N
euroendocrinological studies of patients with depression have focused on the hypothalamic- pituitary-adrenal (HPA) axis, and depressed patients have been reported to have dysfunctional HPA axes [1]. We and others have reported that the chronic adminis-tration of adrenocorticotropic hormone (ACTH) for 14 days to rats counteracted the effects of the tricyclic anti-depressants imipramine and desipramine and decreased the amount of time the rats spent immobile in the forced swim test, a behavioral test that is widely used as a predictor of antidepressive activity [2,3]. The chronictreatment of rats with ACTH might therefore represent an effective animal model of antidepressant treatment- resistant depression.
Ketamine is a non-competitive antagonist of the phencyclidine site of the N-methyl-D-aspartate (NMDA) receptor. Clinically, it is well known that ketamine exerts rapid and sustained antidepressive effects in depressed patients and treatment-resistant depressive patients [4-6]. In 2019, the U.S. Food and Drug Administration approved esketamine as a treatment for treatment-resistant depression. It was reported that in rodents, ketamine significantly decreased immobility in
CopyrightⒸ 2020 by Okayama University Medical School.
http ://escholarship.lib.okayama-u.ac.jp/amo/
Original Article
Immobility-reducing Effects of Ketamine during the Forced Swim
Test on 5-HT
1AReceptor Activity in the Medial Prefrontal Cortex
in an Intractable Depression Model
Kei Takahashi
a, Yoshihisa Kitamura
a,b*, Soichiro Ushio
a, and Toshiaki Sendo
aaDepartment of Clinical Pharmacy, Okayama University Graduate School of Medicine,
Dentistry and Pharmaceutical Sciences, Okayama 700-8558, Japan,
bDepartment of Pharmaceutical Care and Health Sciences, School of Pharmacy,
Shujitsu University, Okayama 703-8516, Japan
Ketamine has been clinically proven to ameliorate depression, including treatment-resistant depression. The detailed mechanism of action of ketamine in treatment-resistant depression remains unclear. We examined the effects of ketamine on the immobility times of adrenocorticotropic hormone (ACTH)-treated rats during the forced swim test, and we explored the mechanism by which ketamine acts in this model. We investigated the neuroanatomical site of action by microinjecting ketamine into the medial prefrontal cortex of rats. A signifi-cant reduction of the rats’ immobility during the forced swim test was observed after the intraperitoneal injec-tion of ketamine in both saline- and ACTH-treated rats. The microinjecinjec-tion of ketamine into the medial pre-frontal cortex also decreased immobility during the forced swim test in both saline- and ACTH-treated rats. The immobility-decreasing effect of intraperitoneally injected ketamine was blocked by administering WAY100635, a 5-HT1A receptor antagonist, into the medial prefrontal cortex. These findings contribute to the
evidence that ketamine can be useful against treatment-resistant depressive conditions. The immobility-reduc-ing effects of ketamine might be mediated by 5-HT1A receptor activity in the medial prefrontal cortex.
Key words: ketamine, adrenocorticotropic hormone, forced swim test, medial prefrontal cortex, 5-HT1A receptor
Received January 23, 2020 ; accepted April 6, 2020.
*Corresponding author. Phone : +81-86-235-7641; Fax : +81-86-235-7974
the forced swim test [7,8], and that ketamine signifi-cantly decreased the amount of time ACTH-treated rats spent immobile during the forced swim test [3]. These results suggested that ketamine could be effective against antidepressant treatment-resistant depression.
The serotonin (5-HT)1A receptor has been implicated in the pathophysiology of major depressive disorder in both animal models and humans [9,10]. We observed that the 5-HT1A receptor full agonist (±)-8-hydroxy-2-(di-n-propylamino)tetralin significantly reduced the amount of time saline- and ACTH-treated rats spent immobile during the forced swim test [11]. Thus, the 5-HT1A receptor might play an important role in depression, particularly in depression that is resistant to tricyclic antidepressant treatment. The antidepressive effects of ketamine might also be mediated by post-syn-aptic 5-HT1A receptor activity in mice [12]. In mice that underwent the novelty-suppressed feeding test, the antidepressive effects of ketamine were attenuated by WAY100635, a 5-HT1A receptor antagonist, but not ritanserin, a 5-HT2A/2C receptor antagonist [13]. These findings suggested that ketamine exerts its effects by indirectly activating postsynaptic 5-HT1A receptors. Ketamine might therefore be effective in animal models of depressive conditions that are resistant to tricyclic antidepressant treatment, and these effects might be mediated by post-synaptic 5-HT1A receptor activity.
The role of the medial prefrontal cortex (MPC) in depression has been extensively studied. Conditioned fear stress selectively increased 5-HT metabolism in the MPC [14]. Ketamine increased the release of 5-HT from the MPC, probably by activating neurons in the dorsal raphe nucleus [15,16]. Following its microinjec-tion into the MPC of rodents, ketamine was observed to exert antidepressive effects in the forced swim test, and these effects were attenuated by depleting 5-HT using p-chlorophenylalanine, an inhibitor of 5-HT synthesis [17]. These findings suggested that the MPC serotoner-gic system is deeply involved in the psychopathology of depression and anxiety.
We conducted the present study to investigate whether ketamine treatment would reduce the amount of time ACTH-treated rats spent immobile during the forced swim test. To explore the role of the 5-HT1A receptor in the antidepressant-like effects of ketamine, we also examined whether the effects of ketamine are mediated via 5-HT1A receptors in the MPC.
Materials and Methods
Animals. Wistar rats (Charles River, Yokohama, Japan) with initial weights of 210-230 g were used. The rats were housed 2 per cage in an air-conditioned room (23±1°C; approximate humidity level: 60%) under a constant light-dark cycle (lights on: 7 : 00 a.m. to 7 : 00 p.m.) and were fed standard laboratory food and tap water. All experiments were conducted according to the guidelines for animal experimentation at Okayama University Medical School. All efforts were made to minimize the number of animals used and the degree of suffering they experienced.
Drug administration. The following drugs were used: ketamine (Ketalar®; Daiichi-Sankyo, Tokyo),
WAY100635 (Sigma-Aldrich, St. Louis, MO, USA), and ACTH-(1-24)-zinc (Cortrosyn Z; Daiichi-Sankyo). The rats were administered ketamine intraperitoneally at a dose of 2 ml/kg body weight. ACTH was injected subcutaneously once daily (at 9 : 00-10 : 00 a.m.) for 14 days at a dose of 100 μg/rat (injection volume: 0.2 ml/ rat). The control rats received an equivalent volume of vehicle (saline, 0.2 ml/rat, subcutaneously injected) for the same period of time. The amount of time the con-trol rats spent immobile was assessed 60 min after the administration of a single dose of ketamine (10-20 mg/ kg, i.p.) to rats that had been treated with ACTH for 14 days. The last injection of ACTH was given immedi-ately after the pre-swim test. Ketamine was adminis-tered the next day, without concurrent ACTH treat-ment. The amount of time the rats spent immobile was assessed 60 min after the administration of ketamine as described below.
Microinjections. Rats were anesthetized by a sin-gle intraperitoneal administration of sodium pentobar-bital (50 mg/kg) and were fixed to a brain stereotactic apparatus (Narishige, Tokyo). For the injection of ket-amine into the MPC, the rat’s brain was implanted with bilateral guide cannulas (AG-8; Eicom, Kyoto, Japan), and the tips were positioned in the MPC (anteroposte-rior: +3.2 mm in front of the bregma suture, midline: ±0.6 mm, dorsoventral: −3.2 mm, angle: 20°). The cannulas were held in place with dental cement. A dummy cannula was inserted into the guide cannula to prevent clogging.
The microinjection of ketamine or WAY100635 was performed on day 2 after surgery. The injection cannula (AMI-8; Eicom Co., Kyoto, Japan) was connected via
Teflon tubing to a Hamilton microsyringe driven by a microinfusion pump (CMA/100 syringe pump; Carne-gie Medicine, Stockholm, Sweden). The injections were performed for 1 min at a rate of 1 µl/min. The injection cannulas were left in position for an additional 1 min before being withdrawn. After the behavioral test, Evans blue dye was infused and then coronal sec-tions were prepared to confirm the locasec-tions of the can-nula tips. The ketamine and WAY100635 were diluted with Ringer’s solution prior to being used for the MPC microinjections.
Assessment of immobility. For the assessment of immobility, individual rats were placed in plastic cylin-ders (height 37 cm, dia. 15.5 cm), containing 20 cm of water at 25°C, as described by Porsolt et al. [18]. Two swimming sessions were conducted in the initial 13-min pretest, and a 6-min test was performed 24 h later. The total amount of time each rat spent immobile during the 6-min test period was recorded by a TARGET series/7M analysis program (Neuroscience Inc., Tokyo).
On the 15th day of treatment, immobility was assessed 60 min after the administration of a single dose of ketamine (10-20 mg/kg, i.p.) without ACTH. In the microinjection experiments, immobility was assessed 15 min after the microinjection of ketamine (1-15 µg/side) into the MPC without ACTH on the 15th day of treatment. In the experiment involving the microinjection of WAY100635, WAY100635 (1 nmol/ side) was administered 20 min before the assessment of mobility.
Locomotor activity. The rats’ locomotor activity was monitored for 6 min with the use of automated activity monitoring chambers (Neuroscience, Inc.). The plastic chambers measured 23 cm (width) by 40 cm (length) by 18 cm (height).
Statistical analyses. All data are expressed as mean±standard error of the mean (SEM) values. The immobility time and locomotor activity were analyzed by a one-way analysis of variance (ANOVA). The group means were compared using Dunnett’s test (Figs.1,2; Table 1) and Tukey’s test (Fig.3) for multiple compari-sons. For the evaluation of the influence of the ket-amine injection into the MPC on the rats’ locomotor activity, comparisons between the 2 groups were per-formed using the unpaired two-tailed Student’s t-test. P-values of <0.05 were considered significant.
Results
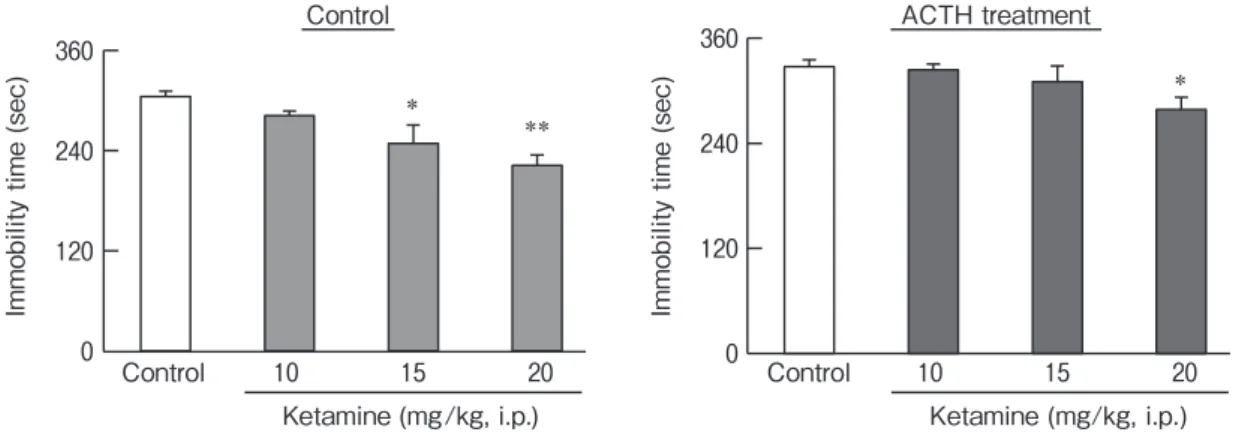
Effects of ketamine on immobility in saline- and ACTH-treated rats. We examined the amount of time that the saline- and ACTH-treated rats spent immobile during the forced swim test after they were treated with ketamine (10-20 mg/kg, i.p.). Ketamine significantly reduced the amount of time spent immo-bile during the forced swim test in both the saline- and ACTH-treated rats (control: F(3,20)=7.31, p<0.01; ACTH: F(3,20)=3.11, p<0.05) (Fig.1).
Effects of the infusion of ketamine into the MPC on the amount of time saline- and ACTH-treated rats
Im m ob ili ty ti m e (s ec ) Control 10 15 20 Ketamine (mg/kg, i.p.) * ** 0 120 240 360 Im m ob ili ty ti m e (s ec ) *
Control ACTH treatment
0 120 240 360 Control 10 15 20 Ketamine (mg/kg, i.p.)
Fig. 1 The effects of ketamine on the amount of time saline- or ACTH-treated rats spent immobile during the forced swim test. Immobility was assessed 60 min after the administration of ketamine (10-20 mg/kg, i.p.) in saline- and ACTH-treated rats. We repeatedly administered ACTH (100 μg/day, s.c., 14 days) to rats for 14 days. On the 15th day, ketamine was administered without ACTH. Each column represents the mean±SEM (n=6 for each group). Data were analyzed with a one-way ANOVA, and group means were compared using Dunnettʼs test for multiple comparisons. *P<0.05 vs. the control group. **P<0.01 vs. the control group.
spent immobile. We examined the effects of ket-amine (1-10 μg/side) injected into the MPC on the amount of time saline- and ACTH-treated rats spent immobile during the forced swim test (Fig.2). The infu-sion of 10 μg/side ketamine into the MPC decreased the amount of time the control rats spent immobile during the forced swim test (F(3,15)=8.77, p<0.01). Similarly, the infusion of 15 μg/side ketamine into the MPC sig-nificantly decreased the amount of time the ACTH-treated rats spent immobile during the forced swim test (F(2,11)=4.48, p<0.05) (Fig.2).
Effects of ketamine on locomotor activity in saline- and ACTH-treated rats. Ketamine (20 mg/kg, i.p.) significantly decreased the locomotor activity of the saline- and ACTH-treated rats (saline: F(3,20)=0.001, p<0.01; ACTH: F(3,20)=3.13, p<0.05) (Table 1). In contrast, the injection of 10 or 15 µg/side ketamine into the MPC did not affect locomotor activity in the saline- or ACTH-treated rats (Table 2).
Effects of the microinjection of WAY100635 into the MPC on the immobility-decreasing effects of ketamine in the forced swim test. The effects of the microin-jection of WAY100635 into the MPC on the ketamine- induced reduction of time the rats spent immobile during the forced swim test are illustrated in Fig.3. The reduction in immobility induced by ketamine (20 mg/ kg, i.p.) was prevented by microinjecting WAY100635 (1 nmol/side) into the MPC (F(3,39)=3.71, p<0.05).
Discussion
Our findings clarify the effects of an intraperitoneal injection of ketamine and a microinjection of ketamine into the MPC on the amount of time ACTH-treated rats spent immobile during the forced swim test. The observed effects of ketamine (i.p.) agree with the results obtained for normal and ACTH-treated rats in previous studies [3,12].
In a study using mice, the microinjection of ket-amine into the MPC decreased immobility [16,17], and Table 1 The effects of ketamine on locomotor activity in saline- and ACTH-treated rats
Dose (mg/kg) Locomotor activity (counts)
Saline ACTH
- 221.3±6.8 222.5±10.0
10 205.1±19.1 202.9±13.7
15 184.1±8.3 182.9±14.1
20 134.4±18.3* 181.9±18.4
ACTH (100 g/rat, s.c.) was administered to the rats once daily for 14 days. Locomotor activity was measured on the day after the final dose of ACTH was administered, 60 min after the ketamine injec-tion. Data are mean±SEM values (n=5-6 rats). The data were analyzed with a one-way ANOVA, and group means were compared using Dunnettʼs test for multiple comparisons. *P<0.05 vs. the
saline-treated group. 0 120 240 360 0 120 240 360 Control 1 3 10 Ketamine (µg/side) ** *
Control ACTH treatment
Control 10 15
Ketamine (µg/side)
Immobility time (sec) Imm
ob ili ty ti m e (s ec )
Fig. 2 The effects of the microinjection of ketamine into the MPC for 14 days on the amount of time saline- or ACTH-treated rats spent immobile during the forced swim test. We examined the effects of injecting a single dose of ketamine (1-15 μg/side) into the MPC on the amount of time rats spent immobile during the forced swim test. Immobility was assessed 15 min after the administration of ketamine. We repeatedly administered ACTH (100 μg/day, s.c., 14 days) to rats for 14 days. On the 15th day, ketamine was administered without ACTH. Each column represents the mean±SEM (n=4-6, each group). Data were analyzed with a one-way ANOVA, and group means were compared using Dunnettʼs test for multiple comparisons. *P<0.05, **P<0.01 vs. the control group.
in the present investigation using rats, the infusion of ketamine at a dose of 10 μg/side into the MPC signifi-cantly reduced the amount of time the control rats spent immobile during the forced swim test. In the ACTH-treated rats, the infusion of ketamine at 15 μg/side into the MPC significantly reduced the amount of time they
spent immobile during the forced swim test. Taken together, the previous and present results suggest that ketamine may improve the antidepressant treatment- resistant depression.
To determine whether the observed changes in immobility were associated with reductions in locomo-tor activity, we examined the effects of ketamine on locomotor activity in saline- and ACTH-treated rats. Only the highest dose of ketamine (20 mg/kg, i.p.) decreased the locomotor activity of both the saline- and ACTH-treated rats. It is therefore unlikely that the ten-dency for ketamine to reduce immobility in the forced swim test was related to the drug’s effect on locomotor activity.
In mice, the antidepressive effects of ketamine appear to be mediated by 5-HT1A receptor activity in the MPC [12]. We reported the effects of 8-OH-DPAT, a 5-HT1A receptor agonist, on the immobility exhibited by saline- or ACTH-treated rats during the forced swim test [11], and we observed that 8-OH-DPAT potently reduced the amount of time the ACTH-treated rats spent immobile during the forced swim test, in a dose- dependent manner. This suggests that the 5-HT1A receptor plays an important role in depression, particu-larly in depression that is resistant to tricyclic antide-pressant treatment. We also observed that the microin-jection of WAY100635 (a 5-HT1A receptor antagonist) into the MPC blocked the immobility-decreasing effects of ketamine. The present results thus indicate that the stimulation of 5-HT1A receptors in the MPC might play an important role in the antidepressive effects of ket-amine.
It was reported that the sustained antidepressant- like effects of ketamine increase the release of 5-HT in the MPC through local mechanisms, such as inhibition of the 5-HT transporter, or through the activation of the dorsal raphe nucleus neurons by MPC projection [12]. A recent study concerning 5-HT1A receptor func-tion revealed that the antidepressant-like effects of ket-amine are mediated by the post-synaptic 5-HT1A recep-tor and the subsequent activation of phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin complex-1 (PI3K/Akt/mTORC1) signaling in the MPC [19]. Ketamine is a non-competitive NMDA receptor antag-onist. In the glutamatergic system, the metabotropic glutamate (mGlu) receptors are known to have modula-tory roles in glutamatergic transmission, and their roles in depression have been investigated [20]. Importantly, Table 2 Influence of the infusion of ketamine into the medial
prefrontal cortex (MPC) on locomotor activity in saline- and ACTH-treated rats
a) Saline
Treatment Locomotor activity (counts)
Saline 165.0±12.2
Ketamine (10 μg/side) 181.2±15.0 b) ACTH
Treatment Locomotor activity (counts)
ACTH 201.5±9.3
ACTH+ketamine (15 μg/side) 189.0±12.3
ACTH (100 g/rat, s.c.) was administered to the rats once daily for 14 days. Locomotor activity was measured on the day after the final dose of ACTH was administered, 15 min after the infusion of ket-amine into the MPC. Data are expressed as the mean±SEM val-ues (n=5-6 rats). The data were analyzed with the unpaired two-tailed Studentʼs t-test.
0 120 240 360 * Control Ketamine WAY100635 (1 nmol/side) Ketamine -Im m ob ili ty ti m e (s ec )
Fig. 3 The effects of microinjecting WAY100635 into the MPC on the immobility-reducing effects of ketamine in rats during the forced swim test. Immobility was assessed 60 min after the admin-istration of ketamine (20 mg/kg, i.p.). WAY100635 (1 nmol/side) was injected into the MPC 20 min before the examination. Each column represents the mean±SEM (n=9-12, each group). Data were analyzed with a one-way ANOVA, and group means were compared using Tukeyʼs test for multiple comparisons. *P<0.05
an mGlu2/3 receptor antagonist had rapid and long-lasting ketamine-like antidepressive effects on treatment-resistant depression in rodents [19,21-23]. Further studies are in progress to clarify the detailed mechanisms underlying the effects of ketamine in ACTH-treated rats.
In conclusion, the present results demonstrated that ketamine exerted antidepressant-like effects via the medial prefrontal cortex in both control and ACTH-treated rats. Ketamine might have important effects on depression that are mediated through 5-HT1A receptors in the medial prefrontal cortex.
Acknowledgments. We thank Ms. Hiroko Nakamura for her assis-tance with the animal experiments.
References
1. Carroll BJ, Curtis GC and Mendels J: Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Arch Gen Psychiatry (1976) 33: 1051-1058.
2. Kitamura Y, Araki H and Gomita Y: Influence of ACTH on the effects of imipramine, desipramine and lithium on duration of immobility of rats in the forced swim test. Pharmacol Biochem Behav (2002) 71: 63-69.
3. Walker AJ, Foley BM, Sutor SL, McGillivray JA, Frye MA and Tye SJ: Peripheral proinflammatory markers associated with ket-amine response in a preclinical model of antidepressant-resistance. Behav Brain Res (2015) 293: 198-202.
4. Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS and Krystal JH: Antidepressant effects of ketamine in depressed patients. Biol Psychiatry (2000) 47: 351-354.
5. Aan Het Rot M, Zarate CA, Jr., Charney DS and Mathew SJ: Ketamine for depression: where do we go from here? Biol Psychiatry (2012) 72: 537-547.
6. Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS and Manji HK: A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry (2006) 63: 856-864.
7. Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K and Chaki S: Antidepressant Potential of (R)-Ketamine in Rodent Models: Comparison with (S)-Ketamine. J Pharmacol Exp Ther (2017) 361: 9-16.
8. Viana GSB, Xavier CC, do Vale EM, Lopes MJP, Alves VJ, Costa RO and Neves KRT: The monoaminergic pathways and inhi-bition of monoamine transporters interfere with the antidepressive- like behavior of ketamine. IBRO Rep (2018) 4: 7-13.
9. Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R and Leonardo ED: 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron (2010) 65: 40-52.
10. Kishi T, Meltzer HY, Matsuda Y and Iwata N: Azapirone 5-HT1A
receptor partial agonist treatment for major depressive disorder: systematic review and meta-analysis. Psychol Med (2014) 44: 2255-2269.
11. Kitamura Y, Araki H, Shibata K, Gomita Y and Tanizaki Y: 5-HT(1A) receptor full agonist, 8-OH-DPAT, exerts antidepressant-like effects in the forced swim test in ACTH-treated rats. Eur J Pharmacol (2003) 481: 75-77.
12. Fukumoto K, Iijima M, Funakoshi T and Chaki S: Role of 5-HT1A Receptor Stimulation in the Medial Prefrontal Cortex in the Sustained Antidepressant Effects of Ketamine. Int J Neuropsy-chopharmacol (2018) 21: 371-381.
13. Fukumoto K, Iijima M and Chaki S: Serotonin-1A receptor stimula-tion mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) (2014) 231: 2291-2298. 14. Inoue T, Koyama T and Yamashita I: Effect of conditioned fear
stress on serotonin metabolism in the rat brain. Pharmacol Biochem Behav (1993) 44: 371-374.
15. Nishitani N, Nagayasu K, Asaoka N, Yamashiro M, Shirakawa H, Nakagawa T and Kaneko S: Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int J Neuropsychopharmacol (2014) 17: 1321-1326.
16. Pham TH, Mendez-David I, Defaix C, Guiard BP, Tritschler L, David DJ and Gardier AM: Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology (2017) 112: 198-209. 17. Fukumoto K, Iijima M and Chaki S: The Antidepressant Effects of
an mGlu2/3 Receptor Antagonist and Ketamine Require AMPA Receptor Stimulation in the mPFC and Subsequent Activation of the 5-HT Neurons in the DRN. Neuropsychopharmacology (2016) 41: 1046-1056.
18. Porsolt RD, Anton G, Blavet N and Jalfre M: Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol (1978) 47: 379-391.
19. Fukumoto K, Iijima M, Funakoshi T and Chaki S: 5-HT1A recep-tor stimulation in the medial prefrontal cortex mediates the antide-pressant effects of mGlu2/3 receptor antagonist in mice. Neuro-pharmacology (2018) 137: 96-103.
20. Chaki S, Ago Y, Palucha-Paniewiera A, Matrisciano F and Pilc A: mGlu2/3 and mGlu5 receptors: potential targets for novel antide-pressants. Neuropharmacology (2013) 66: 40-52.
21. Dong C, Zhang JC, Yao W, Ren Q, Ma M, Yang C, Chaki S and Hashimoto K: Rapid and Sustained Antidepressant Action of the mGlu2/3 Receptor Antagonist MGS0039 in the Social Defeat Stress Model: Comparison with Ketamine. Int J Neuropsycho-pharmacol (2017) 20: 228-236.
22. Dwyer JM, Lepack AE and Duman RS: mGluR2/3 blockade pro-duces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J Mol Psychiatry (2013) 1: 15.
23. Ago Y, Yano K, Araki R, Hiramatsu N, Kita Y, Kawasaki T, Onoe H, Chaki S, Nakazato A, Hashimoto H, Baba A, Takuma K and Matsuda T: Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology (2013) 65: 29-38.