40 Tech ,, Bull. Fac. Agr
.
Kagawa Univ.DRIFTS IN 3"P DISTRIBUTION AND ITS
INCORPORATION INTO NUCLEIC ACID IN
OAT LEAVES AFTER INOCULATION WITH
PUCCrNTA
CORONA
T A
Toshikazu TANI, Takatoshi
ONOE,and
Nakato
NAITOIntroduction
I t has been well established that rust infection causes an increase of phosphorus con- tent in wheat leaves(14), and a marked accumulation of ph~sphorus around the infected sitec3, 4 * 5 3 1 9 ) . The accumulation of phosphorus has been discussed a t the level of respiratory
activity in association with the defense reaction of host cells. It has also been related t o nucleic acid synthesis of host cells or parasite, especially in the obligate parasitism'').
Histochemical studies clearly showed an increase of ribonucleic acid (RNA) concentra- tion in the host cell nuclei, fungal hyphae, and h a u s t ~ r i a ' ~ ? 20), and evidences have been
accumulated t o indicate the enhanced synthesis of RNA in the leaves infected with rust fungi. Total nucleotide content in the wheat leaves infected by Puccznza recondzta was higher than that in healthy control(g). In the susceptible varieties, RNA content and specific 32P activity in RNA increased in the leaves of wheat and bean plants infected respectively by P. graminzs tr~ticz('~. "1 and Uromyces ~haseolz(~). Increased susceptibility t o stem rust in a temperature -sensitive resistant variety of wheat was also correlated with a large increase in RNA content and 32P-RNA(6). On the other hand, in plants showing resistance, neither 32P incorporation into RNA(18) nor RNA -P(I1) increased. Similar results have been reported with powdery mildew(l0> " 2 139 19, and downy mildew(12).
As far a s the authots are aware, little is known about the alterations in nucleic acid metabolism in the crown rust infected oat. This paper presents distribution patterns of 32P, incorporation of 32P into nucleic acid, and content of RNA and deoxyribonucleic acid (DNA) in the leaves of oat exhibiting resistant and susceptible reactions t o crown rust infection.
Material and Method
Three oat varieties, Victoria, Hyuga
-
kairyo- kuro (HKK),
and Shokan No.1 (S-
I ) ,respectively, susceptible, moderately resistant, and resistant to the infection by Puccinza coronata CQRDA, were used a s the host plant. The fungus has been maintained on Victoria in our laboratory. The uredospores were freshly harvested in each run of the experiments by t h e manner described in the previous paper (I5).
About 200 oat seeds germinated on wet filter papers for 24 hr in dark condition a t 25OC were transplanted on vermiculite in plastic trays ( 2 7 x 9.5 cm base and 10 cm height). The plants were grown in a growth chamber controlled at 20-25OC receiving continuous light illumination at 6,000- 8,000 lux from combined incandescent and cool -white
Vol 21 (No. 48) (1970) 41
fluorescent sources. Irrigation was done daily with SO ml of Kasugai solution (NH4NOs 66mg, KMzPO4 38mg, KC1 43 mg, MgS04-7Hz0 245mg, Ca (NOs)z.4Hz0 189mg, FeC13 2mg, deionized water 1 L; adjust with NaOH at pH 7.0).
The under surface of the primary leaf of 7-day -old seedling, prior to emergence of the secondary leaf, was heavily inoculated with uredospores using a hair brush. For distribution experiments of 32P the inoculation was confined to a zone 2-3 cm wide in approximately halfway between the top and the base of the leaves. For other experiments spores were brushed on a whole part of the leaf. The inoculated plants were kept in moist chamber for 20 hr and then transferred into the growth chamber.
82P feedzng procedure: Primary leaves were detached with stems of 1 cm long, put with
their cut ends into deionized water for 30 min, and then transferred to 2.5
mM
of phosphate buffer solution (pH 6.7) containing 82P-labeled orthophosphate in a test tube of 3 cm height. Activity of 82P in 1 ml of the solution was 2 4 for autoradiography, 20 and 40 PC f or radioactive scanning, and 40 for labeling nucleic acid, respectively. The depth of the solution was restricted to be within 0.5 cm. Care was taken to assure uniform tran- spiration during the feeding periods by loosely supporting the leaves in a vertical portion with a wire loop. After being incubated for 4 hr in the growth chamber the stems were cut off and the leaves were harvested. This procedure provided no contamination on the leaf surface with radioactive material.Autwadzagraphy and measurement of radzoactzvzty: For autoradiography the leaves were laid on paper pads and pressed with a flat iron at 130°C for 2-3 min. The radiographs were made on Fuji (No-Screen) X-ray film with exposure periods of 40-60 rnin at room temper- ature. For radioactivity determinations by means of a radioactive scanner the leaves were affixed to a glass plate with strips of cellophane tape. The plate was then subjected to radioactivity measurement with a windowless, gas flow counting system composed of a thin layer chromatographic (TLC) scanner (Aloka, Model TLC-1) and a proportional scaler. Preparation o f crude nuclezc aczd: The modified Schmidt- Thanhauser procedure was employ
-
ed. Twenty leaves of uniform length, about 1 g by fresh weight, were cut into pieces of 4-5 mm length and immediately frozen a t -22'C and stored until use. The reliability of specific activity values for incorporation of 32P and content of RNA and DNA was found to be satisfactory when more than 20 leaves were used in each run of the assay. T h e frozen leaves were homogenized with 20 ml of cold Tris-HC1 buffer solution (50 mM, pH 7.2) containing 50 mM of MgClz and 1 mM of 6-mercaptoethanol for 3 rnin at 20,000 rpm using a homogenizer (Nihon-Seiki, Model HB) . The homogenate was filtered through double sheets of gauze t o remove fibrous material. The filtrate was immediately added to equal volume of 10% cold trichloroacetic acid (TCA), allowed to stand for 30 rnin in ice chilled condition. The pellet was successively washed by centrifugation twice with cold 5% TCA, then once with 40 ml of methanol a t room temperature. The pellet was then incubated twice with 10 ml of ethanol-ethylether-chloroform mixture (2:2:1 v/v) a t 37°C for 15 min, finally washed with ethylether to obtain dry powder.Determznatzon of 32P zn nuclezc acid: The crude preparation of nucleic acid was suspended
42 Tech. Bull,, Fac., Agr.. Kagawa Univ,.
centrifuged a t 4,000 g for !5 min. The precipitate was washed with 5 ml of 5% TCA and the washing was combined with the supernatant. On a planchet 0.5 ml of this solution was plated, dried, and counted in a thin window gas flow counter (Aloka, Model PC-10 E). All counts recorded were corrected to give the activity a t time of rust inocula. tion.
Determinatzon o f KNA and DNA content: The primary leaves were cut into small pieces immediately after being detached from the plants, and used for preparation of the crude powder of nucleic acid by the procedure as mentioned above. For RNA determination the powder material was hydrolyzed for 17 hr in 0.5 N KOH at 30°C. KOH was removed by adding HC104 in ice chilled condition. Hydrolyzate of DNA was obtained from the residue of RNA extraction by hydrolyzing for 20 min in 5% HClOd at 70°C. The orcinol reacton for RNA and the diphenlyamine reaction for DNA were employed using D-ribose and 2-deoxy -D-ribose as respective standards.
Results
Victoria, susceptible variety, reveals light -green flecks 4 days and develops ure- dospores 5 days after inoculation. Vigorous sporulation occurs on 7-8 days. Massive growth of the infection hyphae in Victoria tissues ha; been observed 3 davs after in- oculation and 60% of the mother cells for uredosori are produced 4 days after i~locula- tion(''). In HKK, moderately resistant variety, flecks are not visible and the leaves were yellowing by 4 to 5 days after inoculation. There is no sporulation and the discolo- ration to light brown begins by 6 to 7 days after inoculation. Histological investigations have shown that the infection hyphae develop f o ~ 3 days aftcr inoculation, and no mother cells for uredosori is produced by further incubation(16'. S - 1 reveals a hypersensitive reaction 2 days after inoculation developing the necrotic spots which turn to dark brown in the followjng one or two days. The infection hyphae in the tissue cease their elongation 48 hr after inoculation, resulting in a ~ t o l y s i s ( ' ~ ) .
1
Distribution of 3 2 P activity in infected leavesAutoradiograms of 32P distribution of rusted leaves are summarized in Plate 1 and 2. Striking accumulation was found in Victoria leaves. Distinct imsge on the radiograms a t 5 days after inoculation coincided with the loci of rust pustules. This was accentuated in 6-day-old lesions and became to uniform over the infected area of the leaves by 8 days after inoculatioa. Definite reduction in radioactivity was observed in S - 1 leaves when incubated for more than 3 days. No detectable change was observed on radiograms with HKK leaves throughout the experimental period of 8 days.
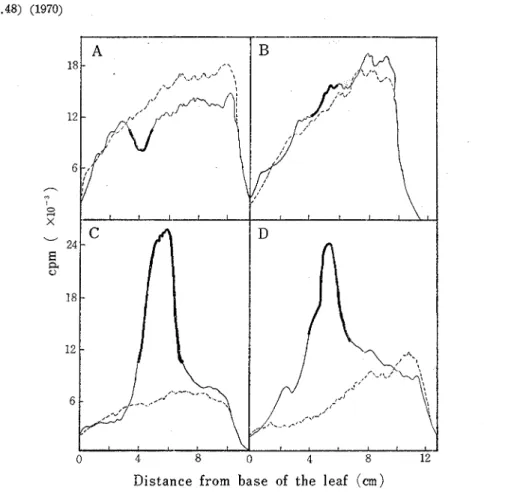
The direct scanning of radioactivity with TLC-scanner gave much more sensitive and reliable data as to the radioactive ratio of infected sites to healthy area of the zone-infected leaves, especially in the early stage of rust infection. The results with Victoria leaves are summarized in Fig. 1 and 2. Clear depression in the radioactive profile was shown a t the infected sites 3 and 4 days after inoculation. This depression was observed to the same extent when feeding time of 32P was shorten to 1 and 2 hr (Fig. 2). The decreased activity in Victoria leaves disappeared 5 days after inoculatin. Pronounced accumula-
Distance from base of the leaf (cm)
Fig. 1 Determination of a2P distribution by means of a TLC-scanner in Victoria leaves a t various times after inoculation. From A to D are shown just prior of flecking (3- day-old), the beginning of sporulation (5-day-old), the early stage of sporulation (8-
day -old), and the late stage of sporulation (13-day-old) , respectively A big line in solid one indicates inoculated sites and dotted line represents uninoculated control.
Distance from base of the leaf (cm)
Fig. 2 Determination of a2P distribution by means of TLC-scanner in Victoria leaves a t the flecking stage (4 days a f t e ~ inoculation). A , B, and C are shown leaves fed for
1
,
2,
and 4 hr, respectively. Concentration of 32P used in this experiment was 4044 Tech. Bull. Fac. Agr. Kagawa Univ.
tion was then deteced in the following period of incubation, with a 4-fold increase at the peak of the curves on 8 to 13 days. Fig.3 shows the representative profiles of radioactivity in HKK and S - 1 leaves demonstrating a distinct fall at resistant reacting sites. The drop in the curves in both varieties occured when rust reactions revealed brown lesions, usually after 4 to 5 days for HKK and 2 days for S-1.
Distance from base of the leaf (cm)
F i g " 3. Determination of 32P distribution by means of a TLC-scanner in inoculated leaves of Hyuga-kair yo-kuro and Shokan No 1 4 days after inoculation A: Hyuga -kair yo-kuro B: Shokan No.1 For other explanations see Fig. 1.
2.
D r i f t s in incorporation o f 8 2 P into nucleic acid and content o f R N A and D N AThe results on the drifts of radioactivity in the nucleic acid fractions in uninoculated leaves are given in Fig. 4-A. The changes in s2P incorporation exhibited a similar be- havior i n the healthy leaves of three oat varieties except in HKK at 5 days. The ratio of incorporation in inoculated leaves to uninoculated control is given in Fig. 4-B. The curve of Victoria is a sigmoid one, rising to 200% of control level within 3 days after inoculation and ammounted to approximately 500% on the next two days. The curve of HKK exhibits 180% rise at 3 days with a plateau on the following two days. Increased ratio in S-1 is about 150% at 2 days after inoculation.
Days after inoculation
Fig,. 4. =P incorporation into nucleic acid in three oat varieties a t various times after in., oculation, A: 32P incorporation in uninoculated leaves B: Ratios of 32P incorporation
in inoculated leaves to uninoculated control ( A ) , Solid, dotted, and broken lines represent Victoria, Hyuga
-
kaix yo - kuro, and Sholran No,.l, respective1 y ,Vo1 .21 (No ,48) (1970) 45
Days a f t e r inoculation
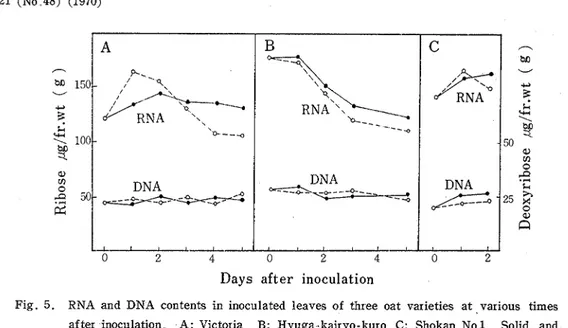
Fig. 5. RNA and DNA contents in inoculated leaves of three oat varieties a t .various times after inoculation A : Victoria B: Hyuga.kairyo-kuro C: Shokan No.1 Solid and dotted lines represent the content in inoculated and uninoculated leaves, respectively.
Fig. 5 shows RNA and DNA contents in inoculated and uninoculated leaves. RNA content in uninoculated control revealed no significant difference among the oat varieties in progressive change during the experimental periods. Apparent increase in RNA, about 12093, was observed in rusted Victoria leaves 4 and 5 days after inoculation. Slight increase, 105%, was found in infected leaves of HKK on 3 and 5 days and i n those of
S - 1 on 2 days, respectively. DNA content of three varieties exhibited a n uniformity through- out tne experimental periods, no essential difference being observed after rust infection.
Discussion
The results of these experiments indicate that three oat varieties employed responded to the rust infection with characteristic behaviors in 32P distribution and s2P incorporation into nucleic acid depending on their rust reactions. The outline of them confirms the find- ings for other rust disease^'^).
The reduction of 32P distribution a t infected sites coincided with the beginning of fleck- ing in Victoria and necrosis in IiKK and S - 1 , respectively (Fig. 1, 2, and 3). Less s2P
distribution in susceptible reaction to Puccznza spp. was not observed by the use of auto- radiographic technique(lg). The present results in Victoria may indicate a reduced metabolic activity rather than RNA synthesis during flecking stage. The fact that photosynthesis decreases within a few days after rust inoculation(') may support this view. In Victoria, however, such a metabolic depression is not accompanied with any damage for rust develop- ment. It i s probable, therefore, that the reduced metabolic activity in the host tissues may induce sporulation of the fungus. Details on this problem will be discussed in a separate paper with further investigations. On the contrary, the reduction in HKK and S - 1 may probably be due t o disintegration of the host tissues associated with subsequent cease of fungal growth.
46 Tech. Bull. Fac, A ~ I
.
Kagawa Univ.Local accumulation of radioactivity in Victoria 5 days after inoculation (Plate 1-4) indicates that vigorous translocation of phosphorus compounds towards pustules occurs when sporulation is initiated. The accumulation seems to be in parallel with the advance- ment of sporulation (Plate 1 - 5 and 6, Fig. 1 -C and D)
.
Increased incorporatin of 32P into nucleic acid on 3 days after inoculation in both Vic- toria and HKK (Fig. 4-B) coincides with rapid development of infection hyphae in host tissues. Dramatic rise of incorporation ratio in Victoriz on 4 days after inoculation may be due to the beginning of uredosor us for mation, because no increase in 32P -nucleic acid is observed in HKK in which no mother cell for uredosori develops. The authors (unpublished) have shown that this large increase in Victoria could be attributed to high molecular weight RNA newly synthesized. Slight increase of incorporation ratio in 2-day -old leaves of
S 1 might probably be due to a resistant response by the affected tissue.
Infection had little or no effect on total RNA and DNA content except RNA concentra- tion in Victoria which showed consistent increase in RNA on 4 and 5 days after inoculation (Fig. 5-A). This increase is mainly attributable to the increased synthesis of ribosomal RNA in the host-parasite complex (unpublished).
Summary
Reduction in 82P distribution at the site infected with Puccinia coronata was observed at the initiation times of flecks in Victoria oat (susceptible) and necrosis in Hyuga
-
kair yo-
kuro (moderately resistant) and Shokan No.1 (resistant).
Striking accumulation of S2P a t the loci of rust pustules in the susceptible leaves was first shown a t the beginning of sporula. tion and was increasingly stimulated during the sporulation period up to 4-fold of the healthy control. No accumulation towards infected sites was observed in the leaves of resistant varieties. 82P incorporation into nucleic acid in both susceptible and moderately resistant leaves increased to 200 and 180% at the time when abundant growth of infection hyphae occured. About 5-fold increase of 32P -nucleic acid was found when uredosori began to develop in susceptible leaves. The resistant variety noted about 150% increase in the incorporation when the hyper sensitive reaction occured. Infection had no or little effect on DNA content of oat Ieaves of three varieties, but RNA content of susceptible variety consistently increased a t the stage of uredosorus development.Acknowledgments
The authors are grateful to Dr. T. Kajiwara and Mr.
T.
Inaba, National Institute of Agricultural Science, Tokyo, for their instruction in the autoradiographic technique; Mr .S.Ouchi, Okayama University, for his suggestion in the preparation of this manuscript. The authors also wish to thank Miss T . Goto of our laboratory for her technical assistance.
Vo1 .21 (No, 48) (1970) 47
Plate 1 Autoradiograms showing a2P distribution in zone-infected primary leaves of Victoria (susceptible variety) Uninoculated control is given in 1. From 2 to 6 are shown leaves 2, 4, 5, 6, and 8 days after inoculation, respectively.
Plate 2 Autoradiograms showing s2P distribution in zone-infected primary leaves From 1 to 3 are shown Hyuga-kair yo-kuro (moderately resistant variety)before inoculation, and 3 and 5 days after inoculation, respectively From 4 to 6 are shown Shokan No 1
(resistant variety) before inoculation, and 2 2nd 3 days after incculation, respectively
Uninoculated leaves in Plate 1 a n d 2 were harvested on the day of inoculation. Arrows point to the ends of inoculation zone,
48 Tech. Bull. Fac. A g r . Kagawa Univ, References
(1) ALLEN, P . J : Physiological aspects of fungus diseases of plants Ann Rev Plant Physzol, 5, 225- 248 (1954).
(2) BHATIACHARYA, P K , NAYLOR, J M , SHAW, M : Nucleic acid and protein changes in wheat leaf nuclei during rust infection Sczence, 150, 1605-1607 (1965)
13) DURBIN, R D : Obligate parasites: Effect on the movement of solutes and water T h e Dynamic Role of Molecular Constituents in Plant-Parasite Interaction (ed MIROCHA, C J , URIIANI, I ), 80- 99, S t Paul, Minnesota, Am Phytopathol.. Soc (1967)
(4) GERWITZ, D L , DURBIN, R.D : The influence of rust on the distribution of s2P in the bean plant.
Phytopathol , 55 57-61 (1965)
15) GOIILIEB, D., GARNER, J.M : Rust and phosphorus distribution in wheat leaves Phytopathol, 36,
557-564 (1946).
16) HEIIEFUSS, R.: Veranderungen im Gehalt a n Ribonucleins2ure in Weizenpflanzen unterschiedlicher und temperatur gesteur ter Resistenz gegen Puccznza gramznzs trztzcz Z Pflanzenkrnkh. Pflanzenschutz,
71, 154-158 (1964).
(7) HEIIEFUSS, R.: Nucleic acid metabolism in obligate parasitism. Ann Rev Phytopathol,
4,
221-244 (1966).(8) HEIIEFUSS, R : Regulation of host-parasite relations in obligate parasitism Biochemical Regulation in Diseased Plants or Injury (ed. HIRAI, T e t a1 ), 223-232, Tokyo, Phytopathol Soc. Japan (1968) (9) JOHNSON, L B , ZSCHEILE, F P., TR , BRANNAMAN, B L. : Effect of Pz~ccznza recondzta infection on
the RNA composition of wheat leaves PhytoPathol, 57, 632-638 (1967)
(10) KLJAJIC, R , PLESNICAR, M : Incorporation of 39P into the acid soluble and phospholipid fraction of wheat leaves infected with powdery mildew. Host-Parasite Relations in Plant Pathology (ed. KIRALY, Z , UBRIZSY, G . ) , 239-241, Budapest, Res. Inst Plant Protection (1964)
(11) MALCA, I., ZSCHEILE, F.P, J R , GULLI, R . : Nucleotide composition of RNA in healthy and mildew- infected leaves of barley Phytopathol, 5 4 , 1112-1116 (1964).
(12) MILLIKAN, D.F., WYLLIE, T D , P I C K E T I , E.E : Some comparative biochemical changes associated with downy mildew infection in soybeans. Phytopathol., 55, 932 (1965).
(13) MOUNI, M.S., ELLINGBOE, A.H.: 32P and
35s
transfer from susceptible wheat to Erys~Phe gramz- nis f . sp. tritici during primary infection. Phytopathol, 59, 235 (1969)(14) MUKHERJEE, E.L., SHAW, M.: T h e physiology of host-parasite relations XI. The effect of stem rust on the phosphate fractions in wheat leaves. Can. J Bot., 40, 975-985 (1962).
(15) NAIIO, N., T A N I , T : Swelling and projection of uredospores of Puccznza coronata during the early stage of germination Ann. Phytopathol Soc. Japan, 32, 26-33 (1966).
(16) NAIIO, N , TANI, T , ARAKI, T . : Trans. Mycol Soc Japan (in press)
07) QUICK, W. A , SHAW, M. : The physiology of host-parasite relations XIV The effect of rust infec- tion on the nucleic acid content of wheat leaves. Can. J Bot.,
42,
1531-1540 (1964).(18) ROHRINGER, R., HEIIEFUSS, R : Incorporation of 32P into ribonucleic acid of rusted wheat leaves
Can J . Bot , 3 9 , 263-267 (1961).
(19) SHAW, M,, SAMBORSKI, D..J. : T h e physiology of host-parasite relations I. The accumulation of radioactive substances a t infections of facultative and obligate parasites including tobacco mosaic virus..
Can. ,J,, Bot,, 34, 389-405 (1956).
(MI1 WHITNEY, H . S., SHAW, M,, N A Y L O R , J.,M,.: The physiology of host parasite relations XII. A cytophotometsic study of the distribution of DNA and RNA in rust-infected leaves.. Can. ,J Bot., 40,
Vol .21 (No. 48) (1970) 49
(21! ZSCHEILE, F P., JR., MOSEMAN, J.G., BRANNAMAN, B L : Content and nucleotide composition of ribonucleic acid in powdery mildew infected leaves of near-isogenic barley lines. Phytopathol., 59, 492-495 (1969)
r5'
1 9 7' (mi3tk) D%&j&B&%l%k b U K HPI&&% (~b%%t%), l%;d 1 % (&@I&) 01 &&f&BL/&W&K M Puccznza coronata @@$ISi$K %fa~\c 32P %JU!;Si&&&Lk R % t ~ L % % ~ l a & l ~ ~ 3 ' % & M h 2 0 ~ % % K k & 2 g v dF
i$tD$BaK32PB&;SiilS@K& b b h , Q@-3W~jliEo@lJU2 2 & K % % M ~ ~ j J D L ~ @ & $ 1 3 0 4 @ K & L k
-2
, t j ~ j E@I&*
L d:%@t%%@ TMQ@7%i%M% b b', ;54%'F%E$BD 32P%%la% bhkfaL\ f%J$i1I6% 1 ~rtl+&@tLL$@ T la, @$83:;s:%&Klrfl%%&G&&@BK'fd:& 2 , $&E@-D 32P tr 92%M#&&K3.JL ? h ? h 2 0 0 % , 180% A W J U L k . ~E@t!k%@ TMJ2l@?J%%fi@K 3 bK@jJUL, @&%o& 5 4 % A S L k . % k , ?EDi'tkkABT4%.,%BE&
Rj&!6& b b h & tr s2P - & @ V % ~ J 150 % K I # L k . 3 &LGK %L\T, DNA ~ E M & ~ @ ~ ~ ~ ~ L ~ L ~ ; S ~ , ] % ' J ~ l t 4 ~ ~ l
B D R N A ~ E ~ ~ M M T J K % ~ ~ & K ~ C . I ~ ~ O % K ~ @ I J O L ~ .