Nodal Merkel Cell Carcinoma in Head and Neck Lesions with an Unknown Primary:
A Case Report in Light of the Literature
Ryoukichi Ikeda,* Nobuo Ohta,* Sachiko Fukaya,† Fumi Shoji,* Takahiro Suzuki,* Naoya Noguchi,* Risako Kakuta,* Kazuhiko Hayashi,‡ Takayoshi Kiba,§ Kazuhiro Murakami† and Yasuhiro Nakamura†
*Division of Otolaryngology, Tohoku Medical and Pharmaceutical University, Sendai 981-0905, Japan, †Division of Pathology, Tohoku Medical and Pharmaceutical University, Sendai 981-0905, Japan, ‡Division of Molecular Pathology, Department of Pathology, Tottori University Faculty of Medicine, Yonago 683-8503, Japan, and §Laboratory of Gastrointestinal Regenerative Medicine, Okayama University of Science, Okayama 700-0005, Japan
ABSTRACT
Merkel cell carcinoma (MCC) is a rare but aggressive neuroendocrine skin cancer. To diagnose nodal MCC with an unknown primary disease is challenging, and it has to be separated from other nodal metastatic neoplasms. We report a unique case of nodal MCC in head and neck lesions with an unknown primary. A 70-year-old woman was admitted to our department with a right submandibular mass. Fine needle aspiration biopsy was performed and indicated malignancy. F-18-fluorodeoxyglucose positron emission tomography (PET) demonstrated abnormal accumulation in the right submandibular lymph node, right palatine tonsil, and right thyroid gland. For diagnostics and treatment, bilateral selective neck lymph node dissection, right tonsillectomy, and right thyroidectomy were performed. Histopathological examination revealed that most parts of the submandibular lymph node were occupied by diffuse sheets of tumor cells. Contrary to our expecta-tion, malignant cells were not detected in the right palatine tonsil and right thyroid. Immunohistochemistry demonstrated a marked positive reaction for AE1/ AE3, chromogranin A, synaptophysin, cytokeratin 20 (CK20) and CD56 and a negative reaction for vimentin, leucocyte common antigen (LCA), thyroid transcription factor-1 (TTF1) and cytokeratin 7 (CK7) in the tumor cells. Immunostaining of Merkel cell polyomavirus-large T antigen (MCPyV-LT) showed a positive reaction and MCPyV-positive MCCs were assessed by PCR analysis, demonstrating that viral copy number was 12.8 copies per cell. These histological findings confirmed the diagnosis of Merkel cell carcinoma of the lymph node. In cases of tumors in the lymph node with a neu-roendocrine appearance in head and neck lesions, it is necessary to eliminate the possibility of metastasis from MCC.
Key words head and neck; lymph node; Merkel cell carcinoma; neck lymph node dissection; neuroendo-crine tumor
Merkel cell carcinoma (MCC) is a rare but aggressive neuroendocrine skin cancer.1–3 Although Merkel cells
are believed to be the source of MCC, the cells of origin in MCC remain a controversial issue. Uncommonly, cases of high-grade neuroendocrine tumors have been encountered in lymph nodes with unknown extra-nodal primary disease, and these tumors are usually described as ‘nodal MCC with unknown primary’.4–7 However,
it has been unclear whether nodal MCC is a primary tumor of the lymph node itself or if it represents a me-tastasis from an occult or regressed extra-nodal lesion. Here, we present a unique case of nodal MCC in a head and neck lesion with an unknown primary.
PATIENT REPORT
A 70-year-old woman was admitted to our department with a right, 3 cm round and immobile submandibular mass. Computed tomography revealed that 3 cm and 2cm mass were observed in the right submandibular. Endoscopic examination did not reveal any primary le-sion in head and neck regions. Fine-needle aspiration bi-opsy (FNA) of submandibular mass was performed and revealed that the gathered individual cells had a nucleus with irregular contour and fine chromatin, with a high nucleo-cytoplasmic and thin perinuclear edges. These findings were not in accordance with typical squamous cell carcinoma (SCC) and malignant lymphoma and me-tastasis of neuroendocrine tumor was firstly considered (Fig. 1). For detection of primary lesion and staging, F-18-fluorodeoxyglucose positron emission tomography
Patient Report Yonago Acta Medica 2019;62(3):258–262 doi: 10.33160/yam.2019.09.003
Corresponding author: Nobuo Ohta, MD, PhD noohta@hosp.tohoku-mpu.ac.jp
Received 2019 May 17 Accepted 2019 July 17
Online published 2019 September 13
Abbreviations: CBDCA, carboplatin; CD, cluster differentiation; CK20, cytokeratin 20; CPT-11, irinotecan; CT, computed tomog-raphy; FNA, fine-needle aspiration biopsy; LCA, leucocyte com-mon antigen; MCC, Merkel cell carcinoma; MCPyV-LT, Merkel cell polyomavirus-large T antigen; MRI, magnetic resonance im-aging; PET, F-18-fluorodeoxyglucose positron emission tomogra-phy; SCC, squamous cell carcinoma; TTF1, thyroid transcription factor-1; VP-16, etoposide
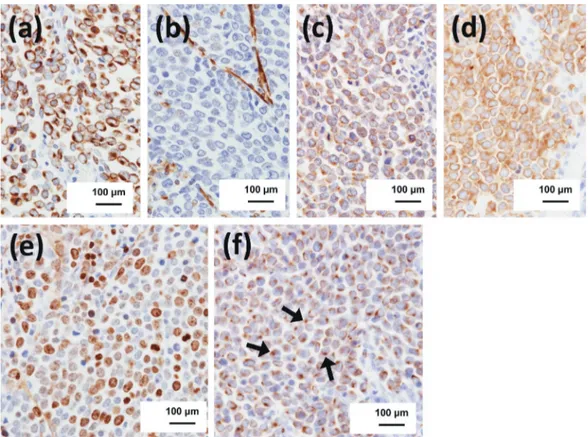
(PET) was conducted. PET demonstrated abnormal accumulation in the right submandibular lymph node, right palatine tonsil, and right thyroid gland (Fig. 2). The serological data showed serum ProGRP 155.8 pg/ mL (baseline: 0–81), NSE13.7 ng/mL (baseline: 0–16.3). For diagnostics and treatment, bilateral selective neck lymph node dissection (I–V), right tonsillectomy and right thyroidectomy were performed. Histopathological examination revealed that most parts of the lymph node were occupied by diffuse sheets of tumor cells. Moreover, the tumor cells were uniformly small rounded cells with scanty cytoplasm and had a round to oval nucleus with dispersed chromatin, and inconspicu-ous nucleoli in right IB, IIA and IIB lymph nodes (Fig. 3). Contrary to our expectation, malignant cells were not detected in the right palatine tonsil and right thyroid gland. Immunohistochemistry demonstrated a marked positive reaction for AE1/AE3, chromogranin A, synap-tophysin, cytokeratin 20 (CK20) and CD56 and a nega-tive reaction for vimentin, leucocyte common antigen (LCA), thyroid transcription factor-1 (TTF1) and CK7 in the tumor cells (Fig. 4). The Ki-67 staining index ranged from 50 to 60%. Immunostaining of Merkel cell polyomavirus-large T antigen (MCPyV-LT) showed a positive reaction and MCPyV-positive MCCs were
assessed by PCR analysis, demonstrating that the viral copy number was 12.8 copies per cell (Fig. 5). These histological findings confirmed the diagnosis of nodal MCC in head and neck lesions with primary unknown (stage IIIA). The patient underwent treatment with carboplatin (CBDCA) and irinotecan (CPT-11) chemo-therapy. Right superior deep lateral cervical lymph node swelling was observed eight months after the initial treatment. Resection of the lymph node was performed and histological findings confirmed the same results as the initial surgery. She has since undergone treatment with CBDCA and etoposide (VP-16) chemotherapy and radiation therapy (60Gy/25 fractions). Two years later, the patient was in good clinical conditions without recurrence.
DISCUSSION
Nodal MCC with unknown primary is defined as neuro-endocrine carcinoma in lymph nodes with microscopic, immunohistochemical, and genetic features similar to those of cutaneous MCC.8–10 In our case, we initially
could not diagnose MCC based on morphological features. However, FNA was very useful as a diagnostic tool in differential diagnosis from SCC and malignant lymphoma. It is generally difficult to diagnose MCC
Fig. 1. Fine-needle aspiration biopsy (FNA) revealed that the individual cells had a nucleus with irregular contours and showed fine chromatin.
R. Ikeda et al.
solely by morphologic characteristics. Therefore, several metastatic tumors should be considered in the differential diagnosis such as metastatic neuroendocrine carcinoma including small cell carcinoma from the lung or genitourinary tract, malignant melanoma, B-cell lymphoblastic lymphoma, and mature B-cell lymphoma. Immunohistochemistry is also a key tool to identify the diagnosis of MCC. MCC is positive for neuroendocrine markers such as synaptophysin, chromogranin A and CD56. However, the specificity of these markers is low. In our case, the cells were positive
for synaptophysin and chromogranin A, but negative for CD56. CK20 and TTF-1 are useful in distinguishing MCC from metastatic small cell carcinoma. CK20 is a low molecular weight cytokeratin that is only expressed in normal gastrointestinal epithelia, urothelia, and Merkel cells. MCC almost always stains with CK20 in contrast to metastatic SCC.11 TTF-1 is described as
a nuclear transcription factor expressed in epithelial cells of the thyroid and lung. It is expressed in a high proportion of SCC and is not expressed by MCC.12
Most cases of MCC are CK7-negative, but a significant
Fig. 2. F-18-fluorodeoxyglucose positron emission tomography (PET) confirmed the presence of abnormal accumulation in the right submandibular lymph node, right palatine tonsil, and right thyroid gland.
Fig. 3. Histology showed most parts of the lymph node were occupied by tumor, nodules and diffuse sheets of eosinophilic cells with imperceptible cytoplasm, a round nuclei and dispersed chromatin (hematoxylin and eosin stain).
Fig. 4. Immunohistochemistry of AE1/AE3 (a), vimentin (b), chromogranin A (c), synaptophysin (d), Ki67 (e) and CK20 (arrows: positive cells) (f).
R. Ikeda et al.
minority exhibit partial CK7 positivity. CK-7 was nega-tive in our case. MCPyV is a non-enveloped double-stranded DNA virus associated with the pathogenesis of MCC.13, 14 It is suggested that clonal integration of
a polyomavirus might be involved in pathogenesis of MCC.13 MCPyV-LT is highly specific for MCC and
the overexpression of MCPyV was also detected in our case. Detection of MCPyV-LT is clinically important and might help physicians in the diagnosis of MCC. These findings may allow us to speculate that MCPyV might contribute to the pathogenesis of MCC and MCPyV-LT might be used as an additional indicator of MCC. Patients with nodal MCC have a better prognosis than those with metastatic nodal MCC and a concurrent primary tumor.15–19 Surgical excision of the primary
le-sion and additional chemotherapy remain central in the treatment of nodal MCC with primary unknown. In re-current cases, chemotherapy and radiotherapy have been standard treatment modalities.18, 19 A different regimen
from initial treatment was selected from consideration of drug sensitivity in our case. However, recent reports demonstrated that PD-1 and PD-L1 inhibitors have been shown to be superior to other systemic treatments for MCC in advanced stages.1, 2 The use of these therapies
are thus first-line treatment in advanced stages, espe-cially because the side effects of these substances are generally easily controlled.2
The authors declare no conflict of interest. REFERENCES
1 Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107-10. PMID: 5009611, DOI: 10.1001/arch-derm.1972.01620040075020
2 Smith VA, Camp ER, Lentsch EJ. Merkel cell carcinoma: identification of prognostic factors unique to tumors located in the head and neck based on analysis of SEER data. Laryn-goscope. 2012;122:1283-90. PMID: 22522673,DOI: 10.1002/ lary.23222
3 Hussain SK, Sundquist J, Hemminki K. Incidence trends of squamous cell and rare skin cancers in the Swedish national cancer registry point to calendar year and age-dependent increases. J Invest Dermatol. 2010;130:1323-8. PMID: 20090766, DOI: 10.1038/jid.2009.426
4 Huber GF, Khalil M, Falck V, Matthews TW, Dort JC. Merkel cell carcinoma with solitary parotid metastasis: diagnostic dilemma in the absence of a primary site. J Otolaryngol Head Neck Surg. 2008;37:E19-21. PMID: 18479622
5 Pan Z, Chen YY, Wu X, Trisal V, Wilczynski SP, Weiss LM, et al. Merkel cell carcinoma of lymph node with unknown primary has a significantly lower association with Merkel cell polyomavirus than its cutaneous counterpart. Mod Pathol. 2014;27:1182-92. PMID: 24406862, DOI: 10.1038/ modpathol.2013.250
6 Wong SQ, Waldeck K, Vergara IA, Schröder J, Madore J, Wilmott JS, et al. UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas. Cancer Res. 2015;75:5228-34. PMID: 26627015, DOI: 10.1158/0008-5472.CAN-15-1877
7 Wong KKS, Oliver GF. Metastatic Merkel cell carcinoma with an unknown primary tumour presenting as lichenoid dermatitis. Australas J Dermatol. 2010;51:202-5. PMID: 20695861,DOI: 10.1111/j.1440-0960.2010.00624.x
8 Adel K. El-Naggar JKC, Jennifer R Grandis, Takashi Takata, Pieter J Slootweg. WHO Classification of Head & Neck Tumours. 4th ed. Geneva: World Health Organization; 2017. 347 p.
9 Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demo-graphics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20-7. PMID: 19638070, DOI: 10.1111/j.1600-0560.2009.01370.x
10 Saini AT, Miles BA. Merkel cell carcinoma of the head and neck: pathogenesis, current and emerging treatment options. OncoTargets Ther. 2015;8:2157-67. PMID: 26316785
11 Leech SN, Kolar AJO, Barrett PD, Sinclair SA, Leonard N. Merkel cell carcinoma can be distinguished from metastatic small cell carcinoma using antibodies to cytokeratin 20 and thyroid transcription factor 1. J Clin Pathol. 2001;54:727-9. PMID: 11533085, DOI: 10.1136/jcp.54.9.727
12 Byrd-Gloster AL, Khoor A, Glass LF, Messina JL, Whitsett JA, Livingston SK, et al. Differential expression of thyroid transcription factor 1 in small cell lung carcinoma and merkel cell tumor. Hum Pathol. 2000;31:58-62. PMID: 10665914, DOI: 10.1016/S0046-8177(00)80199-9
13 Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096-100. PMID: 18202256, DOI: 10.1126/sci-ence.1152586
14 Arora R, Chang Y, Moore PS. MCV and Merkel cell carcino-ma: a molecular success story. Curr Opin Virol. 2012;2:489-98. PMID: 22710026, DOI: 10.1016/j.coviro.2012.05.007 15 Chen KT, Papavasiliou P, Edwards K, Zhu F, Perlis C, Wu
H, et al. A better prognosis for Merkel cell carcinoma of unknown primary origin. Am J Surg. 2013;206:752-7. PMID: 23835211, DOI: 10.1016/j.amjsurg.2013.02.005
16 Tarantola TI, Vallow LA, Halyard MY, Weenig RH, Warschaw KE, Weaver AL, et al. Unknown primary Merkel cell carcinoma: 23 new cases and a review. J Am Acad Dermatol. 2013;68:433-40. PMID: 23182060, DOI: 10.1016/ j.jaad.2012.07.035
17 Yaramada P, Lim BS, Flannery CM, Koh SS, Yaghsezian H. Merkel cell carcinoma of unknown primary with lymph node and mesenteric metastasis involving the pancreas and duodenum. J Gastrointest Oncol. 2016;7(suppl 1):S66-70. PMID: 27034815
18 Harms KL, Healy MA, Nghiem P, Sober AJ, Johnson TM, Bichakjian CK, et al. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann Surg Oncol. 2016;23:3564-71. PMID: 27198511
19 Müller-Richter UDA, Gesierich A, Kübler AC, Hartmann S, Brands RC. Merkel Cell Carcinoma of the Head and Neck: Recommendations for Diagnostics and Treatment. Ann Surg Oncol. 2017;24:3430-7. PMID: 28762116, DOI: 10.1245/ s10434-017-5993-1