radical lobe and the leaving diacetylmethyl group. This indicates that the formation of vinyl radical B follows the kinetic control and the reaction should be fast.
On the other hand, the formation of the vinyl radical C having synperiplanar relationship between the σ-radical lobe and the diacetylmethyl group would be very slow, though it must be thermodynamically stable since the substituents are trans stereochemistry. If the vinyl radical C would be formed during the first stage, the vinyl radical C would react with the dissolved oxygen to give endoper- oxide F via peroxy radical D. However, the possibility of the formation of the vinyl radical C would be ruled out because of the slow reaction of the first step and any pro- duct of the corresponding endoperoxide derived from the vinyl radical C was not isolated. In contrast, the vinyl radical B should take up the dissolved oxygen to provide peroxy radical G. The peroxy radical G could be reduced by manganese(III) species followed by protonation to give hydroperoxide I. Because of geometrical restriction, the hydroperoxide I could not cyclize and would be ob- liged to undergo redox decomposition to afford oxyradi- cal J (Scheme 5).6 It should be delocalized and finally attacked the dissolved oxygen at the α-benzoyl position to produce the corresponding dioxolane 3a.
Manganese(III)-oxidation of other acetylenes. Next,
Me Me
O O
Ph Mn(III)
Me Me
O O
Ph HO
Mn(III) H2O
Me Me
O O
Ph O
7
3a ? 1a + 2
Scheme 2
VC
AcOH, r.t., air Mn(OAc)3
Scheme 3
7 3a + 4a
HO Me
O
O Ph
Ph O
Me O
Me O
O Ac Ac
Ph Ph
O O
8 9 10
Mn(OAc)3 AcOH, r.t., air
ROOH + Mn(II) + Mn(III) + HO
(Reduction of ROOH)
ROOH + Mn(III) + Mn(II) + H
(Oxidation of ROOH)
ROO
2 ROO 2 RO + O2
(Irreversible ejection of O2)
Scheme 5
RO
we explored the reaction of variety of acetylenes 1 with 2,4-pentanedione (2) under the same conditions (Scheme 6). The use of 1-arylacetylenes 1 resulted in the corre- sponding 1,2-dioxolanes 3 in comparable yields together with oxiranes 4 (Table 2, entries 1-6), while diphenylace- tylene and 1-hexyne did not give any products (entries 7 and 8). In addition, the reaction of 2-phenylvinylacetylene gave an intractable mixture (entry 9).
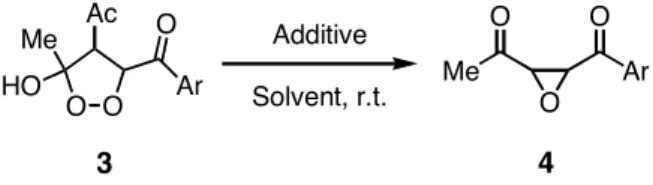
Conversion of dioxolanes into oxiranes. Since the decomposition of 1,2-dioxolanes 3 might afford the corre- sponding oxiranes 4, 1,2-dioxolane 3a was stirred in gla- cial acetic acid at room temperature (Scheme 7). As a result, 4a was obtained in a 18% yield along with a 74%
recovery of 3a (Table 3, entry 1). The reaction of 3a with manganese(II) acetate in glacial acetic acid at room tem-
perature gave 4a in a 59% yield together with a 32%
recovery of 3a (entry 2). This is suggested the fact that 4a should be formed by the decomposition of 3a during the aerobic oxidation. Interestingly, when the crude product of 3a was purified by thin-layer chromatography (silica gel), no 3a was obtained at all, but only 4a was isolated.
This clearly showed that 3a must be converted into 4a on silica gel, which was confirmed by the treatment of 3a with silica gel (Table 3, entries 3-6). Incidentally, it was found that methanol was the most appropriate solvent in the silica gel-mediated decomposition reaction (entry 6).
A similar treatment of other 1,2-dioxolanes 3 with silica gel in methanol quantitatively gave the corresponding oxiranes 4 (Table 3, entries 7-12). Nevertheless, 1,2- dioxolanes 3 were thermally stable at ambient tempera-
Me Me
O O MnIII
O MnIII MnIII
H Ph
O O MnIII
Me Me MnIIIOMnIII O
Me O
Me H
H H
H Ac
Me O
Ph O O
H Me O O Me
Ph O O
O2
MnII
MnIII
A
B C
D E F
- MnII - MnII
AcOH
1a
Ph H
Me O O Me
O O Ph
H Me O O Me
O
Ph Me
O O
Me
O H
Ph H Me
O O
Me
O H
Ph H Me
O O
Me
O H
Ph Me
O O
Me
O H
O O
Ph Me
O O Me
O H
O O O O
Ac O Ph HO
Me
MnII
MnIII
3a
O2
G I
K L
Ph H
Me O O Me
O OH J
Scheme 4
Ph H
Me O O Me
O O H
M MnII MnIII
O2 AcOH
AcOH O O Ac
Ph OH Me
ture even under prolonged storage in air. Several reports describing the decomposition of 1,2-dioxolan-3-ols to oxirane under alkaline conditions were found in the litera-
tures.7 To the best of our knowledge, however, the con- version of 1,2-dioxolan-3-ols into oxiranes mediated by silica gel has never been reported. A plausible mechanism for the formation of 4 is depicted in Scheme 8.
In summary, various 1,2-dioxolanes 3 were obtained in moderate yields, and an alternative mechanism for the formation of 3 was proposed. Furthermore, the silica gel- mediated decomposition of 1,2-dioxolanes 3 quantita- tively gave the corresponding oxiranes 4.
References
1. (a) Casteel, D. A. Nat. Prod. Rep. 1992, 9, 289-312.
(b) Casteel, D. A. Nat. Prod. Rep. 1999, 16, 55-73.
2. Nishino, H. In Bioactive Heterocycles I; Eguchi, S.
Ed.; Springer: Berlin, 2006; pp 39-76.
3. Qian, C.-Y.; Noiri, Y.; Nishino, H.; Kurosawa, K.
Chinese Chem. Lett. 1997, 8, 189-190.
4. (a) Nishino, H. Bull. Chem. Soc. Jpn. 1986, 59, 1733- 1739. (b) Nishino, H.; Tategami, S.; Yamada, T.;
Korp, J. D.; Kurosawa, K. Bull. Chem. Soc. Jpn. 1991, 64, 1800-1809.
5. Snider, B. B.; Merritt, J. E.; Dombroski, M. A.;
Buckman, B. O. J. Org. Chem. 1991, 56, 5544-5553.
6. (a) Sheldon, R. A. and Koichi, J. K. Metal Catalyzed Oxidations in Organic Chemistry; Academic Press:
New York, 1981. (b) Hirao, K.; Sakaguchi, S.; Ishii, Y. Tetrahedron Lett. 2002, 43, 3617-3620. (c) Rah- man, M. T.; Nishino, H. Org. Lett. 2003, 5, 2887- 2890. (b) Rahman, M. T.; Nishino, H. Cryo. Rep.
Kumamoto Univ. 2005, 16, 56-61.
Table 3. Conversion of dioxolanes 3 into oxiranes 4a
Time Recovery Yieldb
Entry Ar Additive Solvent
h 3/% 4/%
1 Ph none AcOH 24 74 18
2c Ph Mn(OAc)2 AcOH 24 32 59
3 Ph silica gel MeCN 24 55 45
4 Ph silica gel Me2CO 24 67 33
5 Ph silica gel CHCl3 24 34 66
6 Ph silica gel MeOH 12 0 quant
7 4-MeC6H4 silica gel MeOH 12 0 quant
8 4-MeOC6H4 silica gel MeOH 12 0 quant
9 4-ClC6H4 silica gel MeOH 12 0 quant
10 4-FC6H4 silica gel MeOH 12 0 quant
11 1-Naphthyl silica gel MeOH 12 0 quant
12 3,4-(MeO)2C6H3 silica gel MeOH 12 0 quant
a The reaction of 3 (0.1 mmol) was carried out in the solvent (10 mL) at room temperature in the presence of silica gel (0.5 g).
b The yield was based on the 1H NMR integration.
c The reaction was conducted at the molar ratio of 3 (0.2 mmol):Mn(OAc)2 = 1:5.
Table 2. Reaction of acetylenes 1 with 2,4-
pentanedione (2) in the presence of manganese(III) acetatea
1 Product (Yield, %)b Entry
R1 R2 3 4
1 4-MeC6H4 H 52 7
2 4-MeOC6H4 H 64 2
3d 4-ClC6H4 H 38 2
4d 4-FC6H4 H 41 6
5e 1-Naphthyl H 18 4
6 3,4-(MeO)2C6H3 H 32 8
7 Ph Ph no reaction
8 n-C4H9 H no reaction
9 PhCH=CH H intractable mixture
a The reaction was carried out at 23 °C for 24 h in glacial acetic acid (30 mL) in air at the molar ratio of 1:2:Mn(OAc)3 = 1:10:10.
b The yield was based on the amount of 1 used.
R1
Me Me
O O
O O
Ac
AcOH r.t., air
+ R1 Me
HO O
Mn(OAc)3
Scheme 6 R2 +
R2
1 2
3 4
O Me
O R2
O R1
O O
Ac
Ar O Me
HO
O
Ar O Me
O
3 4
Scheme 7
Additive Solvent, r.t.
7. (a) Kulinkovich, O. G.; Astashko, D. A.; Tyvorskii, V. I.; Ilyina, N. A. Synthesis 2001, 1453-1455. (b) Barnier, J.-P.; Morisson, V.; Blanco, L. Synth. Com- mun. 2001, 31, 349-357.
O O HO
Me
Ar O Me O
3
O Ar O Me
O
4 O HO O
Me
Ar O Me O
- H
O Ar Me O O
O HO
Me
O Ar O Me
O
- AcOH
H H
Scheme 8
silica gel
silica gel silica gel
silica gel
silica gel silica gel
H