356 Bull. Soc. Sea Water Sci., Jpn., 73, 356− 357(2019)
Bulletin of the Society of Sea Water Science, Japan
(Short Paper)
Influence of Calcium Ions on the Fouling of a Thin-film
Composite Reverse Osmosis Membrane by Alginate
Mifuyu H
ARADA1, Tasuma S
UZUKI1, 2*and Masakazu N
IINAE1Abstract
The objective of this study was to investigate the influence of calcium ions (Ca2+) on the fouling propensity of a thin-film composite reverse osmosis (RO) membrane by alginate. Under the experimental conditions investigated in this study, an increase in Ca2+ of up to 0.2 mmol/L accelerated the fouling of the RO membrane. However, the fouling propensity was not influenced by concentrations of Ca2+ above 0.2 mmol/L. This result could be explained by the content of Ca2+ in the fouling layer, which increased when the concentration of Ca2+ in the feed water was 0.25 mmol/L or less and reached a plateau above 0.25 mmol/L.
Key Words : RO membrane, Polyamide, Fouling, Alginate, Calcium ion
1 Graduate School of Sciences and Technology for Innovation, Yamaguchi University, 2-16-1 Tokiwadai, Ube, Yamaguchi, 755-8611, Japan 2 Blue Energy Center for SGE Technology, Yamaguchi University, Yamaguchi University, 2-16-1 Tokiwadai, Ube, Yamaguchi, 755-8611, Japan * Corresponding author E-mail:tsuzuki@yamaguchi-u.ac.jp Tel:+81-836-85-9690
1.Introduction
Thin-film composite polyamide reverse osmosis (RO) membranes are often used for seawater desalination be-cause they are capable of removing salt and precursors of disinfection by-products (e.g., natural organic matter) in a single treatment step. However, one of the difficulties to overcome in the use of RO membranes is fouling, a deterio-ration of water permeability due to the formation of a fouling layer on top of the RO membrane. In the literature1), it has been reported that biofouling, which is caused by the accu-mulation of microorganisms and biopolymers, is a major type of fouling in seawater desalination. It has also been re-ported that the fouling of RO membranes by acidic polysac-charides2) and proteins3) was enhanced by the presence of calcium ions (Ca2+). However, the influence and corre-sponding mechanisms of Ca2+ on the fouling of RO mem-branes is not fully understood. Based on this background, the objective of this study was to investigate the influence of Ca2+ on the fouling of RO membranes using alginate as a representative biopolymer with the final objective of deepen-ing our understanddeepen-ing of the fouldeepen-ing mechanism.
2.Experimental
All solutions were prepared using ultrapure water with a resistivity greater than 18.2 MΩ cm−1(Direct-Q UV, Merck Ltd., Tokyo, Japan). A LFC3-LD RO membrane produced by Nitto Denko Corp. (Osaka, Japan) was used as a representa-tive RO membrane. The LFC3-LD membrane is a thin-film composite RO membrane with a polyamide active layer. The
surface was coated with polyvinyl alcohol4) to improve foul-ing resistance to organic matter. First-grade sodium alginate with a viscosity of 80-120 cPa·s at a concentration of 10 g/L was purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Other reagents were purchased at
guaran-teed reagent-grade quality from Nacalai Tesque Inc. (Kyoto, Japan) or Wako Pure Chemical Industries and used without further purification.
Fouling experiments were performed using a custom-made stirred cell5) with a LFC3-LD flat sheet circle coupon with an effective area of 7.5 cm2. Before starting the fouling experi-ment, the membrane was compacted at 2 MPa for 3 days us-ing ultrapure water. After confirmus-ing that the water permea-bility was stable, the fouling experiment was started using 1 L of feed solution containing 20 mg/L sodium alginate, a pre-determined concentration of Ca2+(provided by CaCl
2), and NaCl added to adjust the ionic strength of the solution to 10 mmol/L (without considering sodium alginate). After 24 hours, the water permeability of the fouled membrane was measured, and normalized water permeability was calculated from the change in water permeability during the 24 hours of the fouling experiment. The fouled RO membrane was then analyzed by a K-Alpha™+ XPS (X-ray photoelectron spectros-copy) system (Thermo Fisher Scientific Inc., Waltham, MA) to obtain information on the Cacontent in the fouling layer.
It is important to mention that we did not use a feed spac-er during the fouling expspac-eriment. In addition, the hydraulic condition of the stirred cell was significantly different from that of the LFC3-LD element. Therefore, we should regard the fouling experiments performed in this study as
accelera-357
tion tests and the obtained results do not represent the foul-ing resistance of the LFC3-LD element.
3.Results and Discussion
The normalized water permeability after the 24 hour foul-ing experiment as a function of Ca2+ concentration is shown in Fig. 1. Within Ca2+ concentrations of 0.2 mmol/L or less, any increase in Ca2+ concentration accelerated the fouling of the RO membrane, as expected. This result is explained by the ability of Ca2+ to bridge carboxyl groups (R-COO−) in alginate and the corresponding formation of calcium algi-nate gel, which provides increased water resistance com-pared to sodium alginate. However, the fouling propensity was not influenced by concentrations of Ca2+ above 0.2 mmol/L, and 50-60 % of normalized water permeability was obtained regardless of Ca2+ concentration.
As a first step to explore the reason behind this unexpect-ed result, we performunexpect-ed similar experiments and increasunexpect-ed the volumes of feed solution from 1 L to 3 L. We hypothe-sized that all the alginate in 1 L of feed solution was deposit-ed on the RO membrane, resulting in a lack of alginate in the feed solution, which is required to cause a further de-gree of fouling. However, the obtained results disproved this hypothesis because the degree of fouling or normalized wa-ter permeability was statistically identical to that in the ex-periment with 1 L of feed solution.
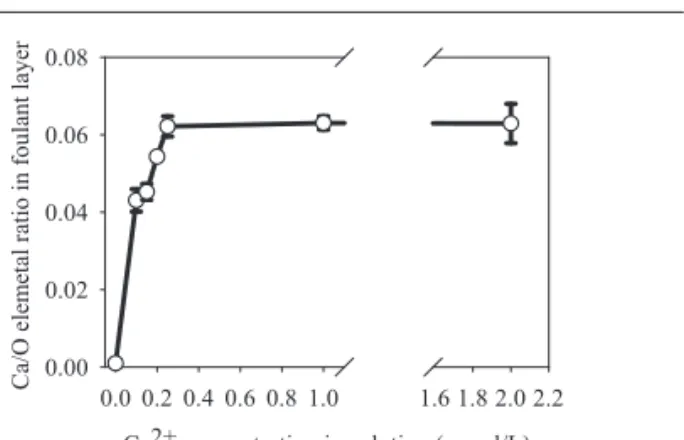
We next measured the Ca content in the foulant layer after the fouling experiment using XPS, and the obtained results are shown in Fig. 2. The Ca content expressed as Ca/O
in-creased when the Ca2+ concentration in the feed solution was 0.25 mmol/L or less but reached a plateau of approximately 0.063 at higher Ca2+ concentrations. The chemical composi-tion of sodium alginate is (NaC6H7O6)n. If we assume 100 % of R-COO− in alginate was saturated with Ca2+ with a molar ratio of R-COO−: Ca2+ = 2:1, we can calculate the Ca/O ratio in calcium alginate as 1/12 or 0.083, which is higher than but close to 0.063. This calculation showed that 0.25 mmol/L of
Ca2+ in the feed solution is sufficient to closely saturate R-COO− in alginate, and additional Ca2+ does not play an im-portant role in the physico-chemical structure of calcium algi-nate deposited on the RO membrane. Consequently, it is reasonable to conclude that the addition of Ca2+ above 0.2 mmol/L did not influence the fouling propensity (Fig. 1) be-cause a majority of alginate was present as calcium alginate within this Ca2+ concentration range. In seawater, the concen-tration of Ca2+ is higher than 0.2 mmol/L. However, the con-centration of sodium ions, which competes with Ca2+ for R-COO− sites, is also higher in seawater than it was in this ex-periment. Therefore, we are currently using artificial seawa-ter to investigate the influence and mechanism of Ca2+ foul-ing on RO membranes by alginate in seawater desalination.
Acknowledgements
This work was partially supported by JSPS KAKENHI (Grant Number 16H01796).
References
1) A. Matin, Z. Khan, S.M.J. Zaidi and M.C. Boyce, Biofouling in Reverse Osmosis Membranes for Seawater Desalination: Phenomena and Prevention, Desalination, 281, 1-16 (2011)
2) W. S. Ang, S. Lee and M. Elimelech, Chemical and physical aspects of cleaning of organic-fouled reverse osmosis mem-branes, Journal of Membrane Science, 272, 198-210 (2006)
3) W. S. Ang and M. Elimelech, Protein (BSA) fouling of re-verse osmosis membranes: Implications for wastewater rec-lamation, Journal of Membrane Science, 296, 83-92 (2007)
4) T. Fujioka, H. Kodamatani, L. D. Nghiem and T. Shintani, Transport of N-nitrosamines through a Reverse Osmosis Membrane: Role of the Molecular Size and Nitrogen Atoms,
Environmental Science & Technology Letters, 6 44-48 (2018)
5) M. Harada, Y. Takao, H. Yamaguchi, T. Suzuki and M. Niinae, An Electrocoagulation/flotation-MF Membrane Pretreat-ment Process for the Mitigation of RO Membrane Fouling by Dissolved Effluent Organic Matter from Municipal Waste-water Treatment Plant, Journal of Japan Society on Water
En-vironment, 42, 185-194(2019) Fig. 1 Influence of Ca2+ concentration on the normalized water
permeability after the 24 hour fouling experiment Fig. 2 Ca content (expressed as Ca/O) in the foulant layer after the fouling experiment