IRUCAA@TDC : Evaluation of high-performance liquid chromatography and mass spectrometry method for pharmacokinetic study of local anesthetic ropivacaine in plasma
全文
(2) Biomedical Research 30 (6) 319-324, 2009. Evaluation of high-performance liquid chromatography and mass spectrometry method for pharmacokinetic study of local anesthetic ropivacaine in plasma Kohei Sawaki1, Migiwa Okubo1, Takashi Shimomiya1, Eri Tukagoshi1, Takayuki Sakai1, Takaki Yamazaki2, Masahiro Kenmochi3, Mariko Miyao3, Yuzuru Kaneko3, Tatsuya Ichinohe3 and Mitsuru Kawaguchi1 1. Department of Pharmacology, 2 Department of Ultrastructural Science, and 3 Department of Dental Anesthesiology, Tokyo Dental College, 1-2-2 Masago, Mihama-ku, Chiba 261-8502, Japan (Received 7 September 2009; and accepted 25 September 2009). ABSTRACT We studied the viability of high-performance liquid chromatography and mass spectrometry (LC/ MS) as a selective and sensitive analytical method for measuring blood concentrations of the local anesthetic ropivacaine. Ropivacaine was effectively separated using a reverse-phase column and monitored at 275 m/z ion. The LC/MS method allowed measurement of concentrations of ropivacaine of lower than 75 ng/mL. The standard curve was linear and in the range of < 1.5 μg/mL. Recovery of ropivacaine in plasma samples was over 90% after precipitation of plasma protein with trichloroacetic acid. The method was tested on the pharmacokinetics of plasma ropivacaine after single intravenous or subcutaneous administration in rabbits. The pharmacokinetic parameters showed a one-compartment model and a mean elimination half-life of 0.54 ± 0.05 h and 2.83 ± 0.51 h after administration at doses of 0.4 mg/kg, i.v. and 5 mg/kg, s.c., respectively. These values were in approximate agreement with previously obtained results in dogs. The results of the present study demonstrated that the LC/MS method was highly selective and sensitive for the measurement of ropivacaine, indicating that it offers a useful tool for monitoring the therapeutic effects and determining the pharmacokinetic parameters of this drug in blood.. Ropivacaine hydrochloride hydrate (Anapeine ®; Fig. 1) is a long-acting amide-type local anesthetic formulated as a pure optical s(-)-enantiomer of 1-propyl-2’,6’-pipecoloxylidide (12). Ropivacaine is indicated for peripheral nerve block and analgesia. It is less toxic to the cardiac system than other anesthetics due to its lower affinity to cardiac sodium channels (2, 5). In addition, it can selectively block sensory fibers, reducing blockage of motor fibers. These advantages are reflected in its popularity as a safe option as a local anesthetic for clinical use. Higher doses of some local anesthetics have the Address correspondence to: Kohei Sawaki, Ph.D. Department of Pharmacology, Tokyo Dental College, 1-2-2 Masago, Mihama-ku, Chiba 261-8502, Japan Tel: +81-43-270-3774, Fax: +81-43-270-3776 E-mail: sawaki@tdc.ac.jp. potential to cause excitation of the central nervous system (CNS), resulting in restlessness and tremor, which may progress to clonic convulsions (5). These adverse effects are directly related to the concentration of the drug in systemic circulation. Excitation of the CNS may be followed by depression and respiratory failure, sometimes leading to death. These effects on the CNS are presumed to result from selective depression of inhibitory neurons and subsequent enhancement of the effect of excitatory neurons (19). A number of studies on the functional role of neurotransmitters in local anesthetic-induced convulsions have noted depression of neuronal activity (1, 6, 14–17, 20) Laboratory studies on the toxicity of ropivacaine to the CNS in dogs noted that average dose and plasma concentration of ropivacaine at convulsive onset were 4.88 ± 0.47 mg/kg and 11.4 ± 0.9 μg/mL,.
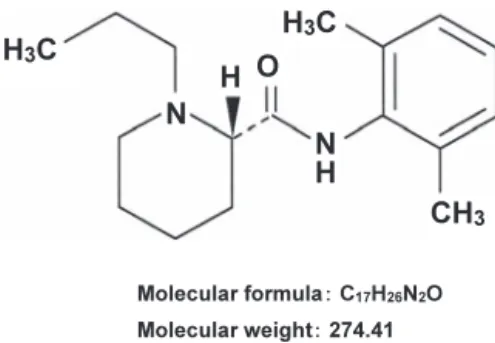
(3) 320. K. Sawaki et al.. animals were treated within the Guidelines for the Treatment of Experimental Animals approved by The Japanese Pharmacological Society and Tokyo Dental College.. Fig. 1 Chemical structure and molecular weight of ropivacaine: (S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2carboxamide.. respectively (8). This indicates that, for safe clinical use, the blood concentration of ropivacaine should be kept below 10.0 μg/mL. When ropivacaine is continuously used as a peripheral nerve block for postoperative analgesia (7), it is necessary to monitor blood ropivacaine concentration to prevent toxicity to the CNS. Accurate measurement of blood ropivacaine concentration would allow prediction of alterations in CNS activity. Several chromatographic assays have been described for determination of plasma concentration of local anesthetics (10, 13). However, a satisfactory method of measuring lower concentrations of ropivacaine remains to be established. In the present study, we investigated the selectivity and sensitivity of a method for measuring blood ropivacaine concentration using high-performance liquid chromatography and mass spectrometry (LC/MS). MATERIALS AND METHODS Chemicals. Ropivacaine hydrochloride hydrate (Anapeine® injection) was purchased from AstraZeneca K.K. (Osaka, Japan). Trichloroacetic acid (TCA), ammonium acetate and acetonitrile were purchased from Wako Pure Chemical Industries (Osaka, Japan). All other reagents used were of high analytical grade. The working solutions of ropivacaine for the basic analysis and the standard curve were prepared by dilution with distilled water. Animals. Male Japanese White Rabbits weighing 3.35 to 3.5 kg each were obtained from Japan SLC, Inc. (Hamamatsu, Japan). All animals were housed in an air-conditioned room (temperature: 21 ± 2°C; humidity: 55 ± 10%) under a 12-h light/dark cycle (lights on between 6 : 00 am and 6 : 00 pm) and maintained on commercial laboratory chow and water for at least one week before being used. All. LC/MS analysis. High performance liquid chromatography was carried out using the Hitachi L-7100 (Hitachi, Tokyo, Japan). Separation of ropivacaine was achieved using a reverse-phase column, Chemcosorb 7-ODS-H (internal diameter, 250 × 4.6 mm; Chemco Co., Osaka, Japan), protected by a precolumn (internal diameter, 10 × 4.6 mm; Eicom Co., Kyoto, Japan) containing AC-ODS. Injection volume was set to 5 μL. The mobile phase consisted of a mixture of acetonitrile and 10 mM ammonium acetate (95 : 5, v/v), with a flow rate of 0.3 mL/min. Column temperature was maintained at 30°C. Mass spectrometry was carried out using the Hitachi M-8000 LC/3DQMS (Hitachi, Tokyo, Japan) equipped with a sonic spray interface (SSI) and operated in the positive ion mode. The MS parameters were as follows: drift volume, 50 V; focus volume, 30 V; ion source temperature, 150°C; drying gas temperature, 250°C; and nitrogen gas flow pressure, 350 KPa. Measurement was carried out in the selected ion monitoring mode and the m/z value of the positive ion of ropivacaine was 275. Ropivacaine administration and blood sampling in rabbits. Ropivacaine was administered via the auricular vein or subcutaneous neck tissue at doses of 0.4 mg/kg, i.v. and 5 mg/kg, s.c., respectively. Blood samples were collected via the auricular vein in heparinized tubes at predetermined times after administration of ropivacaine and plasma was obtained by centrifugation at 1,000 × g for 10 min. In a similar manner, drug-free plasma samples were prepared for the recovery study of standard ropivacaine in plasma with TCA. Samples were stored at −80°C until analysis, which was performed within 2 days of each experiment. Plasma sample (200 μL) was mixed with 16.6 μL of 30% TCA, kept for 1 h and centrifuged for 10 min at 10,500 × g to remove precipitated protein. All procedures were performed at 4°C. Resulting supernatant was immediately filtered with a 0.45-μm filter (Millipore, MA, USA) and subjected to the LC/MS method for measurement of ropivacaine. Plasma ropivacaine concentration was determined from the standard curve and corrected by the recovery ratio of ropivacaine in the presence of TCA. Experiments were usually performed from 10 : 00 am to avoid circadian variations in drug metabolizing factors..
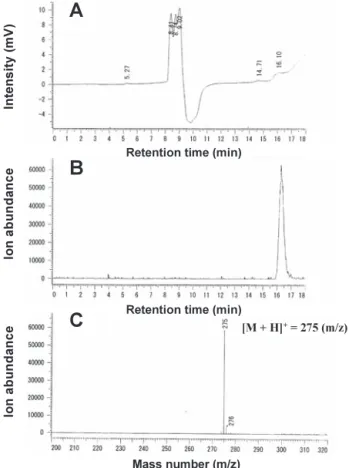
(4) Measurement of ropivacaine by LC/MS. RESULTS Measurement of ropivacaine by LC/MS When pure ropivacaine was injected into the LC/MS system, it was eluted after approximately 16 min under ultraviolet detection at 200 nm (Fig. 2A). Furthermore, when the mass chromatogram was monitored at 275 ([M+H]+) m/z ion for ropivacaine, a single maximal peak was observed at the same retention time (Fig. 2B). This intensity peak was in fair agreement with the mass spectrum for ropivacaine (Fig. 2C). However, no detection was observed with the LC system with a diode array detector (data not shown). These results show that ropivacaine was effectively separated using the reverse-phase column and selectively detected by the mass chromatogram/ spectrum.. 321. concentration of ropivacaine at 0.075 μg/mL was detected with strong intensity in the mass spectrum. This response was linked to ropivacaine concentration. The standard curve was linear and in the range of < 1.5 μg/mL (Fig. 3). Pharmacokinetics of ropivacaine We investigated the pharmacokinetics of ropivacaine using the LC/MS method described here. We first studied recovery of ropivacaine after precipitation of plasma protein with TCA. A recovery study was performed in the ropivacaine-free plasma samples with known amounts of the standard drug and TCA (Table 1). The assay revealed a decrease in recovery with increase in concentration of TCA. However, recovery was found to be over 90% in 2.3% TCA.. Ropivacaine concentration and intensity of mass spectrum When the injection volume was set to 5 μL, a low. Fig. 3 Relationship between ropivacaine concentration and intensity of mass spectrum. Five μL ropivacaine at concentrations indicated was injected into LC/MS system and mass spectrum at 275 m/z ion was measured. Data represent mean ± S.D. of 4–5 samples.. Table 1 Effect of trichloroacetic acid (TCA) on recovery of ropivacaine in plasma. Fig. 2 Measurement of ropivacaine using LC/MS system. Five μL ropivacaine (0.15 μg/mL) was injected into LC/MS system and monitored by UV chromatogram at 200 nm (A), mass chromatogram at 275 m/z ion (B) and mass spectrum at 16.1 min retention time (C).. Final concentration of TCA (%). Recovery of Ropivacaine (%). 1.3. 93.4 ± 2.7. 2.3. 91.8 ± 5.0. 5.0. 86.9 ± 7.6. 6.9. 68.6 ± 10.9. Total volume of 400 μL containing 200 μL rabbit plasma, 30% TCA, and ropivacaine (1.5 μg/mL) was kept on ice for 1 h and centrifuged at 10,500 × g for 10 min at 4°C. After passing through 0.45-μm filter, 5 μL resulting supernatant was injected into LC/MS system. Results indicate percentage of value obtained with TCA, with no-addition condition taken as 100%. Data represent mean ± S.D. of 4–6 samples..
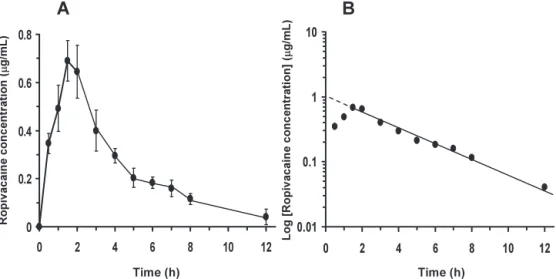
(5) 322. Therefore, a 2.3% concentration of TCA was used in the subsequent experiment. After 15 min i.v. administration of ropivacaine at a dose of 0.4 mg/kg, which did not induce CNS toxicity, the plasma concentration of ropivacaine averaged 2.17 ± 0.18 μg/ mL (Fig. 4A). The pharmacokinetic parameters for ropivacaine showed a one-compartment model and a mean elimination half-life of 0.54 ± 0.05 h (Fig. 4B). On the other hand, the plasma concentrations of ropivacaine after s.c. administration at a dose of 5 mg/kg showed biphasic displacement of absorption and elimination (Fig. 5A). Maximum plasma concentrations of ropivacaine averaged 0.69 ± 0.08 μg/. K. Sawaki et al.. mL after 90 min administration. Mean elimination half-life of ropivacaine was 2.83 ± 0.51 h (Fig. 5B) DISCUSSION Ropivacaine hydrochloride hydrate (Anapeine®) is widely used as a local anesthetic due to its lower toxicity to the CNS and the cardiac system than bupivacaine (4, 5, 18). However, when ropivacaine is used as continuous peripheral nerve block for postoperative analgesia such as in the form of epidural infusion, the monitoring of blood ropivacaine concentration is essential in preventing toxicity to the. Fig. 4 Pharmacokinetics of plasma ropivacaine after intravenous administration. Ropivacaine at dose of 0.4 mg/kg was administered via auricular vein in rabbits and plasma concentrations of ropivacaine (A) and pharmacokinetic phase parameters (B) were measured at times indicated. Values represent mean ± S.D. in 3 rabbits.. Fig. 5 Pharmacokinetics of plasma ropivacaine after subcutaneous administration. Ropivacaine at dose of 5 mg/kg was administered via the neck subcutaneous tissue in rabbits and plasma concentrations of ropivacaine (A) and pharmaco kinetic phase parameters (B) were measured at the times indicated. Values represent mean ± S.D. in 3 rabbits..
(6) Measurement of ropivacaine by LC/MS. CNS. In this study, we investigated a selective and sensitive method for measuring blood ropivacaine concentration using an LC/MS system. We found that ropivacaine was effectively separated using a reverse-phase column and a mobile phase consisting of acetonitrile and ammonium acetate. Ropivacaine was selectively detected by monitoring positive ion of ropivacaine at an m/z value of 275 (Fig. 2). Furthermore, when injection volume was set to 5 μL, we detected a low concentration of ropivacaine at 75 ng/mL with strong intensity. We also found that the intensity of the mass spectrum for ropivacaine correlated proportionately with concentration and that the standard curve was linear and in the range of < 1.5 μg/mL (Fig. 3). This result clearly demonstrates that the described LC/ MS method is highly sensitive for the measurement of ropivacaine and can be utilized for measuring the drug at low concentrations. It also suggests that it is possible to measure concentrations of ropivacaine of below 75 ng/mL when the injection volume is set to > 5 μL. We further examined the specificity of this method for the measurement of blood ropivacaine in rabbits. Since the amount of an unbound drug influences its pharmacological effect, knowledge of the unbound fraction may have significant pharmacodynamic implications (11). Ropivacaine is mainly bound to α1-acid glycoprotein in plasma protein (9). Measurement of recovery of ropivacaine after precipitation of plasma proteins with TCA revealed that recovery was over 90% in 2.3% TCA (Table 1). This result shows that the LC/MS method can accurately measure free ropivacaine in plasma samples. The pharmacokinetic parameters for ropivacaine showed a one-compartment model after i.v. or s.c. administration in rabbits (Figs. 4 and 5). The mean elimination half-life of ropivacaine after i.v. administration was 0.54 ± 0.05 h. This was approximately in agreement with previous results obtained in dogs using gas chromatography and flame-ionization detector, in which the elimination half-life of ropivacaine averaged 25.9 ± 1.7 min (3). This result indicates that the LC/MS method offers a viable tool for the pharmacokinetic study of ropivacaine. It is clear that the LC/MS system is suitable for the analysis of chemical compounds (10). We think that the LC/MS method described here is also applicable to the measurement of other amide-type local anesthetics such as bupivacaine or lidocaine. Further study is necessary to establish the LC/MS system for their measurement in more selectivity. In conclusion, the results of this study demon-. 323. strated that the LC/MS method was highly selective and sensitive for the measurement of ropivacaine, indicating that it offers a useful tool for monitoring the therapeutic effects and determining the pharmacokinetic parameters of this drug in blood. Acknowledgements We would like to thank Associate Professor Jeremy Williams, Tokyo Dental College, for his assistance with the English of the manuscript. REFERENCES 1. Abed WT (1994) Alterations of lidocaine and pentylenetetrazolinduced convulsions by manipulation of brain monoamines. Pharmacol Toxicol 75, 162–165. 2. Arlock P (1988) Actions of three local anaesthetics: lidocaine, bupivacaine and ropivacaine on guinea pig papillary muscle sodium channels (Vmax). Pharmacol Toxicol 63, 96– 104. 3. Arthur GR, Feldman HS and Covino BG (1988) Comparative pharmacokinetics of bupivacaine and ropivacaine, a new amide local anesthetic. Anesth Analg 67, 1053–1058. 4. Björk M, Pettersson KJ and Osterlöf G (1990) Capillary gas chromatographic method for the simultaneous determination of local anaesthetics in plasma samples. J Chromatogr 533, 229–234. 5. Catterall WA and Mackie K (2006) Local anesthetics. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11th ed. (Brunton LL, ed.), pp369–386, The McGraw-Hill Companies, New York. 6. Ciarlone AE and Juras MS (1981) Lidocaine and procaine alter rat brain amines. J Dent Res 60, 1886–1890. 7. Concepcion M, Arthur GR, Steele SM, Bader AM and Covino BG (1990) A new local anesthetic, ropivacaine. Its epidural effects in humans. Anesth Analg 70, 80–85. 8. Feldman HS, Arthur GR and Covino BG (1989) Comparative systemic toxicity of convulsant and supraconvulsant doses of intravenous ropivacaine, bupivacaine, and lidocaine in the conscious dog. Anesth Analg 69, 794–801. 9. Lee A, Fagan D, Lamont M, Tucker GT, Halldin M and Scott DB (1989) Disposition kinetics of ropivacaine in humans. Anesth Analg 69, 736–738. 10. Mathieu O, Hillaire-Buys D, Dadure C, Barnay F, MathieuDaudé JC and Bressolle F (2006) Liquid chromatographyelectrospray mass spectrometry determination of free and total concentrations of ropivacaine in human plasma. J Chromatogr B 831, 91–98. 11. Mazoit JX and Dalens BJ (2004) Pharmacokinetics of local anaesthetics in infants and children. Clin Pharmacokinet 43, 17–32. 12. McClure JH (1996) Ropivacaine. Br J Anaesth 76, 300–307. 13. Rifai N, Hsin O, Hope T and Sakamoto M (2001) Simultaneous measurement of plasma ropivacaine and bupivacaine concentrations by HPLC with UV detection. Ther Drug Monit 23, 182–186. 14. Satoh T, Sawaki K and Kawaguchi M (1996) Pharmacological analysis of local anaesthetic tolycaine-induced convulsions by modification of monoamines in rat brain. Pharmacol Toxicol 79, 305–311. 15. Sawaki K and Kawaguchi M (1989) Some correlations be-.
(7) 324 tween procaine-induced convulsions and monoamines in the spinal cord of rats. Jap J Pharmacol 51, 369–376. 16. Sawaki K, Ohno K, Miyamoto K, Hirai S, Yazaki K and Kawaguchi M (2000) Effects of anticonvulsants on local anaesthetic-induced neurotoxicity in rats. Pharmacol Toxicol 86, 59–62. 17. Sawaki K, Ouchi K, Sato T and Kawaguchi M (1991) Some correlations between local anesthetic-induced convulsions and γ-aminobutyric acid in rat spinal cord. Jap J Pharmacol 56, 327–335.. K. Sawaki et al. 18. Scott DB, Lee A, Fagan D, Bowler GM, Bloomfield P and Lundh R (1989) Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg 69, 563–569. 19. Tanaka K and Yamasaki M (1966) Blocking of cortical inhibitory synapses by intravenous lidocaine. Nature 209, 207– 208. 20. Yoshimura Y, Dohi S, Tanaka S, Tanaka K and Tsujimoto A (1991) Changes in convulsion susceptibility of lidocaine by alteration of brain catecholaminergic functions. Jap J Phamacol 56, 85–91..
(8)
図
関連したドキュメント
A selective, sensitive and rapid method for determining 8-OHdG in human urine was developed using hydrophilic interaction chromatography- tandem mass spectrometry (HILIC-MS/MS)
The re- sults presented in Table 3, showing that total lipase activity (measured in the absence of 1 M NaCl) was similar to HL activity (measured in the presence of 1 M NaCl) in
Abstract Aims: The purpose of this study was to develop high-sensitivity analytical methods for the determination of lansoprazole and 5-hydroxy lansoprazole, glibenclamide and
In this study, a rapid, sensitive and selective LC-MS/MS method using deuterated 1-OHP-glucuronide as an internal standard and an effective pretreatment method for urine samples
All of the above data showed that bufogenin having the 3β-hydroxy-5β-structure is enzymatically metabolized to the inactive metabolite having the 3α-hydroxy-5β-structure (Nambara
established ELISA, liquid chromatography tandem mass spectrometry (LC-MS/MS), and an automated high-throughput mass spectrometry (HT-MS/MS) system (RapidFire) to identify
まず,PREG 及び PROG の重水素標識体をアルカリ条 件下での交換反応により合成し,それぞれを IS として Fig.. 7) .コント
熱力学計算によれば、この地下水中において安定なのは FeSe 2 (cr)で、Se 濃度はこの固相の 溶解度である 10 -9 ~10 -8 mol dm