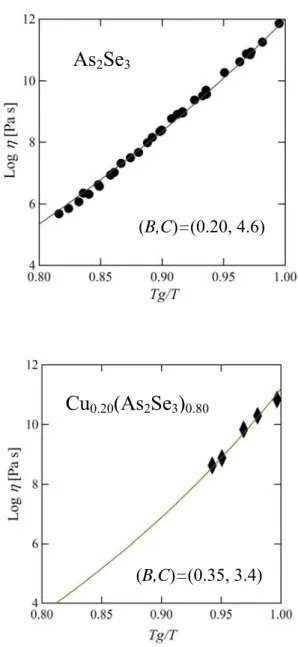
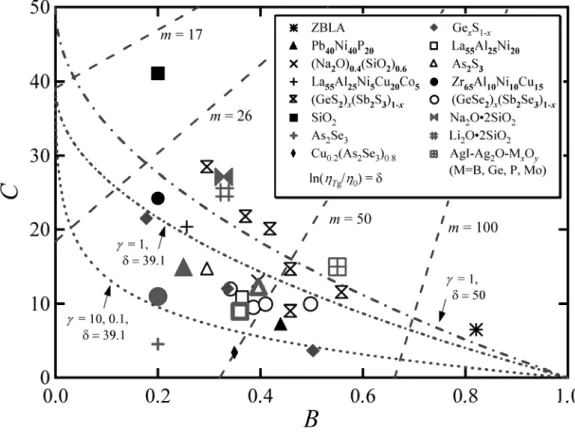
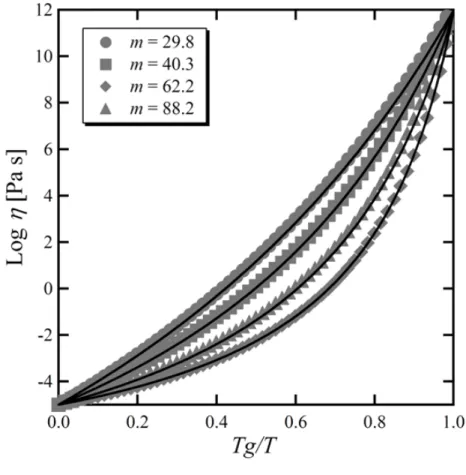
dependence of the viscosity of glass forming liquids. According to a model proposed by one of the
全文
図
![Fig. 1. Temperature dependence of the viscosity in Na 2 O-2SiO 2 and Li 2 O-2SiO 2 . The experimental data are taken from [11]](https://thumb-ap.123doks.com/thumbv2/123deta/5652832.2005719/9.892.226.662.183.761/fig-temperature-dependence-viscosity-sio-sio-experimental-taken.webp)
関連したドキュメント
It is suggested by our method that most of the quadratic algebras for all St¨ ackel equivalence classes of 3D second order quantum superintegrable systems on conformally flat
In particular, we consider a reverse Lee decomposition for the deformation gra- dient and we choose an appropriate state space in which one of the variables, characterizing the
In section 2 we present the model in its original form and establish an equivalent formulation using boundary integrals. This is then used to devise a semi-implicit algorithm
Keywords: continuous time random walk, Brownian motion, collision time, skew Young tableaux, tandem queue.. AMS 2000 Subject Classification: Primary:
Kilbas; Conditions of the existence of a classical solution of a Cauchy type problem for the diffusion equation with the Riemann-Liouville partial derivative, Differential Equations,
Here we continue this line of research and study a quasistatic frictionless contact problem for an electro-viscoelastic material, in the framework of the MTCM, when the foundation
Answering a question of de la Harpe and Bridson in the Kourovka Notebook, we build the explicit embeddings of the additive group of rational numbers Q in a finitely generated group
Maria Cecilia Zanardi, São Paulo State University (UNESP), Guaratinguetá, 12516-410 São Paulo,