Possible Involvement of a bZIP Protein in the
Repression of ABI-/VP+-mediated Chymotrypsin
Inhibitor Gene Expression at the Late-seed Maturation
in Winged Bean
By
Yoichi S
AKATA*, Tomomasa K
UWANO**, Sachiko T
AKAOKA***, Noriko T
AKASHIMA****,
Teruaki T
AJI*, Hiroshi T
AKENAGA***** and Shigeo T
ANAKA*
῍Received May ,0, ,**0/Accepted July +-, ,**0῎Summary : ABI-/VP+ is an important transcription factor, which regulates the expression of many kinds of genes during plant embryo maturation via ABA responsive elements or conserved RY
re-peats. The RY repeats are frequently found in /῍ upstream regions of seed-specific genes. Through an
extensive study of temporally- and spatially-regulated expression of a winged bean chymotrypsin in-hibitor (WCI) gene, we have demonstrated that the RY repeat is necessary but not su$cient for the seed-specific expression. In this study, we have cloned cDNAs encoding an ABI-/VP+ like factor (WbABI-) and a bZIP DNA binding protein from winged bean to investigate the participation of ABI-/ VP+ and bZIP-type transcription factors in WCI gene expression. The deduced protein sequence of the bZIP protein (WbZIP+) was highly homologous to ROM,, which is shown to be a repressor against ABI-/ VP+-activated transcription of MAT class genes during late-seed maturation in French bean. Bacterial recombinant WbZIP+ protein was prepared and tested in a gel mobility shift assay to verify its bind-ing to the promoter region of the WCI-- gene, which encodes a major WCI protein of wbind-inged bean. The recombinant WbZIP+ protein proved to show a high a$nity for specific fragments containing /῍-ACGT--῍ sequences from the WCI-- promoter. Enhanced expression of the WbZIP+ gene was observed during late-stage seed maturation after the transient expression of WCI-- and WbABI- in mid-stage seed maturation. These results suggest that WbABI- and WbZIP+ may function antagonisitically to tune the level of WCI gene expression from mid- through late-stage seed maturation in winged bean. Key words : ABI-/VP+, bZIP protein, chymotrypsin inhibitor, temporal gene regulation, winged bean
ῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌῌ
Introduction
Legumes are known as one of the foremost crops in the world because of their striking ability to accumu-late large amounts of proteins in the seeds. Molecular studies on this special ability of legumes to store pro-teins will a#ord a better understanding of production and accumulation of proteins in plants, contributing
toward the development of agriculture. A legume,
winged bean [Psophocarpus tetragonolobus L. (DC.)], was found to accumulate a protein, chymotrypsin inhibitor
(WCI), largely in the storage organs such as seeds and tuberous roots, although a small amount of WCI is de-tectable in the stem+῎
. In addition, accumulation of WCI was observed only during mid- to late- stage
matura-tion of the seed development+῎
. To elucidate how stor-age organ-specific or maturation-specific genes are regu-lated in legumes, we have investigated the unique ex-pression of the WCI gene in detail. As a result, we have shown that transcriptional regulation is an important step for the seed-specific expression of the WCI-- gene which encodes a major WCI protein, and that the
pro-論 文 Articles * ** *** **** *****
Department of Bioscience, Faculty of Applied Bio-Science, Tokyo University of Agriculture FUSHIMI Phamaceutical Co., Ltd.
Fuji Pharma Co., Ltd.
Gunma Prefectural Isesaki Koyo High School Professor Emeritus, Tokyo University of Agriculture
moter resides within +.* kbp of the /ῌ upstream region of the WCI-- gene,, -ῌ
.
Arabidopsis seeds of abi- mutants and maize seeds of vp+ mutants show deficiencies in normal induction of maturation (MAT) and late embryogenesis abundant protein (LEA) genes, resulting in reduction of storage
protein accumulation.ῌ1ῌ
. These mutant seeds are also
non-dormant and lack desiccation tolerance., 0ῌ
. Molec-ular cloning of ABI- and VP+ genes revealed both genes were orthologous and encoded transcription regula-tors1, 2ῌ
. Ectopic expression of ABI- gene can induce
several seed-specific genes in Arabidopsis leaves upon
treatment with ABA/ῌ
. Therefore ABI-/VP+ had been believed to be one of the most important regulators of seed maturation in dicots and monocots, respectively. Although the ingenious mechanism by which ABI-/VP+ factor regulates the transcription of MAT and LEA genes is still unclear, at least two independent
cis-acting elements have been identified3, +*ῌ
. One is the
ACGT-containing ABA responsive element (ABRE) for
ABA-dependent transactivation by ABI-/VP+. The
other is the sequence /ῌ-CATGCATG--ῌ, known as the
RY repeat conserved in the /ῌ upstream region of
seed-specific genes++ῌ
. Unlike the ABRE, the RY repeat is essential for ABA-independent transactivation by ABI-/VP++*, +,ῌ
. Our transgenic experiments
demon-strated that the RY repeat was responsible for the increased expression of the WCI-- gene in the develop-ing seeds-ῌ
, suggesting that the WCI-- gene expression might be regulated by ABI-/VP+ like factor via the RY repeat in winged bean.
The RY repeat was necessary but not su$cient for
the activation of the WCI-- gene promoter-ῌ
, indicating the participation of other factor(s) for the full activa-tion of WCI-- gene in seeds. In this point, it is notewor-thy that involvement of basic leucine zipper (bZIP) proteins in the regulation of seed-specific gene expres-sion has been demonstrated in several plants, including
Opaque, for ,,-kDa zein genes in maize+-ῌ
, SPA for
pro-lamin genes in wheat+.ῌ
, and ROM+ and ROM, for the
b-phaseolin gene in French bean+/, +0ῌ
. Recently it has been reported that Opaque,-related bZIP proteins syn-ergistically activate seed storage protein genes with
ABI- in Arabidopsis developing seeds+1ῌ
. Moreover, a rice bZIP type protein, TRAB+, was reported to interact physically with rice ABI-/VP+ factor, binding to the
ABRE in the promoter of a LEA gene+2ῌ
. Also it has been ascertained that Arabidopsis ABI/, a regulator of certain LEA gene expression during seed maturation,
is a member of the TRAB+-type bZIP family+3ῌ,+ῌ
. The
WCI-- gene promoter has several /ῌ-ACGT--ῌ core motifs,
possible binding sites of plant bZIP proteins,,ῌ
,
suggest-ing that a bZIP protein may be involved in the tran-scription regulation of the WCI gene. These facts led us to investigate the relationship between the ABI-/VP+ factor and bZIP protein with regard to the mechanism of WCI-- gene regulation in winged bean.
Here we report the cloning and characterization of cDNAs encoding an ABI-/VP+ like factor and a bZIP protein from winged bean. We also discuss a general gene regulation mechanism during legume seed devel-opment, in which mid-stage maturation specific gene activation by ABI-/VP+ is repressed at late-stage mat-uration by the expression of bZIP transcription factor.
Materials and Methods
Cloning of WbABI- and WbZIP+
Total RNA was isolated from seeds of winged bean
-/days after flowering (DAF) by acid
guanidium-phenol-chloroform method,-ῌ
. Poly(A)῍RNA was selected by
Poly(A) Tract (Promega, USA) according to the instruc-tion manual. Poly(A)῍RNA (/** ng) was subjected to the first strand cDNA synthesis. RNA was denatured
at 0/῎ for +* min, annealed with Oligo (dT)-P1 primer
(TOYOBO, Japan) at -*῎ for +* min. The first strand
was synthesized at .,῎ for , hr in a bu#er supplied with M-MLV RTase (TOYOBO, Japan). The cDNA was purified by repeating a combination of dilution in .** ml of TE bu#er (+* mM Tris-HCl pH 2.*, + mM EDTA) and filtration through a Suprec-*, (Takara Shuzo, Japan) four times. The cDNA was diluted to a final volume of
/*ml with TE, and subjected to the -ῌ rapid
amplific-ation of cDNA ends (-ῌRACE),.ῌ
.
Conserved amino acid sequences in the B- domain of ABI-/VP+ (MEDIGTSRVWNMRY) and bZIP region
(RK[Q/E/L]SNRESARR) were used for designing
ABI-/VP+- and bZIP protein-specific degenerate primers, respectively. The sequences of primers used are ALF+ (/ῌ-ATG GAR GAY ATH GGN AC--ῌ) and ALF, (/ῌ-GTN
TGG AAY ATG MGN TA--ῌ) for ABI-/VP+, and ZIP+
(/ῌ-WSI AAY MGI GAR WSY GC--ῌ) and ZIP, (/ῌ-GAR
WSI GCI MGI WSI MG--ῌ) for the bZIP protein. Ampli-fication was carried out by two nested PCR protocols
using one specific adapter primer (P1 ; /ῌ-CGC CAG GGT
TTT CCC AGT CAC GA--ῌ) and two degenerate primers.
Primary PCR was performed with + ml of cDNA, ,** mM dNTP, , mM ALF+ or ZIP+ primer, *., mM P1 adapter primer and /U rTaq (Takara Shuzo, Japan) in a bu#er supplied with rTaq. The reaction was first set up with-out P1 primer, and the second strand was extended at
3.῎ for . min, ./῎ for / min, and 1, ῎ for ,* min. After
adding P1 adapter primer, PCR was repeated for -/
cy-cles of 3.῎ for + min, ./῎ for , min, and 1,῎ for , min.
TE by Suprec-*, as mentioned above. The secondary PCR was carried out with ALF, or ZIP, primer and P1 primer using + ml of the primary PCR product as a temp-late under the same condition except annealing tem-perature of .0῎. The PCR products were cloned by
pPCR-script Amp SK(῍) cloning kit (Stratagene, USA)
according to the instruction manual.
Several pools of the amplified cDNA library, con-structed in lambdaZAPII (Stratagene, USA), were
sub-jected to PCR using primers corresponding to the /῍
end sequence of the partial cDNA clone and pBlue-script sequencing SK primer to amplify the /῍ up-stream region of the cDNA clone. The PCR products were separated by agarose gel electrophoresis, the li-brary pool containing the longest amplified DNA frag-ment was used for infection of E. coli strain XL-+ Blue MRF’, and one-tenth titer of original pool was plated on each plate to make +* plates for next screening. The lysate fractions from these plates were subjected to PCR again. This procedure was repeated until the num-ber of phage plaques was reduced to /**. The final
phage pool was subjected to plaque hybridization,/ῌ
using partial cDNAs as probes. The cDNA inserts were excised from positive phage clones by the ExAssist Helper/SOLR system (Stratagene, USA).
DNA sequence analysis
Sequence analyses of cloned DNA fragments were per-formed by dideoxy sequencing using the AmpliTaq FS sequencing kit (Perkin Elmer, USA) and an ABI -1-S autosequencer (Perkin Elmer, USA). Homology search
of the sequence was done by BLAST program,0ῌ
. Align-ments of amino acid sequences are performed by
T-co#ee program,1ῌ
Southern- and RNA- gel blot analysis
Total DNA was prepared from leaves of winged bean
using cetyltrimethyl ammonium bromide as described,2ῌ
and purified by CsCl density gradient ultracentrifuga-tion,/ῌ
. Ten mg of DNA was digested by restriction en-zymes, resolved by agarose gel electrophoresis, and blotted onto Zeta probe nylon membrane (Bio-Rad,
USA). The membrane blot was hybridized with the
WbABI- or WbZIP+ cDNA labeled with [a--,P]dCTP by
Random Prime labeling kit (Amersham, USA) in a bu#er
containing /*ῌ (V/V) formamide, 1,* mM NaCl, .* mM
phosphate bu#er (pH 1..), . mM EDTA, *.+ῌ Ficol, *.+ῌ
BSA, *.+ῌ polyvinylpyrrolidone, +ῌ SDS, and +** mg/ ml denatured salmon sperm DNA at .,῎ for +0ῌ,. hr. After hybridization, the membrane was subjected to subsequent wash in ,xSSC (+xSSC ; +/* mM NaCl, +/ mM trisodium citrate) containing *.+ῌ SDS for -* min
at room temperature, *.+xSSC containing *.+ῌ SDS for
-*min at room temperature, and *.+xSSC containing
*.+ῌ SDS for -* min at //῎.
Total RNA was isolated from leaves and seeds of winged bean by acid guanidium-phenol-chloroform method,-ῌ
. Each total RNA (-* mg) was separated by
formaldehyde-denaturing agarose gel electrophoresis, and transferred onto GeneScreenPlus (DuPont, USA). Prehybridization was performed in a bu#er containing
/*ῌ formamide, +ῌ SDS, +*ῌ dextransulfate, +M NaCl,
and +** mg/ml denatured salmon sperm DNA at .,῎
for , hr. After prehybridization, P-,
-labeled cDNA was added to the prehybridization solution to perform
hy-bridization at .,῎ for +0ῌ,. hr. The membrane was
washed once in ,xSSC for / min at room temperature, twice in ,xSSC containing +ῌSDS for -* min at 0*῎, and once in *.+xSSC for -* min at room temperature. The membranes were analyzed by BAS-*** (Fuji Photo Film, Japan).
Gel mobility shift assay
A cDNA fragment from῍13+ to poly(A)
correspond-ing to the C-terminal region of WbZIP+ was amplified
by PCR. The amplified fragment was sequenced to
confirm accurate amplification and subcloned into
pET-+/b vector (Novagen, Germany). This construct was
used to transform Escherichia coli strain BL,+ (DE-)/ pLysS. The transformant was pre-cultured overnight in LB medium containing /* mg/ml ampicillin at ,/῎. The culture was diluted ten-fold with the fresh LB
medium and cultured for + hr at ,/῎, then IPTG was
added to a final concentration of + mM to induce the production of the recombinant WbZIP+ protein. After
a --hr culture at ,/῎, cells were harvested, resuspended
in ice-cold sonication bu#er (,* mM sodium phosphate,
+*mM imidazol, /** mM NaCl, pH 1..), and sonicated.
The cell debris was spun down, and the supernatant was applied to a HisTrap Chelating column (Amersham Pharmacia Biotech, USA), according to the manufac-ture’s instruction. WbZIP+ protein was eluted from the column with -** mM imidazol. The purity of recom-binant WbZIP+ was confirmed as a single band by Coomassie blue staining after SDS-PAGE. The binding reaction was carried out in a solution containing , mM sodium phosphate, /* mM NaCl, *.*,/ῌ (W/V) BSA and
,/mg/ml poly(dI-dC) : poly(dI-dC) with -fmol of P-,
-labeled probe. The protein-DNA complex was
sepa-rated by -.0ῌ polyacrylamide gel in *./x Tris-borate bu#er (.../ mM Tris-base, .../ mM boric acid, +.+ mM EDTA). After electrophoresis, the gel was dried and analyzed by BAS-***.
Results and Discussion
Cloning of winged bean ABI-/VP+-like factor WbABI-To gain a clearer understanding of transcription reg-ulation of the WCI-- gene via the RY repeat, we searched for a cDNA encoding the winged bean ortholog of
ABI-/VP+. The cloning was performed using a -῍ RACE
method with cDNAs as the templates prepared from mid-maturation stage seeds of winged bean. The de-generate primers were designed from the conserved amino acid sequence in B- domain of ABI-/VP+, which
was reported to be the RY repeat binding domain,3ῌ
. After the secondary -῍ RACE, we detected a DNA frag-ment of appropriate size that was about /** bp in length (data not shown). Sequencing analysis and homology search of this fragment convinced us that the fragment is a partial sequence of a novel winged bean ABI-/VP+ like factor (WbABI- ; accession number AB+0..,0). To obtain the whole sequence information of WbABI- gene, the cDNA library was screened by plaque hybridiza-tion using the WbABI- partial sequence as a probe. The longest cDNA clone was ,/,/ bp in length and encoded 1/+ amino acids as the open reading frame
started with an ATG (Fig. +A). In comparison with
known ABI-/VP+ factors from various plant species, WbABI- presented the highest similarity to PvALF, a
French bean ABI-/VP+ like factor-*ῌ
. WbABI- was iden-tical to PvALF at an extremely high level of 2*ῌ through the entire amino acid sequence, while the
identities with ABI- and VP+ were .3ῌ and .*ῌ,
re-spectively. Especially, extensive homology was
ob-served between the B- domain of WbABI- and those of PvALF, ABI- and VP+ at 31ῌ, 3.ῌ and 22ῌ, respec-tively. It is noteworthy that the conserved domains of WbABI- are almost identical to that of PvALF (Fig. +B), which has proved to transactivate seed-specific gene
promoters via the RY repeats-+ῌ
. These results strongly suggested that WbABI- is an ABI-/VP+ ortholog that regulates the transcription activity of seed maturation specific WCI gene, via the RY repeat in winged bean. A winged bean bZIP protein WbZIP+ belongs to a repressor type bZIP family
To isolate cDNA clones encoding bZIP proteins ex-pressed in developing seeds of winged bean, we used , sets of degenerate primers corresponding to a highly conserved basic DNA binding domain in N-terminal side of the leucine zipper region of plant bZIP proteins for -῍ RACE method. Although various species of DNA fragments were amplified in the primary PCR, three DNA fragments of *.2, +.* and +.+ kbp, were specifically amplified in the secondary PCR (data not shown). These
fragments were cloned and sequenced. The protein
encoded by the *.2 kbp fragment started with ESARR which is a part of the conserved amino acid sequence, suggesting that the *.2 kb fragment encodes a bZIP protein. We isolated a longer clone of the cDNA from the winged bean cDNA library using the *.2 kb frag-ment as a probe. This cDNA was +.0 kb in length, and the longest open reading frame started with ATG,
sug-Fig. + The deduced amino acid sequence of WbABI-.
(A) An N-terminal serine domain and three basic domains (B+-B-) conserved in ABI-/VP+ like factors are shaded and underlined,
respec-tively. (B) Sequence comparison of the
con-served domains between WbABI-, PvALF (French bean), ABI- (Arabidopsis) and VP+ (maize). Align-ments were performed by T-co#ee program. The numbers of identical amino acids/total amino acids in each domain of PvALF, ABI-and VP+ to WbABI- are indicated.
gesting that it encodes the total sequence of the protein (Fig. ,). The cDNA encoded .,. amino acids, and the expected molecular mass is ./kDa. A basic region and a leucine zipper domain characteristic of bZIP protein are well conserved in the deduced amino acid sequence. The zipper region of the winged bean bZIP protein (WbZIP+ ; accession number AB+0..,1) contains five leu-cines and one methionine (Fig. ,). The homology search by BLAST revealed that the deduced amino acid se-quence encoded by the clone is highly homologous to French bean bZIP protein ROM,, which is reported to repress a PvALF-dependent activation of MAT genes
in late-maturation stage seeds of French bean+/ῌ
. Be-cause the amino acid identity between the winged bean bZIP protein and ROM, is 23ῌ through their entire pro-tein sequence (data not shown), and only three amino acids are di#erent from ROM, in the basic zipper re-gion (located between ,11 and -.- in Fig. ,), WbZIP+ might be orthologous with the repressor protein ROM,. Gene organization of WbABI- and WbZIP+ in winged bean genome
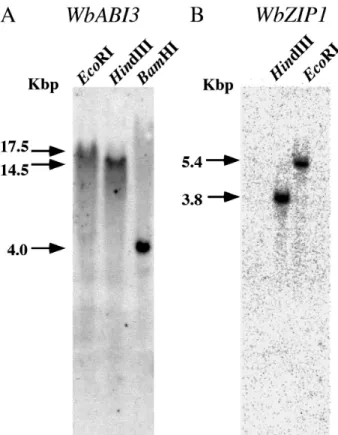
Southern blot analysis of winged bean DNA digested with EcoRI, HindIII or BamHI using the WbABI- cDNA as a probe resulted in single bands, indicated WbABI-is encoded by a single copy gene (Fig. -A). As far as we know, genes encoding ABI-/VP+ and their homologues in angiosperms are single copy in their genomes. This pattern is also conserved in winged bean.
The gene organization of WbZIP+ was also predicted as a single copy like those of ROM, in French bean, because only a single band was detected when EcoRI-or HindIII-digested DNA was probed by WbZIP+ cDNA (Fig. -B).
Binding ability of WbZIP+ to the WCI gene promoter Because WbZIP+ was cloned by the homology-based strategy, it was unclear whether WbZIP+ is involved in the WCI-- gene regulation. To address the question whether WbZIP+ is a candidate for a transcription factor of the WCI-- gene, we investigated the binding ability of WbZIP+ to the WCI-- gene promoter. The His-tagged C-terminal region including the bZIP do-main of WbZIP+ was expressed in E. coli, and purified by a nickel column. The purified protein was used for
gel mobility shift assays. Although the WCI-- gene
promoter contains several /῍-ACGT--῍ core motifs (Fig.
.A), these motifs do not exactly match with the
pro-posed binding motif of ROM,, which is /῍-GCCACG/
CTCAG/AYY--῍+/ῌ
. Therefore, the region between῍22,
and ῍3+ relative to the transcription initiation site
of the WCI-- gene, which was shown to contain
cis-Fig. , The deduced amino acid sequence of WbZIP+.
The basic region and the leucine zipper re-gion are underlined and boxed, respectively. The leucine residues in the zipper region are marked with dots. The fifth zipper is not leu-cine but methionine. Three amino acids which are di#erent from those of ROM, in the basic-and zipper region are shown by double under-line.
Fig. - Southern blot analysis of winged bean DNA
with WbABI- and WbZIP+ probe. Ten mg of winged bean DNA was digested with EcoRI, HindIII or BamHI for WbABI-, and HindIII or EcoRI for WbZIP+ probe. The digested DNAs were separated by electrophoresis. DNA frag-ments were transferred onto a nylon mem-brane and the filter was subjected to hybridi-zation using the labeled WbABI- cDNA or
WbZIP+ cDNA probes. The estimated
frag-ment length of the signals is shown on the left side.
elements for transcription in seeds-ῌ
, was divided into four segments (DNA fragments I-IV, Fig. .A), and these were used as probes for gel mobility shift assay. The labeled DNA fragments were separately incubated with WbZIP+ protein and analyzed by polyacrylamide gel
electrophoresis (Fig. .B). When the amount of WbZIP+ protein was increased, retarded bands were observed in each probe, however, the DNA fragments I and II seemed to have higher binding a$nity to the WbZIP+. To con-firm this, we performed a competition assay in which
+**or ,** fold-molar excess of each unlabeled fragment
(I, II, II, or IV) was added to the binding mixture of the labeled fragment II and WbZIP+ (Fig. .C). Competition e#ect was observed when the fragment I or II was used as a competitor, whereas the fragments III and IV had no competition e#ect even at ,** fold-molar excess. These results indicated that WbZIP+ binds to the
WCI--gene promoter in a sequence-specific manner. This
also agreed well with the fact that the putative binding sequences (/ῌ-ACGT--ῌ) were observed only in the frag-ments I and II (Fig. .A).
Expression analysis of WbABI-, WbZIP+ and WCI Since the B- domain of WbABI- is almost the same as that of PvALF which activates MAT gene promoters via the RY repeats, and WbZIP+ was also shown to bind to the promoter region of WCI-- gene, it is sug-gested that WbABI- and WbZIP+ are involved in the
Fig. . DNA binding activity of WbZIP+ to the
WCI--gene promoter. A) Schematic representation
of the WCI-- gene promoter from -22, to -3+ used in the study. The fragments I-IV from -22, to -0,- (I), -0,, to --11 (II), --10 to -+3. (III), and -+3- to -3+ (IV) were generated by PCR. Numbers are shown relative to the
transcrip-tion initiatranscrip-tion site (shown by arrow). ACGT
motifs are shown by asterisks. The RY
se-quence in the region II is shown by a filled
tri-angle. B) Gel mobility shift assay with P-,
-labeled DNA fragments (I-IV) of the WCI-- moter region and recombinant WbZIP+
pro-tein. - ; no protein, ῌ and ῌῌ ; / and +* ng
of recombinant protein. The bound bands are
indicated by open triangles. C) Competition
experiments of gel mobility shift assay using the DNA fragment II as a labeled probe. Each DNA fragment was added to the binding reac-tion mixture in +**- or ,**-molar excess to the probe.
Fig. / RNA gel blot analyses of WbABI-, WbZIP+ and
WCI-- mRNA expression during the seed devel-opment. Total RNAs were isolated from seeds at various developing stages. Ten mg of each RNA was separated by electrophoresis in a de-naturing agarose gel and transferred onto a nylon membrane. The filter was subjected to the hybridization experiment with the labeled WbABI-, WbZIP+ or WCI-- cDNA. An ethidium bromide-stained gel image of rRNA was shown as a standard of comparison with the RNA amount loaded in each lane. DAF ; days after flowering.
regulation of WCI-- gene expression during the seed development of winged bean. To gain a deeper insight into the roles of WbABI- and WbZIP+, we examined their expression pattern in the developing seeds of winged bean (Fig. /). We reported in the previous pa-per+ῌ
that the transcription activation of WCI-- gene occurred transiently at the mid-maturation stage of seeds. To put it more precisely, transcripts of the
WCI--gene started to accumulate at -* days after flowering
(DAF) and peaked in .* DAF, then decreased to be a negligible amount at 0* DAF. Taking these results into account, we collected RNA samples for RNA blot anal-yses from seeds at the early-maturation stage (,/ DAF), mid-maturation stage (-/ DAF), late-maturation stage (/* DAF) and matured stage (1* DAF). The RNA blot analyses were performed with labeled cDNAs of WbABI-, WbZIP+ and WCI-- as probes. As was observed in the previous report+ῌ
, the transient accumulation of WCI mRNA took place at -/ DAF, followed by a great reduc-tion at /* DAF. On the other hand, WbABI- mRNA was expressed from -/ DAF to /* DAF at a high level. This agrees very well with previous reports that ABI-/VP+ regulates not only MAT genes expressed transiently at the mid-maturation stage, but also LEA genes ex-pressed at the late-maturation stage.ῌ0, -,ῌ-.ῌ
.
Interest-ingly, enhanced accumulation of the WbZIP+ mRNA was observed at /* DAF, although weak accumulation was detected in the earlier stage of ,/ DAF when the WCI and WbABI- mRNAs were undetectable. Thus, the study on mRNA expression also supports the prob-able repression of WbABI--mediated activation of WCI--gene by WbZIP+ at the late-maturation stage.
A bZIP protein named EEL has been reported in Arabidopsis to repress the transcriptional activity of AtEm+ gene by competing a critical bZIP factor ABI/, that is essential to recruit ABI- to the promoter, for the ABRE-/ῌ
. In the seeds of Arabidopsis, the maximum
level of EEL expression was observed at the mid-matu-ration stage, suggesting that it prevents the precocious activation of the AtEm+ gene by ABI-.
From the data showed here and the results from
French bean PvALF-*ῌ
and ROM,+/ῌ
, it seems to be a general system in legumes in which ROM,-related bZIP protein family actively represses the MAT genes ac-tivation by ABI-/VP+ at the late-stage maturation seeds. Plants seem to have worked out a strategy for utilizing bZIP transcription factors as repressors, as well as ac-tivators, to tune the level of ABI-/VP+-dependent gene expression during seed maturation. It will be interest-ing from the view of protein production in legumes whether disruption of target sequence of ROM, family or repression of these bZIP proteins by RNAi will
in-crease the accumulation of storage protein in seeds. FUS- and LEC,, other seed developmental regulators in Arabidopsis, have been shown also to encode B- do-main transcription factors-0, -1ῌ
. FUS- proved to inter-act directly with the RY repeat of legumin gene pro-moter. Furthermore, FUS- can activate the promoter
in concert with ABI- via the RY repeat-2ῌ
. These data suggest that ABI-/VP+ is not an exclusive factor for RY repeat-mediated gene regulation. Further cloning and analysis of B- domain transcription factors, such as FUS- and LEC,, from winged bean would reveal the detail of regulation mechanism in WCI gene expression during the seed development.
Acknowledgements
We thank Dr. Gregg CLARK(Washington University)
for his critical reading of the manuscript. We also thank
Dr. Shunsuke YAJIMA (Tokyo University of
Agricul-ture) for his advice on the production of bacterial pro-tein.
References
+ῌ HABU, Y., PEYACHOKNAGUL, S., UMEMOTO, K., SAKATA, Y.
and OHNO, T. +33,. Structure and regulated expression of Kunitz chymotrypsin inhibitor genes in winged bean [Psophocarpus tetragonolobus (L.) DC.]. J Biochem (Tokyo) +++, ,.3ῌ,/2.
,ῌ SAKATA, Y., FUKUSHIMA, H., HABU, Y., FURUYA, S., NAITO, S. and OHNO, T. +33.. Transcriptional activities of a winged
bean Kunitz chymotrypsin inhibitor gene promoter in stable and transient expression systems. Biosci Biotech-nol Biochem /2, ,+*.ῌ,+*0.
-ῌ SAKATA, Y., CHIBA, Y., FUKUSHIMA, H., MATSUBARA, N.,
HABU, Y., NAITO, S. and OHNO, T. +331. The RY sequence is necessary but not su$cient for the transcription ac-tivation of a winged bean chymotrypsin inhibitor gene in developing seeds. Plant Mol Biol -., +3+ῌ+31. .ῌ NAMBARA, E., KEITH, K., MCCOURT, P. and NAITO, S. +33/. A
regulatory role for the ABI- gene in the establishment of embryo maturation in Arabidopsis thaliana. Develop-ment +,+, 0,3ῌ0-0.
/ῌ PARCY, F., VALON, C., RAYNAL, M., GAUBIER-COMELLA, P., DELSENY, M. and GIRAUDAT, J. +33.. Regulation of gene
expression programs during Arabidopsis seed develop-ment : roles of the ABI- locus and of endogenous ab-scisic acid. Plant Cell 0, +/01ῌ+/2,.
0ῌ MCCARTY, D.R., CARSON, C.B., STINARD, P.S. and R OBERT-SON, D.S. +323. Molecular analysis of viviparous-+ : An abscisic acid-insensitive mutant of maize. Plant Cell +, /,-ῌ/-,.
1ῌ MCCARTY, D.R., HATTORI, T., CARSON, C.B., VASIL, V., LAZAR,
M. and VASIL, I.K. +33+. The Viviparous-+ developmental gene of maize encodes a novel transcriptional activator. Cell 00, 23/ῌ3*/.
2ῌ GIRAUDAT, J., HAUGE, B.M., VALON, C., SMALLE, J., PARCY,
F. and GOODMAN, H.M. +33,. Isolation of the Arabidopsis ABI- gene by positional cloning. Plant Cell ., +,/+ῌ+,0+. 3ῌ EZCURRA, I., ELLERSTROM, M., WYCLIFFE, P., STALBERG, K. Chymotrypsin Inhibitor Gene Expression at the Late-seed Maturation in Winged Bean 119
and RASK, L. +333. Interaction between composite ele-ments in the napA promoter : both the B-box ABA-responsive complex and the RY/G complex are neces-sary for seed-specific expression. Plant Mol Biol .*, 033ῌ 1*3.
+*ῌ EZCURRA, I., WYCLIFFE, P., NEHLIN, L., ELLERSTROM, M. and RASK, L. ,***. Transactivation of the Brassica napus napin promoter by ABI- requires interaction of the conserved B, and B- domains of ABI- with di#erent cis-elements : B, mediates activation through an ABRE, whereas B- interacts with an RY/G-box. Plant J ,., /1ῌ 00.
++ῌ DICKINSON, C.D., EVANS, R.P. and NIELSEN, N.C. +322. RY repeats are conserved in the /’-flanking regions of leg-ume seed-protein genes. Nucleic Acids Res +0, -1+. +,ῌ HATTORI, T., VASIL, V., ROSENKRANS, L., HANNAH, L.C., M
C-CARTY, D.R. and VASIL, I.K. +33,. The Viviparous-+ gene and abscisic acid activate the C+ regulatory gene for anthocyanin biosynthesis during seed maturation in maize. Genes Dev 0, 0*3ῌ0+2.
+-ῌ SCHMIDT, R. J., KETUDAT, M., AUKERMAN, M. J. and HOSCHEK, G. +33,. Opaque-, is a transcriptional activator that rec-ognizes a specific target site in ,,-kD zein genes. Plant Cell ., 023ῌ1**.
+.ῌ ALBANI, D., HAMMOND-KOSACK, M.C., SMITH, C., CONLAN, S., COLOT, V., HOLDSWORTH, M., and BEVAN, M.W. +331. The wheat transcriptional activator SPA : a seed-specific bZIP protein that recognizes the GCN.-like motif in the bifactorial endosperm box of prolamin genes. Plant Cell 3, +1+ῌ+2..
+/ῌ CHERN, M.S., BOBB, A. J. and BUSTOS, M.M. +330. The regu-lator of MAT, (ROM,) protein binds to early maturation promoters and represses PvALF-activated transcrip-tion. Plant Cell 2, -*/ῌ-,+.
+0ῌ CHERN, M.S., EIBEN, H.G. and BUSTOS, M.M. +330. The de-velopmentally regulated bZIP factor ROM+ modulates transcription from lectin and storage protein genes in bean embryos. Plant J +*, +-/ῌ+.2.
+1ῌ LARA, P., ONATE-SANCHEZ, L., ABRAHAM, Z., FERRANDIZ, C., DIAZ, I., CARBONERO, P. and VICENTE-CARBAJOSA, J. ,**-. Synergistic activation of seed storage protein gene ex-pression in Arabidopsis by ABI- and two bZIPs related to OPAQUE,. J Biol Chem ,12, ,+**-ῌ,+*++.
+2ῌ HOBO, T., KOWYAMA, Y. and HATTORI, T. +333. A bZIP fac-tor, TRAB+, interacts with VP+ and mediates abscisic acid-induced transcription. Proc Natl Acad Sci U S A 30, +/-.2ῌ+/-/-.
+3ῌ FINKELSTEIN, R.R. and LYNCH, T. J. ,***. The Arabidopsis abscisic acid response gene ABI/ encodes a basic leucine zipper transcription factor. Plant Cell +,, /33ῌ0*3. ,*ῌ CASARETTO, J. and HO, T.H. ,**-. The transcription
fac-tors HvABI/ and HvVP+ are required for the abscisic acid induction of gene expression in barley aleurone cells. Plant Cell +/, ,1+ῌ,2..
,+ῌ FINKELSTEIN, R.R., GAMPALA, S.S. and ROCK, C.D. ,**,. Abscisic acid signaling in seeds and seedlings. Plant Cell +.Suppl, S+/ῌ./.
,,ῌ IZAWA, T., FOSTER, R. and CHUA, N.H. +33-. Plant bZIP protein DNA binding specificity. J Mol Biol ,-*, ++-+ῌ ++...
,-ῌ CHOMCZYNSKI, P. and SACCHI, N. +321. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem +0,, +/0ῌ
+/3.
,.ῌ FROHMAN, M.A., DUSH, M.K. and MARTIN, G.R. +322. Rapid production of full-length cDNAs from rare transcripts : amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A 2/, 2332ῌ3**,. ,/ῌ SAMBROOK, J., FRITSCH, E.F. and MANIATIS, T. +323.
Molec-ular cloning : a laboratory manual. (Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.).
,0ῌ ALTSCHUL, S.F., GISH, W., MILLER, W., MYERS, E.W. and LIPMAN, D. J. +33*. Basic local alignment search tool. J Mol Biol ,+/, .*-ῌ.+*.
,1ῌ NOTREDAME, C., HIGGINS, D.G. and HERINGA, J. ,***. T-Co#ee : A novel method for fast and accurate multiple sequence alignment. J Mol Biol -*,, ,*/ῌ,+1.
,2ῌ WILSON, Z.A. ,***. Arabidopsis : a practical approach. (Ox-ford University Press, Ox(Ox-ford ; New York).
,3ῌ SUZUKI, M., KAO, C.Y. and MCCARTY, D.R. +331. The con-served B- domain of VIVIPAROUS+ has a cooperative DNA binding activity. Plant Cell 3, 133ῌ2*1.
-*ῌ BOBB, A. J., EIBEN, H.G. and BUSTOS, M.M. +33/. PvAlf, an embryo-specific acidic transcriptional activator enhances gene expression from phaseolin and phytohemagglutinin promoters. Plant J 2, --+ῌ-.-.
-+ῌ BOBB, A. J., CHERN, M.S. and BUSTOS, M.M. +331. Conserved RY-repeats mediate transactivation of seed-specific pro-moters by the developmental regulator PvALF. Nucleic Acids Res ,/, 0.+ῌ0.1.
-,ῌ VASIL, V., MARCOTTE, W.R., JR., ROSENKRANS, L., C OCCIO-LONE, S.M., VASIL, I.K., QUATRANO, R.S. and MCCARTY, D.R. +33/. Overlap of Viviparous+ (VP+) and abscisic acid response elements in the Em promoter : G-box elements are su$cient but not necessary for VP+ transactivation. Plant Cell 1, +/++ῌ+/+2.
--ῌ SUZUKI, M., KETTERLING, M.G., LI, Q.B. and MCCARTY, D.R. ,**-. Viviparous+ alters global gene expression patterns through regulation of abscisic acid signaling. Plant Physiol +-,, +00.ῌ+011.
-.ῌ NAKASHIMA, K., FUJITA, Y., KATSURA, K., MARUYAMA, K., NARUSAKA, Y., SEKI, M., SHINOZAKI, K. and YAMAGUCHI -SHINOZAKI, K. ,**0. Transcriptional regulation of ABI--and ABA-responsive genes including RD,3B ABI--and RD,3A in seeds, germinating embryos, and seedlings of Arabi-dopsis. Plant Mol Biol 0*, /+ῌ02.
-/ῌ BENSMIHEN, S., RIPPA, S., LAMBERT, G., JUBLOT, D., PAUTOT, V., GRANIER, F., GIRAUDAT, J. and PARCY, F. ,**,. The homologous ABI/ and EEL transcription factors func-tion antagonistically to fine-tune gene expression during late embryogenesis. Plant Cell +., +-3+ῌ+.*-.
-0ῌ LUERSSEN, H., KIRIK, V., HERRMANN, P. and MISERA, S. +332. FUSCA- encodes a protein with a conserved VP+/AB+--like B- domain which is of functional importance for the regulation of seed maturation in Arabidopsis thaliana. Plant J +/, 1//ῌ10..
-1ῌ STONE, S.L., KWONG, L.W., YEE, K.M., PELLETIER, J., L EPI-NIEC, L., FISCHER, R.L., GOLDBERG, R.B. and HARADA, J. J. ,**+. LEAFY COTYLEDON, encodes a B- domain tran-scription factor that induces embryo development. Proc Natl Acad Sci U S A 32, ++2*0ῌ++2++.
-2ῌ REIDT, W., WOHLFARTH, T., ELLERSTROM, M., CZIHAL, A., TEWES, A., EZCURRA, I., RASK, L. and BAUMLEIN, H. ,***. Gene regulation during late embryogenesis : the RY motif of maturation-specific gene promoters is a direct target of the FUS- gene product. Plant J ,+, .*+ῌ.*2.
シカクマメ種子成熟後期における ABI-
ῌVP+ を
介したキモトリプシンインヒビタ
῏遺伝子の
発現抑制における bZIP 型転写因子の関与
坂田洋一*
ῌ桑野知昌**ῌ高岡幸子***ῌ高島範子****ῌ
太治輝昭*
ῌ武長 宏*****ῌ田中重雄*
ῐ平成 +2 年 / 月 ,0 日受付ῌ平成 +2 年 1 月 +- 日受理ῑ 要約 : ABI-ῌVP+ は ABA 応答配列あるいは保存された RY 配列を介して῍ 植物の種子成熟において多くの 遺伝子発現制御を行う重要な転写因子であるῌ シカクマメ Kunitz 型キモトリプシンインヒビタ῏ ῐWCIῑ 遺伝子の時期および器官特異的な遺伝子発現制御の解析から῍ 我῎は RY 配列が WCI 遺伝子の発現制御に 必須ではあるが十分ではないことを示したῌ 本研究では῍ シカクマメから ABI-ῌVP+ 様のタンパク質ῐWbABI-ῑ と bZIP 型転写因子をクロ῏ニングし῍ これらの因子が WCI 遺伝子の発現制御に関与している
か調査したῌ 推定されるアミノ酸配列の解析から῍ この bZIP 型転写因子 ῐWbZIP+ῑ は῍ インゲンマメにお ける ABI-ῌVP+ を介した種子タンパク質遺伝子の転写活性化を抑制する転写因子 ROM, と高い相同性を示 すことが明らかとなったῌ 大腸菌を用いて発現させた組換え WbZIP タンパク質を用いたゲルシフト解析か
ら῍ このタンパク質は WCI-- 遺伝子プロモ῏タ῏の /῍-ACGT--῍を含む DNA 断片に結合することが示され
たῌ ノザンブロット解析を行なったところ῍ WCI-- と WbABI- の mRNA が一過的に蓄積する種子成熟中期
の後に῍ WbZIP+ mRNA の蓄積が増えることが明らかとなったῌ これらの結果から῍ WbABI- と WbZIP+
はそれぞれ正の制御因子および負の制御因子として῍ WCI 遺伝子の種子成熟中期から後期にかけての一過 的な発現を制御していることが考えられたῌ キῌワῌド : ABI-ῌVP+, bZIP 型転写因子῍ キモトリプシンインヒビタ῏῍ シカクマメ῍ 時期特異的遺伝子発現 * ** *** **** ***** 東京農業大学応用生物科学部バイオサイエンス学科 ῐ株ῑ伏見製薬所 富士製薬工業株式会社 群馬県立伊勢崎興陽高等学校 東京農業大学名誉教授