Mem. Institute of Advanced Technology, Kinki University No. 6 : 1 ^- I 2 (2001) 1
Review
Lysyl-tRNA Synthetase of Bacillus stearothermophilus.
A Few Aspects on the Primary Structures of the Gene and the Enzyme'.
Teisuke Takita2 and Ben`ichiro Tonomura 3 4
Summary
The gene of lysyl-tRNA synthetase of Bacillus stearothermophilus NCA1503 (abbreviated as B.s.LysRS) consists of 1485bp nucleotides with the ATG start codon and the TAA stop codon. The amino acid sequence of B.s.LysRS, a homodimer, deduced from the gene nucleotide sequence reveals 493 amino acid residues with the molecular weight of 57274, the N-terminal Met being deleted. The codon usage of B.s.LysRS gene was compared with those for other aminoacyl-tRNA synthetases of the same organism and with those for lysyl-tRNA synthetase of Escherichia colt and Thermus thermophilus. The amino acid composition of B.s.LysRS was compared with those of IysyI-tRNA synthetases of E. colt and T. thermophilus. A strategy for the thermostability of those organisms was speculated on the amino acid compositions.
Key words
Aminoacyl-tRNA synthetase, Bacillus stearothermophilus, Lysyl-tRNA synthetase, Codon usage, Amino compostion.
acid
Introduction : An aminoacyl-tRNA synthetase (abbreviated as ARS), a member of ligases, catalyzes the aminoac- ylation of tRNA, the binding of an amino acid to the cognate tRNA. In order to guarantee the fidelity of translation of the genetic information into the structure of a protein, ARS must have a high degree of substrate specificity for each heterogeneous substrate: amino acids, nucleotides, and tRNAs. Though all ARSs catalyze the similar aminoac- ylation reaction and the sizes of tRNAs are similar, ARSs exhibit a wide diversity in subunit size and quaternary structure. With E. coli ARSs, the subunit sizes range from 303 amino acids for the a subunit of GIyRS to 951 amino acids for VaIRS, and the diversity of quaternary structure involves a , a 2, a4 , and a212, and the molecular weights range from 52,000 for CysRS to 380,000 for AIaRS (1 ^- 3). Each organism, except a few cases, has the specific ARS for each twenty amino acids that constitute protein. Based on the sequence alignment analysis, the twenty ARSs were divided into two classes, Class I and Class IL consisting of ten ARSs each (4), and this classification has been found consistent with that of the three dimensional structure of ARSs revealed by X-ray crystallographic analysis (3).
I. This study was supported in part by Grants-in-Aid for Scientific Researches from the Ministry of Education, Science, Sports and Culture of Japan, and from Takeda Science Foundation.
2. Division of Applied Life Sciences, Graduate School of Agriculture, Kyoto University, Kitashirakawa, Kyoto 606-8502, Japan 3. Research Institute of Biology-Oriented Science and Technology, Kinki University, Uchita, Wakayama 649-6493, Japan 4. To whom correspondence should be addressed.
Abbreviations: ARS, aminoacyl-tRNA synthetase; B.s. ARS, ARS from Bacillus stearothermophilus, LysRS, lysyl-tRNA synthetase; T.t. ARS, ARS from Thermus thermophilus. The other aminoacyl-tRNA synthetases are also abbreviated as the three-letter symbols of their specific
amino acid followed by RS.
2 Memoirs of institute of Advanced Technology, Kinki University No. 6 (2001)
We have maintained a strong interest in elucidating the molecular mechanism of ARS catalytic reaction, and have chosen lysyl-tRNA synthetase (L-Iysine:tRNA`'`s ligase (AMP forming);EC6.1.1.6), a Class II enzyme, from Bacillus stearothermophilus (abbreviated as B.s. LysRS) as the research target. We have studied some fundamental charac- ters: static, kinetic, and fluorophotometric properties of the enzyme with a highly purified preparation (5 — 8).
In order to investigate further the structure-function relationship of the enzyme, we have cloned the gene of B.s.
LysRS and established the expression system of the gene in E. coli (9). Thus far the gene of LysRS has been reported for the following microorganisms: E. coli (IysS and lysU) (10, 11), Bacillus subtilis (12), yeast (cytoplasm (13) and mitochondria (14)), Mycoplasma hominis (15), Campylobacter jejuni (16), Thermus thermophilus (17), Myco- plasma fermentans (18), and Methanococcus maripaludis (19).
In the present review, we wish to make some characterization of the primary structure of the gene and the protein of B.s.LysRS in comparison with those of the enzymes of E. coil and T. thermophilus.
The nucleotide sequence of B.s. LysRS gene and the amino acid sequence of the enzyme deduced from the gene:
The nucleotide sequence of LysRS gene isolated from Bacillus stearothermophilus NCA1503 was determined (9).
The sequence (Fig. 1) contains an open reading frame with 1482 nucleotide residues and the termination codon, TAA.
The amino acid sequence of B.s. LysRS is deduced from the nucleotide sequence of the gene, together with the consideration on the results of amino acid analysis, as shown also in Fig. 1. However, the possibility remains that the C-terminal residue, Lys, may be modified, because we failed the determination of the residue by carboxypeptidase digestion. The total number of amino acid residues of the enzyme monomer is 493, and the amino acid composition is shown in Table I. The enzyme contains no Cys residue. The molecular weight of the enzyme monomer calculated from the composition is 57,274, and the molar absorption coefficient at 280nm, ~ Z$o, of the dimer enzyme is cal- culated as 63,000 M-'cm' at pH 7.1 and 72,800 M-'cm"' in 0.1N KOH (20).B.sLysRS is a homodimer at pH 8.0 as judged by gel-chromatography and gel-electrophoresis (8). The three motifs characteristic to Class II ARS can be seen: motif 1, position 184 -- 201; motif 2, position 243 — 276; motif 3, position 456 — 488 (Fig. 2). The N-terminal Met residue encoded by the start codon is deleted.
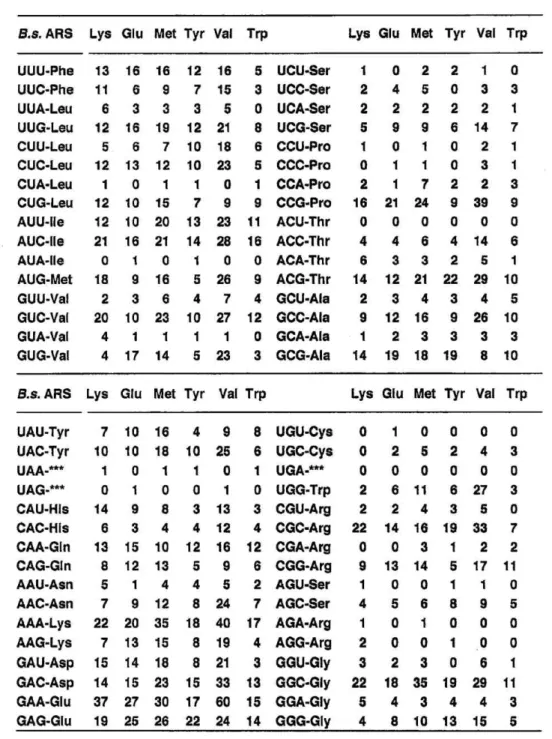
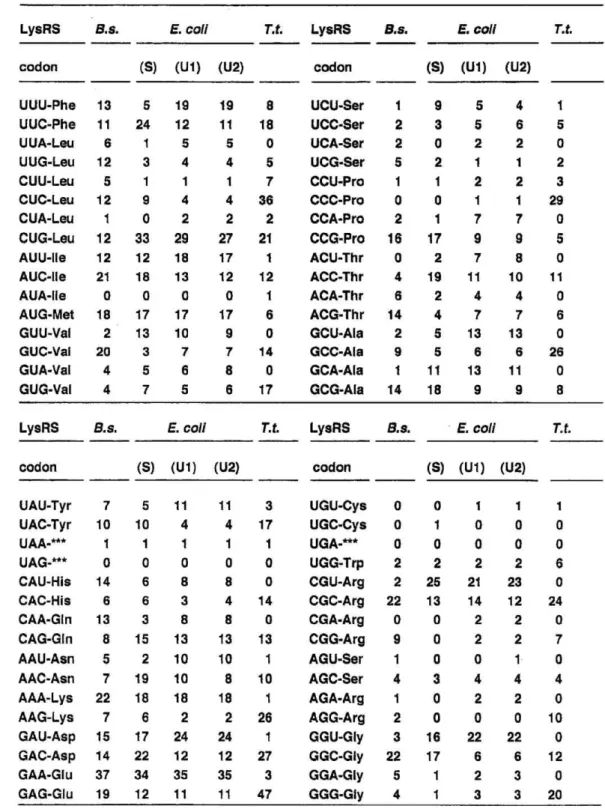
Comparison of the codon usage and the G+C content of B.s. LysRS gene with those of the other B.s. ARSs, E. coli LysRSs, and T.t. LysRS: The codon usage of B.s. LysRS gene is very similar to the codon usage found for the genes of five other ARSs of B. stearothermophilus studied so far (21 25), which are all the Class I enzymes (Table II), though the Class I and the Class II enzymes are considered to have evolved via different routes (2 ^- 4). B. stearo- thermophilus ARS genes show a preference for the codons, GTC(Val), CCG (Pro), ACG (Thr), CGC and CGG (Arg), GGC (Gly). The codon ACT (Thr) is never used in the six B.s. ARS genes. The codon usage of B.s. LysRS gene is distinctly different from those of the genes of E. coli LysRSs and T thermophilus LysRS (abbreviated as T.t. LysRS) (Table III), and yet the recombinant B.s. LysRS was amply expressed in E. coil cells.
The G+C content of B.s. LysRS gene (51.6%) is slightly lower than those of GIuRS (54.6%) (21), MetRS (53.6%) (22), TyrRS (55.1%) (23), VaIRS (54.9%) (24), and TrpRS (54.2%) (25) of the same organism, and it is between the two E. coli LysRS genes (LysRS (U) (47.5%) (10, 11) and LysRS (S) (53.1%) (10)). This is in contrast with the trend that the G+C content of B.s. ARS genes other than LysRS gene are all higher than those of the corresponding E. coil ARS genes (10,11, 26 —' 30).
Fig. 1 Nucleotide sequence of the gene encoding B.s. LysRS and the deduced amino acid sequence of the enzyme.
The deduced amino acid sequences that were confirmed by Edman sequencing of the native B.s. LysRS and the partially digested peptide fragments are indicated with black boxes and underlines. Black boxes show the amino acid sequence used for synthesizing PCR primers. Taken from (9) by permission with some modifications.
Table I
Amino acid compositions of B.s. LysRS, E. coli LysRSs, and T.t. LysRS.
The amino acid compositions were deduced from the reported nucleotide sequences of E.coli lysS (12),E.coil lysU 1 (10), E. coil lysU2 (1 I), and T t. lysS (17). The numbers of Met do not contain the first Met encoded by the start codon ATG.
* The nucleotide sequence of lysU1 (10) was revised and deposited in the EMBL/
GenBankTM/DDBJ by Dessen, P., September 9, 1993.
Fig. 2 Amino acid sequence alignments of B.s. LysRS, E. coil, LysRS, and Tt. LysRS.
Boxes show the residues conserved in the three LysRSs and in any two LysRSs. The marks * and + at top of amino acid residues indicate the residues making interaction with substrate L-lysine and ATP, respectively, in the three-dimensional structure of E.
coil LysRS (U) (40, 41). The motifs 1, 2, and 3 are indicated by lines and three arrows at right side. Taken from (9) by permission with some modifications.
Table II
Codon usages of ARS genes from B. stearothermophilus.
The nucleotide sequences of B.s. ARSs so far reported are: LysRS (9), GIuRS (21), MetRS (22), TyrRS (23), VaIRS (24), and TrpRS (25). The numbers of AUG codon (Met) do not contain the start codon.
Table III
Codon usages of LysRS genes from B. stearothermophilus, E. coli, and T thermophilus.
The table is constructed based on Fig. 1 and the reported nucleotide sequences for E. coil IysS (10), E. coil lysU1 (10), E. colt lysU2 (11), and T t. lysS (17).
The numbers of AUG codon (Met) do not contain the start codon ATG.
"***"
means stop codons.
8 Memoirs of Institute of Advanced Technology, Kinki University No. 6 (2001)
Amino acid compositions of B.s. LysRS, E. col•i LysRSs, and T thermophilus LysRS: The amino acid composi- tions of B.s. LysRS, E. coli LysRSs, and T t. LysRS are compared (Table I). The content of hydrophilic-uncharged amino acid residues is in the order of: E. coli LysRSs, B.s. LysRS, and T t. LysRS. On the other hand, the content of hydrophobic amino acid residues is the lowest, while that of hydrophilic-charged amino acid residues is the highest in B.s. LysRS. These comparisons suggest the possibility that the strategy for thermostability of ARSs in a mesothermophile prokaryote B. stearothermophilus may differ from that of an extreme thermophile prokaryote T thermophilus.
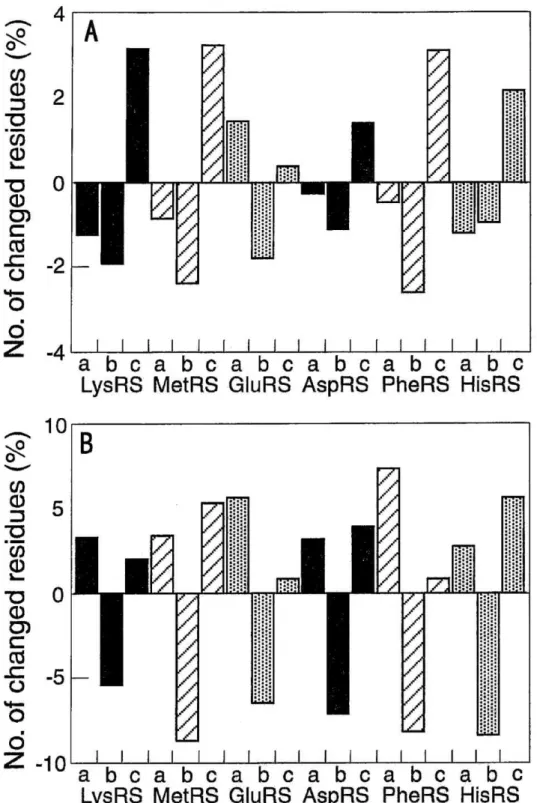
Figures 3 (A) and 3 (B), which were made based on the amino acid sequences of E. coli ARSs (10, 26 -' 33), B.s.
ARSs (21 -- 25), andT.t. ARSs (17, 34 j-- 39), show the difference in the content (%) of each group of amino acid residues of B.s. ARSs and that ofT.t. ARSs against the corresponding content of E. coli ARSs. The trends observed between B.s. LysRS and E. coli LysRSs: a decrease in the hydrophobic and the hydrophilic-uncharged amino acid residues and an increase in the hydrophilic-charged residues in B.s. LysRS can be applied to all cases except B.s.
G1uRS (Fig. 3 (A)). On the other hand, allT.t. ARSs examined have the different trends: a decrease in hydrophilic- uncharged amino acid residues and an increase in both the hydrophobic and the hydrophilic-charged residues (Fig.
3 (B)). These results may indicate that in order to adapt to the growth-temperature, B.s. ARSs have increased ionic interactions, whereasT..t. ARSs have done so by both ionic and hydrophobic interactions.
The amino acid sequence of B.s. LysRS and its homology with the other LysRSs: The amino acid sequence homologies of B.s. LysRS with the other LysRSs were as follows: B. subtilis (77.9%) (12); E. coli lysU (54.1%) (10); E. coli lysS (53.3%) (10); E. colilysU (52.9%) (11); M. hominis (51.0%) (15); M. fermentans (48.8%) (18); T.
therinophilus (48.0%) (17); C. jejuni (46.7%) (16); yeast cytoplasm (39.9%) (13); yeast mitochondria (28.8%) (14);
M. maripaludis (18.0%) (19). The homology is the least with M. maripaludi LysRS which has motifs characteristic of the Rossmann fold usually found in Class I ARSs (19).
The difference in the number of amino acid residues between B.s. LysRS (493 residues) and B. subtilis LysRS (498 residues) is entirely derived from the deletion of Gln2 ^-Asn6 of B. subtilis LysRS, and that between B.s. LysRS and E. coli LysRS (U) (504 residues) is mainly derived from the deletion of 9 amino acid residues in B.s. LysRS that cor- respond to Thr5 ^- Asp 13 of E. coli LysRS (Fig. 2). Further, the fact that N-terminal Asn of T. t. LysRS corresponds to Asn6 in B.s. LysRS (Fig. 2) suggests that the N-terminal region of B.s. LysRS is functionaIIy insignificant. It is consistent with the result that two recombinant B.s. LysRSs with or without T7 tag sequence at the N-terminous have similar kinetic parameters in the aminoacylation reaction (9).
Fig. 3 Difference in the amino acid compositions of B.s. ARSs and T.t. ARSs compared with those of E. coil ARSs.
(A) Difference between B.s. ARSs and E. coli ARSs.
(B) Difference betweenT.t. ARSs and E. coil ARSs.
In both cases, the differences in the content (%) of each group of amino acid residues relative to the values of E. coil ARSs are represented. The symbols a, b, and c show the groups of amino acid residues: a, hydrophobic; b, hydrophilic-uncharged; c, hydrophilic- charged. LysRS (U1) (10) was chosen as E. coil LysRS.
10 Memoirs of Institute of Advanced Technology, Kinki University No. 6 (2001)
REFERENCES
1. Schimmel, P. (1987) AminoacyI tRNA synthetases: General scheme of structure-function relationships in the polypeptides and recognition of RNAs. Annu. Rev. Biochem. 56, 125-158
2. Delarue, M. and Moras, D. (1992) Aminoacyl-tRNA synthetase: Partition into two classes. In Nucleic Acid and Molecular Biology (Eckstein, F. and Lilley, D. M. J., eds.) Vol. 6, pp. 203-224, Springer-Verlag, Berlin, Heidel-
berg
3. Cusack, S. (1995) Eleven down and nine to go. Nature Structural Biology 2, 824-831
4. Eriani, G., Delarue, M., Poch, 0., Gangloff, J., and Moras, D. (1990) Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature 347, 203-206
5. Takita, T., Ohkubo, Y., Shima, H., Muto, T., Shimizu, N., Sukata, T., Ito, H., Saito, Y., Inouye, K., Hiromi, K., and Tonomura, B. (1996) Lysyl-tRNA synthetase from Bacillus stearothermophilus. Purification, and fluorometric
and kinetic analysis of the binding of substrates, L-lysine and ATP. J. Biochem. 119, 680-689 [Corrections: J.
Biochem. 123, 1218 (1998)].
6. Takita, T., Hashimoto, S., Ohkubo, Y., Muto, T., Shimizu, N., Sukata, T., Inouye, K., Hiromi, K., and Tonomura, B. (1997) Lysyl-tRNA synthetase from Bacillus stearothermophilus. Formation and isolation of the enzyme•
lysyladenylate complex and its analogue. J. Biochem. 121, 244-250
7. Takita, T., Hashimoto, S., Inouye, K., and Tonomura, B. (1997) A new filter assay in the study of lysyl-tRNA
synthetase from Bacillus stearothermophilus. Biosci. Biotechnol. Biochem. 61, 726-728
8. Takita, T., Akita, E., Inouye, K., and Tonomura, B. (1998) Lysyl-tRNA synthetase from Bacillus stearothermophi-
lus. Stopped-flow kinetic analysis of enzyme.lysyladenylate formation. J. Biochem. 124, 45-50
9. Takita, T., Shimizu, N., Hashimoto, S., Akita, E., Yokota, Y., Esaki, N., Soda, K., Inouye, K., and Tonomura, B. (2000) Lysyl tRNA synthetase of Bacillus stearothermophilus. Molecular cloning and expression of the gene. Biosci.
Biotechnol. Biochem. 64, 432-437
10. Leveque, F., Plateau, P., Dessen, P., and Blanquet, S. (1990) Homology of lysS and lysU, the two Escherichia coli genes encoding distinct lysyl-tRNA synthetase species. Nucleic Acids Res. 18, 305-312
11. Clark, R. L. and Neidhardt, F. C. (1990) Roles of the two lysyl-tRNA synthetases of Escherichia coli: Analysis of nucleotide sequence and mutant behavior. J. Bacteriol. 172, 3237-3243
12. Kunst, F., Ogasawara, N., Moszer, I., and 148 authors (1997) The complete genome sequence of the gram- positive bacterium Bacillus subtilis. Nature 390, 249-256
13. Mirande, M and Waller, J. P. (1988) The yeast lysyl-tRNA synthetase gene. Evidence for general amino acid control of its expression and domain structure of the encoded protein. J. Biol. Chem. 263, 18443-18451 14. Gatti, D. L. and Tzagoloff, A. (1991) Structure and evolution of a group of related aminoacyl-tRNA synthetase.
J. Mol. Biol. 218, 557-568
15. Ozkokmen, D., Birkelund, S., and Christiansen, G. (1994) Characterization of a Mycoplasma hominis gene encod- ing lysyl-tRNA synthetase (LysRS). FEMS Microbiology Letters 116, 277-282
16. Chan, V. L. and Bingham, H. L. (1992) Lysyl-tRNA synthetase gene of Campyrobacter jejuni. J. Bacterial. 174, 695-701
17. Chen, J., Brevet, A., Lapadat-Tapolsky, M., Blanquet, S., and Plateau, P. (1994) Properties of the lysyl-tRNA synthe- tase gene and product from the extreme thermophile Thermus thermophilus. J. Bacteriol. 176, 2699-2705
11
18. Theiss, P., Karpas, A., and Wise, K. S. (1996) Antigenic topology of the P29 surface lipoprotein of Mycoplasma
fermentans: differential display of epitopes results in high-frequency phase variation. Infect. Immun. 64, 1800-1809
19. Ibba, M., Morgan, S., Curnow, A., W., Pridmore, D. R., Vothknecht, U. C., Gardner, W., Lin, W., Woese, C. R., and SO 11, D. (1997) A euryarchaeal lysyl-tRNA synthetase: resemblance to class I synthetase. Science 278, 1119-1122 20. Mihalyi, E. (1968) Numerical values of the absorbances of the aromatic amino acids in acid, neutral, and alkaline
solutions. J. Chem. Eng. Data 13, 179-182
21. Breton, R., Watson, D., Yaguchi, M., and Lapointe, J. (1990) Glutamyl-tRNA synthetases of Bacillus subtilis 168T and of Bacillus stearothermophilus. J. Biol. Chem. 265, 18248-18255
22. Mechulam, Y., Schmitt, E., Panvert, M., Schmitter, J-M., Lapadat-Tapolsky, M., Meinnel, T., Dessen, P., Blanquet, S., Fayat, G. (1991) Methionyl-tRNA synthetase from Bacillus stearothermophilus: structural and function iden-
tities with the Escherichia coli enzyme. Nucleic Acid Res. 19, 3673-3681
23. Winter, G., Koch, G. L., Hartley, B. S., and Barker, D. G. (1983) The amino acid sequence of the tyrosyl-tRNA
synthetase from Bacillus stearothermophilus. Eur. J. Biochem. 132, 383-387
24. Borgford, T. J., Brand, N. J., Gray, T. E., and Fersht, A. R. (1987) The valyl-tRNA synthetase from Bacillus stearo- thermophilus has considerable sequence homology with the isoleucyl-tRNA synthetase from Escherichia coli.
Biochemistry 26, 2480-2486
25. Barstow, D. A., Sharman, A. F., Atkinson, T., and Minton, N. P. (1986) Cloning and complete nucleotide sequence of the Bacillus stearothermophilus tryptophanyl tRNA synthetase gene. Gene 46, 37-45
26. Barker, D. G., Ebel, J. -P., Jakes, R., and Bruton, C. J. (1982) Methionyl-tRNA synthetase from E. coll: primary structure of the active crystallized tryptic fragment. Eur. J. Biochem. 127, 449-457
27. Heck, J. D. and Hatfield, G. W. (1988) Valyl-tRNA synthetase gene of Escherichia coli K12: Molecular genetic characterization. J. Biol. Chem. 263, 857-867
28. Breton, R., Sanfacon, H., Papayannopoulos, I., Biemann, K., and Lapointe, J. (1986) Glutamyl-tRNA synthetase of Escherichia coll. Isolation and primary structure of the gltX gene and homology with other aminoacyl-tRNA
synthetases. J. Biol. Chem. 261, 10610-10617
29. Hall, C. V. and Yanofsky, C. (1981) Cloning and characterization of the gene for Escherichia colt tryptophanyl-
transfer ribonucleic acid synthetase. J. Bacteriol. 148, 941-949
30. Barker, D. G., Bruton, C. J. and Winter, G. (1982) The tyrosyl-tRNA synthetase from Escherichia coil: complete
nucleotide sequence of the structural gene. FEBS Lett. 150, 419-423
31. Miller, H. I. (1984) Primary structure of the himA gene of Escherichia coli: homology with DNA-binding protein HU and association with the phenylalanyl-tRNA synthetase operon. Cold Spring Harb. Symp. Quant. Biol. 49, 691-698
32. Eriani, G., Dirheimer, G. and Gangloff, J. (1990) Aspartyl-tRNA synthetase from Escherichia coil: cloning and characterization of the gene, homologies of its translated amino acid sequence with asparaginyl- and lysyl-tRNA
synthetases. Nucleic Acids Res. 18, 7109-7118
33. Freedman, R., Gibson, B., Donovan, D., Biemann, K., Eisenbeis, S., Parker, J. and Schimmel, P. R. (1985) Primary structure of histidine-tRNA synthetase and characterization of hisS transcripts. J. BioI. Chem. 260,
10063-10068
12 Memoirs of Institute of Advanced Technology, Kinki University No. 6 (2001)
34. Nureki, 0., Muramatsu, T., Suzuki, K., Kohda, D., Matsuzawa, H., Ohta, T., Miyazawa, T. and Yokoyama, S. (1991) Methionyl-tRNA synthetase gene from an extreme thermophile, Thermus thermophilus HB8. J. Biol. Chem.
266, 3268-3277
35. Nureki, 0., Suzuki, K., Hara-Yokoyama, M., Kohno, T., Matsuzawa, H., Ohta, T., Shimizu, T., Morikawa, K., Miyazawa, T., and Yokoyama, S (1992). Glutamyl-tRNA synthetase from Thermus thermophilus HB8. Molecular cloning of the gltX gene and crystallization of the overproduced protein. Eur J. Biochem. 204, 465-472 36. Kreutzer, R., Kruft, V., Bobkova, E. V., Lavrik, 0. I., and Sprinzl, M.(1992) Structure of the phenylalanyl-tRNA
synthetase genes from Thernus thermophilus 1-1B8 and their expression in Escherichia coli. Nucleic Acids Res.
20, 4173-4178
37. Keller, B., Kast, P. and Hennecke, H. (1992) Cloning and sequence analysis of the phenylalanyl-tRNA synthetase genes (pheST) from Therms thermophilus. FEBS Lett. 301, 83-88
38. Poterszman, A., Plateau, P., Moras, D., Blanquet, S., Mazauric, M. H., Kreutzer, R. and Kern, D. (1993) Sequence, overproduction and crystallization of aspartyl-tRNA synthetase from Thermus thermophilus. Implications for
the structure of prokaryotic aspartyl-tRNA synthetases. FEBS Lett. 325, 183-186
39. Aberg, A., Yaremchuk, A., Tukalo, M., Rasmussen, B., and S. Cusack. (1997) Crystal structure analysis of the activation of histidine by 7'hermus thernwphilus histidyI-tRNA synthetase. Biochemistry 36, 3084-3094 40. Onesti, S., Miller, A. D., and Brick, P. (1995) The crystal structure of the lysyl-tRNA synthetase (LysU) from
Escherichia coli. Structure 3, 163-176
41. Desougus, G., Todone, F., Brick, P., and Onesti, S. (2000) Active site of lysyl-tRNA synthetase: structural studies of the adenylation reaction. Biochemistry 39, 8418-8425.