Title Endocrinological Studies on the Regulation of Reproductive andMetabolic Functions in Trained Yearling Horses( 本文(Fulltext) )
Author(s) Siriwan TANGYUENYONG
Report No.(Doctoral Degree) 博士(獣医学) 甲第492号 Issue Date 2017-09-22 Type 博士論文 Version ETD URL http://hdl.handle.net/20.500.12099/73117 ※この資料の著作権は、各資料の著者・学協会・出版社等に帰属します。
Endocrinological Studies on the Regulation of
Reproductive and Metabolic Functions in
Trained Yearling Horses
㸦
㸦୍ṓ㤿࠾ࡅࡿ⏕Ṫᶵ⬟௦ㅰᶵ⬟ࡢㄪ⠇㛵ࡍࡿෆศἪᏛⓗ◊✲㸧
2017
The United Graduate School of Veterinary Sciences,
Gifu University
(Tokyo University of Agriculture and Technology)
II
Content
Page
Chapter 1. Introduction 1
1.1 Horse 1
1.1.1 Horse and interaction with human 1
1.1.2 Horse age 2
1.2 Equine Growth 3
1.2.1 Lifespan and life cycle 3
1.2.2 Endocrinology of growth and metabolism 3
1.2.2.1 Thyroid hormone 5
1.2.2.2 Insulin-like growth factor I (IGF-1) 6
1.2.3 Other hormone (Cortisol) 7
1.3 Equine Reproduction 8
1.3.1 Estrous cycle in mare 8
1.3.2 Endocrine control of reproduction 8
1.3.3 Onset of puberty 10
1.3.4 Photoperiod and reproduction 11
1.3.4.1 Pineal gland-melatonin and regulation on reproduction 11 1.3.4.2 Light supplemental manipulation in horse 12 1.3.5 Endocrinology of reproduction in horse 13
1.3.5.1 Prolactin 13
1.3.5.2 Estradiol-17E and progesterone 13
1.3.5.3 Testosterone 14
1.4 Objectives of the study 15
Chapter 2. General methodology 16
2.1 Animals 16
2.2 Light supplementation program 18
2.3 Diets and exercise program 19
III
2.4.1 Extraction for insulin-like growth factor I (IGF-1) 20
2.4.2 Extraction for cortisol 20
2.5 Radioimmunoassay (RIA) of hormones 21
2.5.1 RIA of prolactin 21
2.5.2 RIA of immunoreactive (ir)-inhibin 21
2.5.3 RIA of IGF-1 21
2.5.4 RIA of cortisol 22
2.6 Fluoroimmunoassay (FIA) of hormones 22
2.6.1 FIA of estradiol-17E, progesterone and testosterone 22
Chapter 3. Establishment of a novel radioimmunoassay system 29 for total thyroxine (T4) measurement in horse
3.1 Background 29
3.2 Materials and methods 31
3.3 Results 37
3.4 Discussion 39
Chapter 4. Comparison of metabolic and reproductive endocrine 49 functions and body physical growth between north
and south climates of Japan in Thoroughbred trained yearling horses under natural condition
4.1 Background 49
4.2 Materials and methods 52
4.3 Results 55
IV
Chapter 5. Effect of light supplementation on metabolic and 66 reproductive endocrine functions and body physical
growth in Thoroughbred trained yearling horses in different climate conditions
5.1 Background 66
5.2 Materials and methods 67
5.3 Results 69
5.4 Discussion 72
Chapter 6. Comparison of metabolic and reproductive endocrine 86 functions between north and south climates of Japan
in Thoroughbred trained yearling horses under light supplementation
6.1 Background 86
6.2 Materials and methods 87
6.3 Results 88
6.4 Discussion 90
Chapter 7. General discussion 99
Chapter 8. Summary 105
8.1 Establishment of a novel radioimmunoassay system for 105 total thyroxine (T4) measurement in horse
8.2 Comparison of metabolic and reproductive endocrine functions 107 between north and south climates of Japan in Thoroughbred
trained yearling horses under natural and light supplementation conditions
Acknowledgement 110
1
Chapter 1
Introduction
1.1
Horse
1.1.1 Horse and interaction with humans
The horse (Equus caballus), odd-toed ungulate mammal which has single-digit oval- shaped hoof, is taxonomically classified to the order Perissodactyla and family Equidae (Grubb 2005). Wild horses were likely domesticated by human prior to 4000 BC with the earliest archaeological evidence on approximately 3500–4000 BC (Outram et. al 2009; Matossian 1997). The domestication was completed by 3000 BC and widely distributed throughout Europe continent by 2000 BC (Evans 1992). Domestication of wild horses led to horse breed variations and the different characteristics in horses (Bennett and Hoffmann 1999; Ensminger 1969). Horses are endothermic herbivores which are naturally grazing animals and have 64 chromosomes with 2.7 billion DNA base pairs (Antczak and McConville 2006). Horses can be categorized to hot-, warm- and cold-bloods. These words are widely used for equine terminology to describe in temperament not body temperature of horses. The characteristics of hot-blooded horses such as racehorses show more sensitivity and energy whereas the cold-bloods such as draft horses are quieter and calmer (Belknap 2004). The term of warm blood nowadays means to any cross between cold-blooded and hot-blooded breeds (Belknap 2004) which are used for competition in dressage and show jumping (Price and Shiers 2007). Since the old time until today horses have been used for many purposes by human e.g. leisure activities, sports (racing, show jumping dressage riding, endurance riding and competitions), and working (war, agriculture, horse police,
2
etc.). Recently, hippotherapy, term are used for different physical, occupational, and speech therapy treatment strategies that utilize equine movement. In hippotherapy, a therapist uses the horse's movement to improve their patient's cognitive, coordination, balance, and fine motor skills, whereas therapeutic horseback riding uses specific riding skills.
1.1.2 Horse age
In equine field, there is the use of terminology to describe horses in various ages as follows:
x Foal is a horse of either sex less than one year old. A nursing foal is sometimes called a suckling and a foal that has been weaned is called a weanling (Ensminger 1969). Most domesticated foals are weaned at five to seven months of age while weaning in natural wild foals are eight to nine months old. However, the domesticated foals can be weaned at four months with no adverse physical effects (Giffin and Gore 1998).
x Colt means a young male horse under four years old (Ensminger 1990).
x Filly refers to a young female horse under the age of four (Ensminger 1969).
x Yearling refers to horse of either sex that is between one and two years old (Ensminger 1969). In horse racing, these definitions may differ. For example, in the British Isles, Thoroughbred horse racing defines colts and fillies as less than five years old. However, Australian Thoroughbred racing defines colts and fillies as less than four years old (Yuille 2008).
x Gelding means a male horse of any age which is castrated (Ensminger 1969).
x Mare refers to a female horse from four years old and older (Ensminger 1969).
3
1.2
Equine growth
1.2.1 Lifespan and life cycleThe lifespan of domestic horse has been reported around 25 to 30 years depending on breed, environment, management and career (Ensminger 1969). Life stage or life cycle of horse can be divided into 4 phases e.g. foal, adolescence, adulthood and geriatrics. There are differences of growth and development among those phases. In foal, 11 months of gestation termed foal was born and most developed rapidly from birth to 12 months of age (Fig. 1.1) attaining 50 to 60% of mature weight and height in the first year and 80% to 90% of mature weight and height by 24 months of age. In yearling horse, in this stage horse’s legs almost grow in the length, and the body trunk is filled out. The two year of age, horse will reach his or her adult height and weight. The four years old horse will become adulthood and be completely growth. Aged horse shows signs of aging. This stage horse will have rapid deterioration and become death (Freeman and Topliff 2002).
1.2.2 Endocrinology of growth and metabolism
The development and growth of all living organisms from the simplest single cell to the most complex mammal require intrinsic and extrinsic factors and also need to adapt to environment for maintenance and survival of life. Endocrine hormone is one type of communication systems which cells, tissues and organs of the body used for coordination of their activities. The endocrine hormones are carried by the circulatory system to cells throughout the body where they bind with receptor and initiate many reactions (Guyton and Hall 2006). Some endocrine hormones affect most cells of the body such as growth hormone causing growth in most parts of the body, and thyroxine increasing the rate of many chemical reactions in almost all the body’s cell. Other hormones affect only specific tissues
4
called target tissue, because only these tissues have receptors for the hormone. For example, adrenocorticotropic hormone specifically stimulates the adrenal cortex, causing it to secrete adrenocortical hormones. The multiple hormone systems of the body play a key role in regulating almost all it functions. Besides growth hormone, thyroid hormone and insulin-like growth factor I are the important endocrine hormone which is involved in growth and metabolism (Guyton and Hall 2006).
Fig. 1.1 Development of Thoroughbred horse from foal to yearling in Hidaka Training and Research Center, Hokkaido; (A) 2 days; (B) 2 months; (C) 4 months; (D) 12 months of age.
A B C
5
1.2.2.1 Thyroid hormone
The thyroid gland secrets two significant hormones, thyroxine and triiodothyronine, commonly called T4 and T3, respectively in response to the stimulations of thyrotropin-releasing hormone (TRH) from hypothalamus and thyroid-stimulating hormone (TSH) from anterior pituitary gland. Both of these hormones have the profound effect of increasing the metabolic rate of the body and promote growth and development (Guyton and Hall 2006; Chen and Riley 1981; McGuire et al. 1991). All of the circulating T4 is derived directly from thyroid gland. Only 10%-20% of the circulating T3 is secreted directly from thyroid gland (Reed et al. 2004). Nevertheless, most of T4 is finally converted to T3 in the tissues. Over 99% of thyroid hormones circulating in blood bind to several type of plasma proteins which synthesized by the liver (Guyton and Hall 2006). Thyroid hormone combines mainly (70%) with thyroid hormone-binding globulin (TBG), thyroxin-binding prealbumin (TBPA, transthyretin) and albumin, bind to the lesser degree. In horse, the percentages of circulating T4 bound to TBG, TBPA and albumin were found to be 61%, 22% and 17%, respectively (Larsson et al. 1985). T3 is bound to TBG and albumin but not to TBPA (Reed et al. 2004).
The thyroid hormone receptors belong to the superfamily of nuclear receptors that work as transcription factors. The two types of thyroid hormone receptors are TR-D and TR-E. T3, whether directly transported into the cell or derived intercellularly from T4, is considered to be the effector hormone in target cells. The thyroid receptors interact with specific DNA sequences (T3-response elements), regulating gene expression. Growth and thermogenesis depend on the gene expression of thyroid hormones. Thyroid hormone decreases the expression of the D- and E-subunit genes of TSH and the TRH gene. From its effects on gene expression, T3 actions result in thermogenesis; increased oxygen consumption; increased
6
protein synthesis; increased metabolic rate; increased carbohydrate absorption and glucose metabolism; increased lipid metabolism, increased sensitivity of adipose tissue to lipolysis, increased neural transmission; and cerebral and neuronal development in young animals (Kaneko 1989).
1.2.2.2 Insulin-like growth factor I (IGF-1)
The insulin-like growth factor I is the somatomedin C, (small protein from the liver) which has effect on growth similarly to the effects of insulin on growth. Therefore, somatomedins are also called insulin-like growth factors. IGF-1 mainly secreted by liver is induced by growth hormone from pituitary gland. The IGF-1 concentration in the plasma normally follows closely the rate of secretion of growth hormone. Some aspects of the somatomedin hypothesis are still questionable. One possibility is that growth hormone can also cause formation of enough IGF-1 in the local tissue resulting in the local growth. It is also possible that growth hormone itself is directly responsible for increased growth in some tissues and that the somatomedin mechanism is an alternative means of increasing growth but not always a necessary one (Guyton and Hall 2006).
Several researches have been conducted about IGF-1 functions in various animal species. For example, IGF-1 plays role to promote body growth in animals including the period of embryonic development (Doherty et al. 1994; Herrler et al. 1998; Fabian et al. 2004; Wang et al. 2009). It is well-known as endocrine and autocrine/paracrine factor in local tissue such as testis and epididymis of various species (Brokaw et al. 2007; Yoon and Roser 2010) and is involved in steroidogenesis in rat, mice and pig Leydig cells (Lin et al. 1986; Kasson and Hsueh 1987; Wang and Hardy 2004). In horses, IGF-1 and its receptor has immunolocalized stronger in post-pubertal than pre-pubertal stallions (Yoon and Roser 2010) and also improve sperm longevity of equine spermatozoa (Champion et al. 2002).
7
Furthermore, IGF-1 and its receptor are localized in equine Leydig cells in aged dependency of stallions (Yoon et al. 2011) and also promote follicular growth and enhanced ovarian activity in mares (Derar et al. 2005; Hammond et al. 1991).
1.2.3 Other hormone (Cortisol)
Cortisol is steroid hormone which has glucocorticoid and mineralocorticoid activities. The principal glucocorticoid is secreted by adrenal cortex. Cortisol is very potent and accounts for 95% of all glucocorticoid activity. The key for control of cortisol secretion is the excitation of the hypothalamus by different types of stress which stimulate anterior pituitary gland resulting in adrenocorticotropic hormone (ACTH) releasing. The effects of cortisol are quoted in several aspects as follow; reduced in cellular protein stores in body cells except those of liver, increased the liver and plasma protein, increased blood amino acids, promoted mobilization for fatty acid from adipose tissue, reduced the inflammation in the body and blocked the inflammatory response to allergic reactions (Guyton and Hall 2006).
In addition, cortisol has been used as the indicator of stress. Almost any type of physical or mental stress can lead within minutes to greatly enhanced secretion of ACTH and consequently cortisol as well, often increasing cortisol secretion as much as 20-fold. Pain stimuli, caused by any type of physical stress or tissue damage are transmitted first upward through the brain stem and eventually to the median eminence of the hypothalamus. Corticotropin-releasing hormone (CRH) is secreted into hypophysial portal system. Within minutes the entire control sequence leads to large quantities of cortisol in the blood. For mental stress, it can cause an equally rapid increase in ACTH secretion. This is believed to result from increased activity in the limbic system, especially in the region of the amygdala and hippocampus, both of these then transmitting signals to the posterior medial hypothalamus (Guyton and Hall 2006).
8
1.3
Equine reproduction
1.3.1 Estrous cycle in mareBoth mare and stallion is seasonal polyestrous breeder. In breeding season, spring and summer, the ovary and testes are active. During this period, non-pregnant mare can show a series of repeated spontaneous estrous cycles and recurring ovulation. Each estrous cycle is 21 to 22 days (range, approximately 18 to 24 days), with estrus comprising 4 to 7 of these days and diestrus remains relatively constant at 14 to 15 days (Davies Morel 2015).
1.3.2 Endocrine control of reproduction
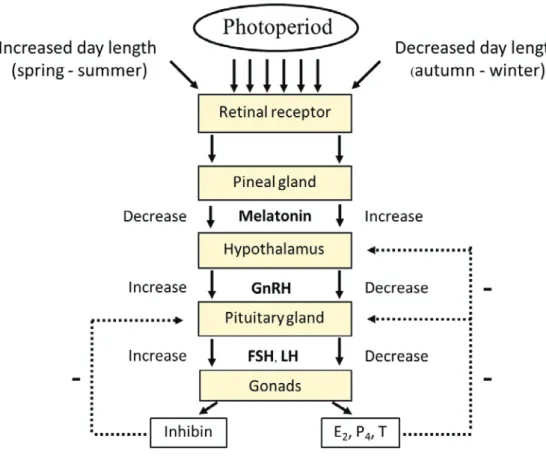
The endocrinological control of the reproduction on both female and male horses is regulated by the pineal gland and hypothalamic-pituitary-gonadal (HPG) axis. Day light is perceived by photoreceptor on horse retina, and then the neurosignals are sent to the pineal gland in the base of the brain. Normally, melatonin is produced nocturnally by the pineal gland, dominates the reproductive system by inhibiting HPG axis’s activity. When day length increasing, inhibition of the axis is removed allowing GnRH produced by the hypothalamus to reach anterior pituitary gland through portal blood vessels. The pituitary hormones, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) which are produced by gonadotrophs, are released and then act to the gonads in male and female horses. The FSH stimulates the follicular growth resulting in estrogen secretion and heat in female whereas promote spermatogenesis in male testes. LH released in a pulsatile manner, stimulates further development of growing follicles and also leads to ovulation in female horses while stimulates the testosterone secretion from Leydig cells of testes in male horses. After ovulation, LH helps to promote corpus luteum (CL) formation which stimulates progesterone, relaxin and inhibin productions (Tortora and Derrickson 2008; Davies Morel 2015). In
9
controlling of this pathway, the releasing and inhibiting hormone of hypothalamus will stimulate and suppress the release of pituitary hormones. Also, the hormone secretion of pituitary gland will be regulated by negative feedback from gonadal hormones (see Fig. 1.2).
Fig. 1.2 The hypothalamic-pituitary-gonadal (HPG) axis. E2: estradiol, P4: progesterone and T: testosterone.
10
1.3.3 Onset of puberty
Onset of puberty is influenced by many factors; endocrine hormone, nutrition, environment, etc. Some theory said that the onset of puberty is regulated by the maturity of the hypothalamic-pituitary-gonadal axis interconnections rather than by the pituitary not producing gonadotropins or by gonadal insensitivity (Squires 1993). The pre-pubertal animals maintain LH pulses in low amplitudes and frequencies and their GnRH pulse generator are very sensitive to negative feedback effects of estradiol and testosterone. At the onset of puberty, the increases in frequency of LH pulse leads to follicular development to more advanced stage resulting in secretion of higher estrogen concentration and stimulation of uterine growth. LH pulse increases and reaches a threshold for enhancing the ovarian growth to pre-ovulatory stage, estrogen induces a pre-ovulatory stage of gonadotropins and ovulation occurs (Squires 1993). Moreover, nutrition condition, body composition and metabolic factors may have a stimulatory or inhibitory effect on puberty in animals and human of sufficient age and weight (Kirkwood and Aherne 1985; Hopwood et al 1990). Photoperiod has influenced on the onset of puberty in seasonal breeder such as horse. Naturally, horse gives birth in the spring season with plenty of food sources. The puberty of horse will occur in the second spring of life at 12 to 15 months of age approximately (Wesson and Ginther 1981). In horse, fillies will reach puberty at 10-25 months of age (Wesson and Ginther 1981; Camillo et al. 2002). Spring-born fillies show heat as yearlings, and has first ovulation during late spring or early summer while those born later in the year generally do not cycle until they are two years old (McGing et al. 2016). The puberty in colt has been reported between 14-24 months of age by determination of semen characteristics (Skinner and Bowen 1968). However, the age of puberty has variation among breed of horse.
11
1.3.4 Photoperiod and reproduction
A photoperiod is the duration of light within a 24-hour period which plant or animal is exposed. Generally, most living things have their biological clock which use for response to the light and dark cycle of 24-hour rhythms in order to coordinate their biology and behavior with daily environmental changes. Photoperiod which is an important environmental factor regulate circadian cycles (Goldman and Nelson 1993). Besides the suprachiasmatic nucleus (SCN) in hypothalamus, retina and pineal gland is the internal clock of vertebrates (Kacsoh 2000).
1.3.4.1 Pineal gland-melatonin and regulation on reproduction
Pineal gland which has been viewed as a third eye (Eakin 1973), lies in the epithalamus, near the center of the brain, between the two hemispheres, tucked in a groove. The pineal gland produces melatonin which modulates sleep patterns in both circadian and seasonal cycles (Macchi and Bruce 2004). The darkness stimulates melatonin production while light will inhibit (Axelrod 1970; Lowrey and Takahashi 2000). Light perceived by photoreceptor in the retina of eye and pass as electrical signals to the SCN through the nerve fibers. Then, the nerve fibers relay the electrical impulses (daylight information) from the SCN via the paraventricular nuclei (PVN) in the hypothalamus and the spinal cord to the superior cervical ganglia (SCG) of sympathetic nervous system, and finally reach to the pineal gland (Yu and Reiter 1993). In pineal gland, melatonin is synthesized from tryptophan, amino acid, which converted to serotonin and then catalyzed by two specific enzymes. After converting by N-acetyltransferase, N-acetyl serotonin eventually converted to melatonin (Reiter 1980). In mammals, circulating melatonin concentrations increase in the dark time whereas become lowest during daylight (Kacsoh 2000; Brennanet al. 2007).
12
1.3.4.2 Light supplemental manipulation in horse
Horse is long-day animal with the highest reproductive functions in long-day season but shows anestrous or transient subfertility during short-day period. Artificial light is commonly performed in horses for advance onset of breeding season or enhance the anovulation during winter anestrus and vernal transition in non-breeding season. Accordingly, the differences of latitude lead to large variation of day length. At equator, daylight is 12 hours per day constantly. Temperate climate zone or the poles have fluctuation of photoperiod between summer and winter season. The minimal length of light exposure necessary has not been critically established, but field experience indicates that 14 to 16 hours of light stimulation (artificial plus natural light) per day is adequate (Brinsko et al. 2011). The lighting programs have traditionally been required a minimum of 8 to 10 weeks for response, mares in the northern hemisphere are exposed to the lighting system by December 1 to establish normal cyclic activity by mid-February. For individual stall-lighting systems, mare should be within 7 to 8 feet of a 200-watt light bulb to provide adequate light exposure and the stall should have sufficient window space to permit the same exposure during daylight (Kenney et al. 1975). The intensity of light, 107 lux approximately using the 100 watt bulb in the center of 12 x 12 feet horse stall is sufficient to improve reproductive function (Sharp and Cleaver 1993). However, various lighting programs suggested best results are obtained when the supplemental light is either added to the end of the day or split and added to both the beginning and the end of the day, instead of adding the supplemental light only at the beginning of the day (Sharp and Cleaver 1993). Recently, the manipulation of light supplementation using 100 watt white bulb in the horse stall (3.6 x 3.6 meter) providing of 14.5 hour light and 9.5 hour dark help to advance molting of winter to
13
summer coats, induce early ovulation and promote growth in yearling horses (Nambo et al. 2010; Kunii et al. 2015; Suzuki et al. 2015; Harada et al. 2015).
1.3.5 Endocrinology of reproduction in horse 1.3.5.1 Prolactin
Prolactin, one important pituitary hormone plays major role in lactation, hair shedding and reproduction. Prolactin is secreted by pituitary gland in higher level during long daylength period but lower in short-day period on both mares and stallions. In mares, prolactin and its receptor are found in follicular fluid, follicles and CL (Daoud and Ezzo 2014; Henderson et al. 2006; Yoon and Roser 2010). In male horses, prolactin involved in testosterone secretion via the stimulation of LH receptor expression in rodent Leydig cells (Hair et al 2002). Moreover, it is thought to be responsible for changes in metabolic rate, increasing the efficiency of food conversion during the winter months with evidence in the native breeds of horses (Argo and Smith, 1983; Morley et al. 1983; Evans et al. 1991).
1.3.5.2 Estradiol and progesterone
Estradiol-17E which is sex steroid hormone and the most potent in three estrogens (Guyton and Hall 2006), produced from cholesterol by interrelationship between the theca and the granulosa cells within developing follicle of ovary. The theca cells convert cholesterol to testosterone, which diffuses across to the neighbouring granulosa cells, where it is converted to estradiol-17. This final conversion within the granulosa cells depends upon the enzyme aromatase, whose activity is FSH-dependent. Estradiol-17E has various effects on several parts of body. Regarding the reproduction in mare, estradiol-17E is secreted into the blood circulation and 24-48 hours prior to ovulation reaches a peak of 10–15 pg/ml. Then, the levels will drop to basal levels immediately after estrus. The increase of
estradiol-14
17E concentration is responsible for the behavioral changes in the mare showing estrous sign or heat and accepting of copulation by stallion (Davies Morel 2015). In male horse, estradiol-17E is derived from testosterone by Sertoli cell of testes probably essential for spermatogenesis (Guyton and Hall 2006).
Progesterone is another sex steroid hormone which is synthesized by the ovary. After ovulation, CL forms within the collapsed follicle lumen. The luteal tissue is derived from the old theca cells. Progesterone is secreted by CL so that the level of progesterone rises after ovulation within 24–48 hours. Maximal progesterone concentrations reach to 10 ng/ml after 5-6 days post-ovulation and are maintained until day 15-16 of estrous cycle. If the mare has not conceived, progesterone levels will drop dramatically 4-5 days prior to the next ovulation to give basal levels again during estrus (Davies Morel 2015). Progesterone promotes uterine endometrium in preparing of uterus for implantation and also helps to increase secretion in fallopian tubes to nourish the fertilized ovum before implantation (Guyton and Hall 2006).
1.3.5.3 Testosterone
Testosterone, sex steroid hormone is secreted by Leydig cell of the testes. The Leydig cells are located in the intertubular spaces or interstitial tissue of the testis and are responsible for testosterone production by controlling of LH (Amann 1981). LH is produced in a pulsatile fashion. Therefore such a pulsatile release of testosterone means that a single blood sample for the hormone can give erroneous results; a hormone profile taken over a period of time and averaged is a much more accurate indication of true testosterone levels (Amann 1993). The FSH has been known as hormone to start the process of spermatogenesis, developing spermatogonia to secondary spermatocytes. Then, testosterone completes from secondary spermatocytes to spermatozoa which are ready for passage to the epididymis for
15
maturation (Davies Morel 1999). Furthermore, testosterone controls the development of male sex organs in the fetus or neonate, pubertal changes and maintains function of the accessory sex glands. Moreover, it is also responsible for male libido and sexual behavior in stallion. In addition, testosterone involves in growth acceleration and also muscular development (Irvine et al., 1986; Flink, 1988).
1.4
Objectives of the study
Regarding growth and reproduction, some connection among thyroid hormone, prolactin and reproductive organs have been reported (Evans et al 1991; Kunii et al 2015; Suzuki et al 2015). However the definite mechanism is still being unclear in young horses. Therefore, the study in this thesis covers a range of studies in development, growth and reproduction by investigation in yearling horses.
The objectives of the study were as follows:
a) To establish a novel radioimmunoassay system that can provide the reasonable total thyroxine values in Thoroughbred trained yearling horses.
b) To determine changes in body physical growth, metabolic and reproductive hormones in Thoroughbred trained yearling horses and compare those changes between Hokkaido and Miyazaki horses under natural condition.
c) To investigate the effect of light supplementation on changes in body physical growth, metabolic and reproductive hormones in Thoroughbred-trained yearling horses which were raised in different climate; the north Hokkaido and the south Miyazaki of Japan.
d) To compare body physical growth, metabolic and reproductive hormones between Hokkaido and Miyazaki horses under light supplementation condition.
16
Chapter 2
General methodology
2.1
Animals
All procedures in the studies were carried out in according with approval of the Institutional Animal Welfare and Experiment Management Committee of Japan Racing Association (JRA) Hidaka Training and Research Center.
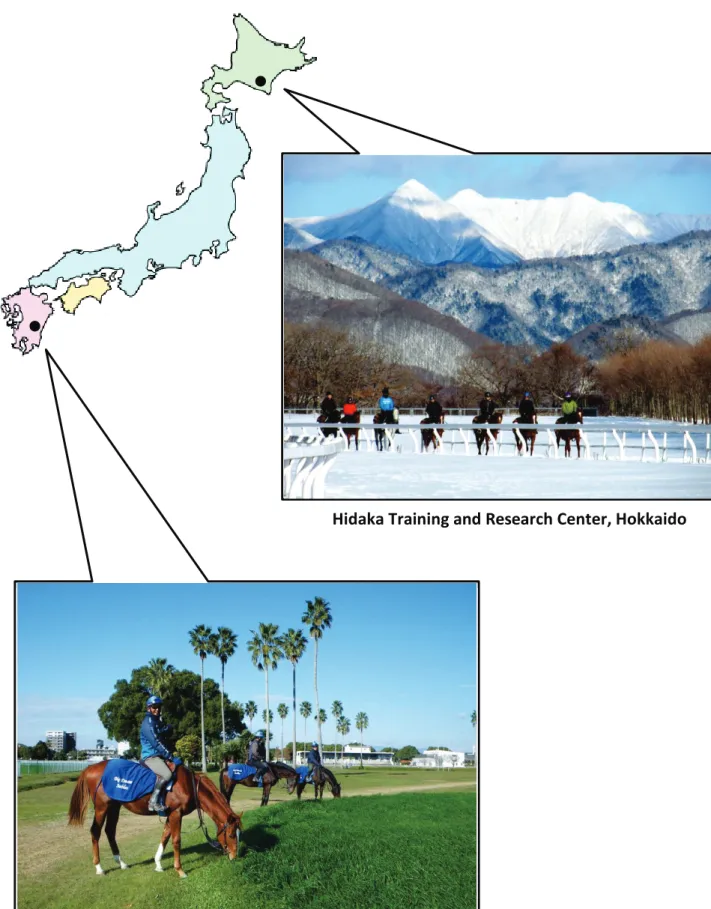
Thoroughbred trained-yearling horses from 1 year of age to less than 2 years of age raised in two facilities of JRA (Fig. 2.1) which were located in the north; Hidaka Training and Research Center in Hokkaido (latitude 42.2q and longitude 142.8q) and the south; Miyazaki Training Yearling Farm in Miyazaki prefecture (latitude 31.9q and longitude 131.4q), were used to be subjects of the studies for two year seasons from 2012-2013 and 2013-2014. Total 160 yearling horses were randomly divided into two groups as follow:
x Control group
The subjects in control group composed of 25 Hokkaido yearlings; 12 colts and 13 fillies, and 22 Miyazaki yearlings; 10 colts and 12 fillies. All horses in control groups only exposed to natural light without artificial light supplementation throughout experimental periods.
x Light supplementation (LS) group
The subjects in LS group consisted of 91 Hokkaido yearlings; 44 colts and 47 fillies, and 22 Miyazaki yearlings; 11 colts and 11 fillies. All horses in LS groups received supplemental lighting throughout experimental periods.
17
Fig. 2.1 The facilities of JRA in Hokkaido and Miyazaki prefectures, Japan.
Hidaka Training and Research Center, Hokkaido
18
2.2
Light supplementation program
In light treatment groups, artificial light supplementation was conducted by using timer-linked 100 watt white light bulb which was set in the ceiling of the horse stall (3.6 x 3.6 m) (Fig. 2.2). The light supplementation was performed 2 times per day as follow;
x At morning, starting before sunrise from 5.30 h to 8.00 h x At evening, starting before sunset from 16.30 h to 20.00 h
The photoperiod was extended equally as summer in 14.5 h daylight and 9.5 h of dark period (Northern Hemisphere) from December 25th to April 16th in each year for two year seasons.
19
2.3
Diets and exercise program
All horses were fed by hay (Poa pratensis), oats, and pellet feed (JRA original 10, NOSAN Corporation, Kanagawa, Japan) containing vitamin and trace mineral supplementation in each individual stall 4 times per day. Water was provided ad libitum. Through the end of December, Miyazaki horses were let to pasturing and fresh grass grazing daily while Hokkaido horses were pastured in small paddocks and ate dried Timothy grass. For winter pasturing since January, hay and water by heated automatic waterer were available for free access individually by Hokkaido and Miyazaki horses.
Training system was conducted in accordance with JRA traditional training programs for growing horse. Both Hokkaido and Miyazaki horses, horses warmed up in a walking machine for 30 min/day through the end of January while by trotting 800 m in February to March. Low- and high-intensity training were performed by canter and gallop on 800 m, 1600 m flat-track and 1000 m slope courses in Hokkaido (Fig. 2.3A), and 1600 m flat-track course in Miyazaki (Fig. 2.3B), then cooled down by walking.
Fig. 2.3 Slope course in Hidaka Training and Research Center (A) and flat-track course in Miyazaki Training Yearling Farm (B).
20
2.4
Method of hormone extraction
2.4.1 Extraction for insulin-like growth factor I (IGF-1)
Prior to measurement of IGF-1, the extraction of sample and standard had been required. “Acid ethanol cryo-precipitation”, a common procedure for extraction, was modified from the original method as previously described (Daughaday et al. 1980). Standards or samples (100 Pl) with the acid ethanol mixture (400 Pl), which was prepared with 2 M HCl and 99.5% ethanol in a ratio of 12.5%:87.5% (v/v) were mixed in 12 x 75 mm glass tubes and incubated at room temperature for 30 min. After centrifugation at 4qC, 2100 x g for 30 min, the supernatant was decanted, neutralized with 0.855 M Tris (hydroxymethyl) aminomethane (Sigma-Aldrich Co. LLC., St. Louis, MO, USA) solution at a ratio of 5:2, mixed thoroughly, and then stored at -20qC for 1 hr. After storage, all tubes were immediately centrifuged at 4qC, 2100 x g for 30 min. The supernatants were collected into fresh tubes and diluted with gelatin-PBS (phosphate buffered saline) which was composed of 0.05 M PBS containing 0.1 % sodium azide (Wako Pure Chemical Industries, Ltd., Osaka, Japan), 0.1% gelatin and 0.1% Triton X-100 (polyoxyethylene octylphenylether), pH 7.4 (Sigma-Aldrich Co. LLC.) to achieve at final solution, and then stored at -20qC until assayed. Herein, the final extracted solution (the supernatant diluted with gelatin-PBS) needed to be calculated for appropriate dose in order to obtain hormone levels which were expected in different age or status of each species, and adjusted to 50 Pl/tube for RIA, which was typically in the middle of the standard curve. The protocol of extraction for IGF-1 is shown in Fig. 2.4.
2.4.2 Extraction for cortisol
Sample (50 Pl) mixed with 1% bovine serum albumin; BSA (Sigma-Aldrich Co. LLC) 350 ul in total as 400 ul or serial standard solutions in 1% BSA (400 Pl) were prepared into glass
21
tube (13 x 100 mm), and added 2 ml diethyl ether (Dojindo laboratories, Kumamoto, Japan). After mixing for 3 min, all tubes were bathed in dry ice (99.5%) ethanol until water phase freezing (white spike appearance). The ether phase was decanted into fresh glass tube (12 x 75 mm) and was dried at 60qC in chamber. After cooling, wall of the tubes were rinsed with 0.5 ml ether and were then dried at 60qC in chamber. All tubes were reconstituted with 1% BSA. The protocol of extraction for cortisol is shown in Fig. 2.5.
2.5
Radioimmunoassay (RIA) of hormones
2.5.1 RIA of prolactinSerum or plasma concentrations of prolactin were measured by the equine prolactin RIA as previously described (Mizukami et al. 2015), using rat antisera against equine prolactin (Lot No. AFP-261987), purified equine prolactin (Lot No. AFP-8794B) for radioiodination, and equine prolactin (Lot No. AFP-7730B) for reference standard. The protocol for RIA is shown in Fig. 2.6.
2.5.2 RIA of immunoreactive (ir)-inhibin
Serum or plasma concentrations of ir-inhibin were measured by the bovine inhibin RIA as previously described (Hamada et al. 1989), using a rabbit antiserum against bovine inhibin (Lot No. H-1), purified 32-kDa bovine inhibin for radioiodination and standard. The protocol for RIA is shown in Fig. 2.6.
2.5.3 RIA of IGF-1
Serum or plasma concentrations of insulin-like growth factor I were measured by the RIA as previously described (Mizukami et al. 2015), using rabbit anti-serum against human
22
IGF-1 (Lot No. AFP4892898) and recombinant human IGF-1 (Lot No. 090701) for radioiodination and reference standard. The protocol for RIA is shown in Fig. 2.6.
2.5.4 RIA of cortisol
Serum or plasma concentrations of cortisol were measured by RIA system as previously described (Arai et al. 1995), using rabbit anti-cortisol (Lot No. HAC-AA71-02RBP85), Cortisol-3-CMO-BSA (Cat. No. 80-IC20) for radioiodination, and Hydrocortisone (Cat. No. H-4001) for reference standard. The protocol for cortisol RIA is shown in Fig. 2.7.
2.6
Fluoroimmunoassay (FIA) of hormones
2.6.1 FIA of estradiol-17EE, progesterone and testosteroneSerum or plasma concentrations of estradiol-17E, progesterone and testosterone were measured by time-resolved fluorescence immunoassay using commercial kit (DELFIA). Rabbit antiserum against estradiol, progesterone and testosterone; estradiol-, progesterone- and testosterone-europium tracer; and anti-rabbit IgG (goat) were used for assaying. Fluorescence was measured using the time-resolved fluormeter (1420 multilabel counter). The protocol for estradiol-17E, progesterone and testosterone FIA are shown in Fig. 2.8-2.9
.
23
100 Pl standard or sample were mixed with 400 Pl acid ethanol mixture in glass tube (12 x 75 mm) at a ratio of 1:4
Incubation at room temperature for 30 min
Centrifugation at 4qC, 2100 x g for 30 min
Decantation of supernatant and neutralization with 0.855 M Tris solution at a ratio of 5:2
Mixing and incubation at -20qC for 1 h
Centrifugation at 4qC, 2100 x g for 30 min
Decantation of supernatant and dilution with gelatin-PBS
Final extracted solution
Fig. 2.4 Extraction procedure for insulin-like growth factor I
24
400 Pl serial standard solutions in 1% BSA or 400 Pl sample solution (50 Pl sample was mixed with 1% BSA) in glass tube (13 x 100 mm)
Adding with 2 ml diethyl ether and mixing for 3 min
Freezing water phase with dry ice ethanol
Decantation of ether phase to fresh glass tube (12 x 75 mm)
Drying at 60qC in chamber
Rinsing the wall of the tube with 0.5 ml diethyl ether
Drying at 60qC in chamber
Reconstitution with 1% BSA
Fig. 2.5 Extraction procedure for cortisol
25
100 Pl standard or sample preparations in 0.05M PBS containing 5%BSA1
Adding 50 Pl primary antibody in PBS containing 0.4% normal serum2 and 0.05M EDTA
Incubation3
Adding 50 Pl 125I-labeled radioligands in 0.05M PBS containing 5%BSA4
Incubation5 for 24 h
Adding 50 Pl secondary antibody6 diluted with 0.05M PBS containing 5% polyethylene glycol
Incubation at 4qC for 24 h
Decantation of supernatant, swabbing of remaining liquid drops, and counting radioactivity in gamma counter
1
SPS (mixture of acidic ethanol, Tris, and gelatin-PBS at ratio of 2:1:2) for IGF-1 2
Normal rat serum for PRL, and Normal rabbit serum for ir-inhibin and IGF-1 3
4qC, 24 h for PRL; 37qC, 24 h for ir-inhibin; and room temperature, 1 h for IGF-1 4
Gelatin-PBS for IGF-1 5
4qC for PRL and IGF-1, and 37qC for ir-inhibin 6
anti-rat gamma globulin for PRL, and anti-rabbit gamma globulin for ir-inhibin and IGF-1
26
100 Pl standard or 50 Pl sample preparations in 0.05M PBS containing 1%BSA
Adding 100 Pl antibody in PBS containing 0.4% normal rabbit serum and 0.05M EDTA
Mixing for 3 min
Adding 100 Pl 125I-labeled radioligands in gelatin-PBS
Incubation 4qCfor 24 h
Adding 100 Pl anti-rabbit gamma globulin (ARGG) diluted with 0.05M PBS containing 5% polyethylene glycol
Incubation at 4qC for 24 h
Decantation of supernatant, swabbing of remaining liquid drops, and counting radioactivity in gamma counter
27
Pipetting 25 Pl standard solution or sample preparations into the strip wells
Adding 100 Pl diluted primary antibody in assay buffer
Incubation at room temperaturefor 30 min with slow shaking
Adding 100 Pl diluted Europium-labeled tracer
Incubation at room temperaturefor 2 h with slow shaking
Aspiration and wash each strip with washing buffer using DELFIA Platewash program
Adding 200 Pl enhancement solution and slow shaking for 5 min
Measurement of fluorescence in time-resolved fluorometer
28
Pipetting 25 Pl standard solution or sample preparations into the strip wells
Adding 100 Pl diluted Europium-labeled tracer
Adding 100 Pl diluted primary antibody in assay buffer
Incubation at room temperaturefor 2 h with slow shaking
Aspiration and wash each strip with washing buffer using DELFIA Platewash program
Adding 200 Pl enhancement solution and slow shaking for 5 min
Measurement of fluorescence in time-resolved fluorometer
29
Chapter 3
Establishment of a novel radioimmunoassay system for total
thyroxine (T4) measurement in horse
3.1
Background
The thyroid gland synthesizes and secretes the thyroid hormones 3, 3’, 5, 5’-tetraiodothyronine (or thyroxine; T4) and 3, 3’, 5-triiodothyronine (T3), which play important roles in thermogenesis, growth, and metabolism in animals (Breuhaus 2006; Chen and Riley 1981; Bird et al. 1998; Todini et al. 2015). T4 is transformed to T3, the active form, by monodeiodination in peripheral tissues (Reed et al. 2004). Although over 99% of thyroid hormones circulating in blood are bound to plasma proteins, only the free forms, fT3 and fT4, active in binding thyroid hormone receptors (Chopra et al. 1975; Evinger and Nelson 1984; Messer 1993; Reed et al. 2004).
Several techniques have been routinely used to evaluate thyroid hormone concentrations in various samples (e.g., plasma, serum, saliva, feces) for research and the diagnosis of thyroidal or non-thyroidal illness in humans and animals. Radioimmunoassay (RIA) has been considered to be the gold standard method, exhibiting high sensitivity and specificity, and low detection limits (Breuhaus et al. 2006; Fitzgerald and Davison 1998; Graves et al. 2006; Huszenicza et al. 2000; Kadunc et al. 2003; Slebodzinski et al. 1998; Sojka et al. 1993). Furthermore, equilibrium dialysis (Breuhaus et al. 2006), ultrafiltration (Sophianopoulos et al. 1980), enzyme immunoassay (EIA) (Brinkman et al. 2006; Medica et al. 2001; Todini et al. 2010; Todini et al. 2015), chemiluminescence immunoassay (CLIA) (Eshratkhah et al. 2011; Singh et al. 1997), chemiluminescent enzyme immunoassay (CLEIA)
30
(Esquivel and Ramírez 2016), and electrochemiluminescence immunoassay (ECLIA) (Eshratkhah et al. 2011; Wheeler 2013) are available for the evaluation of thyroid function in many species. Nonetheless, reference values and intervals of thyroid hormone concentrations substantially vary among measurement techniques and laboratories.
Recently, various commercial immunoassay kits that were developed for human samples are being widely applied to determine T4 and T3 concentrations for biomedical research and clinical hospital practice in animals, including horses, due to the simplicity of measurement procedure. Thyroid hormones are chemically common in human and horse. However, thyroid hormone binding proteins such as thyroid hormone-binding globulin (TBG), albumin, and thyroxin-binding prealbumin (TBPA, transthyretin) are different between species in their quantity and binding affinity (Engelking 2002). Although circulating concentrations of total T4 are the combination of T4 bound to the binding proteins and a small free portion (about 0.05% of total T4), importantly, determination of serum fT4 levels in horses does not provide any information regarding thyroid gland function that is additional to that of total T4 measurement (Sojka et al 1993). In addition, species difference in binding proteins can affect accurate detection of T4 by the kits that have been produced specifically for humans. Therefore, assay protocol optimized for total T4 of horses including dissociation of T4 from binding proteins is necessary to determine true value of circulating T4 in horses.
The aims of this study were: i) to establish a reliable and sensitive RI assay protocol, ii) to develop a method for binding protein separation that is simple and more convenient for equine T4 measurements, and iii) to evaluate the assay by comparing total T4 concentrations in horses among different chronologic ages and geographic climates.
31
3.2
Materials and methods
3.2.1 AnimalsTwenty one Thoroughbred-trained yearling and 2 adult horses (in estrus or diestrus) were used in this study. The yearlings were raised on Hokkaido (in the temperate north) and Miyazaki (in the subtropical south) in Japan with and without artificial light supplementation from October to April. Blood samples were collected from the jugular veins into non-anticoagulant and heparinized vacutainers. For different climate comparison, blood sample collection were performed one week before light supplementation (mid-December) and then once a month in late January, late February, mid-March and early April. After centrifugation, sera and plasma were harvested and stored at -20qC until assayed. To compare the two methods in binding protein separation, and differences between yearlings and adults, we used plasma samples from Hokkaido yearlings under light supplementation. Sera collected from adult horses in their estrous and diestrous periods were used for testing in sodium salicylate method. Plasma from Hokkaido and sera from Miyazaki yearlings under natural light conditions were also employed for different climate comparisons.
3.2.2 Separation of binding proteins
The separations of binding protein in this study were categorized to 3 methods as follows:
Sodium salicylate method. The use of sodium salicylate for binding protein separation had been described previously in thyroxine evaluation (Tohei et al. 1997). A glycine-gelatin buffer (GGB) containing 2% sodium salicylate (w/v) was typically used as T4 assay buffer to dilute with standard and samples. In the present study, the original GGB was used in preparation of standard and sample solutions. Then, GGB which was modified from Tohei et
32
al. by adjusting gelatin to 0.1% (w/v); with sodium azide 0.1% (w/v) added, pH titrated to 7.4, and without sodium salicylate, was required to mix with standard and sample solutions for RIA. In addition, to investigate the effect of sodium salicylate on the binding of T4 radioligand to the first antibody, serial dilution of the original GGB were performed with modified GGB in range from 1% to 0.002% sodium salicylate containing and then were assayed.
Acid ethanol cryo-precipitation. The following extraction method was modified from the original method which was commonly performed before IGF-1 measurement (Daughaday et al. 1980). The protocol for acid ethanol method has been demonstrated in Chapter 2 (see Fig. 2.4). Standards and samples (100 Pl) with the acid ethanol mixture (400 Pl), which was prepared with 2 M HCl and 99.5% ethanol in a ratio of 12.5%:87.5% (v/v), were mixed in 12x75 mm glass tubes and incubated at room temperature for 30 min. After centrifugation at 4qC, 2100 g for 30 min, the supernatant was collected by decantation and neutralized with 0.855 M Tris (hydroxymethyl) aminomethane (Sigma-Aldrich Co. LLC., St. Louis, MO, USA) solution at a ratio of 5:2, mixed thoroughly, and then stored at -20qC for 1 hr. After storage, all tubes were immediately centrifuged at 4qC, 2100 g for 30 min. The supernatants were collected into fresh tubes and diluted with gelatin-PBS which was composed of 0.05 M PBS containing 0.1 % sodium azide (Wako Pure Chemical Industries, Ltd., Osaka, Japan), 0.1% gelatin and 0.1% Triton X-100 (polyoxyethylene octylphenylether), pH 7.4 (Sigma-Aldrich Co. LLC., USA) to achieve at final solution; and then stored at -20qC until assayed.
Sodium acetate ethanol method. This technique is commonly used for protein precipitation using alcohol and sodium acetate (Nomura et al. 2003). The standard and
33
samples (100 Pl) were transferred to glass tubes. Sodium acetate ethanol mixture (300 Pl) which was a mixture of 2 M sodium acetate (Wako Pure Chemical Industries, Ltd.) and 99.9% ethanol in a ratio of 5%:95% (v/v) was added to each tube, carefully mixed, and then centrifuged at 4qC, 2100 g for 30 min. The supernatant was harvested and mixed with gelatin-PBS Triton X as the final solution. The extracted standards and samples were kept at -20qC until analysis. The protocol for sodium acetate ethanol method is shown in Fig. 3.1.
Herein, the final extracted solution for both methods (the supernatant diluted with gelatin-PBS), needed to be calculated and adjusted to 50 Pl/tube for RIA, which was typically in the middle of the standard curve. In addition, T4 concentrations in yearlings during growth and development were relatively high, so that a further ten-fold dilution of the final solution with modified GGB without sodium salicylate was required.
34
100 Pl standard solutions in modified GGB or samples were transferred to glass tubes (12 x 75 mm)
Adding with 300 Pl sodium acetate ethanol mixture
Mixing for 3 min
Centrifugation at 4qC, 2100 g for 30 min
Harvesting of supernatant to fresh glass tube (12 x 75 mm)
Adding with gelatin-PBS Triton X
Mixing for 3 min
35
3.2.3 Radioimmunoassay of T4
In radioiodination, tracer was prepared from thyroxine-BSA conjugate (Cat. No. 8960, Bio-Rad AbD Serotec Limited, Raleigh, NC, USA) labeled with 125I (NEZ033A, PerkinElmer, Inc., Waltham, MA, USA) by the chloramine T method as the previously described (Hasegawa et al. 1986). Total thyroxine (T4) concentrations were determined by a double-antibody RIA system. All samples and standard (L-thyroxine, T2376, Sigma-Aldrich Co. LLC.) doses were measured in duplicate and triplicate in the same assay. The diluted (1:1000) primary polyclonal antibody (lyophilized rabbit anti-thyroxine BSA serum, Cat No. 65850, MP Biomedicals, LLC., Santa Ana, CA, USA) in modified GGB without sodium salicylate containing 0.4% normal rabbit serum (NRS) was added (50 Pl/tube) to disposable polypropylene tubes (1.2 ml microtiter tubes for RIA, Thermo Scientific, San Diego, CA, USA). After mixing, all tubes were incubated at 4qC for 24 h. Tracer T4 labeled with 125I in modified GGB without sodium salicylate (50 Pl; approximately 5000 counts per minute) was added and briefly mixed. After incubation at 4qC for 24 h, the diluted (1:40) secondary antibody (anti-rabbit-gamma-globulin (ARGG), Veterinary Physiology Laboratory, Tokyo University of Agriculture and Technology) in modified GGB without sodium salicylate containing 7% polyethylene glycol (Wako Pure Chemical Industries, Ltd.) was added (50 Pl/tube) to the tubes. After mixing, the tubes were then incubated at 4qC for 24 h. Thereafter, bound and free ligands were separated by centrifugation at 1700 g for 30 min, at 4qC. The supernatant was decanted and the precipitate was counted in a gamma counter (Cobra Quantum, PerkinElmer, Inc.) for 1 min. The protocol for sodium salicylate method is shown in Fig. 3.2.
36
50 Pl extracted standard or sample were mixed with 50 Pl GGB without sodium salicylate
Adding 50 Pl primary antibody in GGB without sodium salicylate containing 0.4% NRS
Incubation at 4qC for 24 h
Adding 50 Pl 125I-labeled radioligands in GGB without sodium salicylate
Incubationat 4qC for 24 h
Adding 50 Pl anti-rabbit-gamma-globulin diluted with GGB without sodium salicylate containing 7% polyethylene glycol
Incubation at 4qC for 24 h
Decanting of supernatant, swabbing of remaining liquid drops, and counting radioactivity in gamma counter
37
3.2.4 Statistical analysis
All results are expressed as means ± standard errors of the means (SEM). Linear regression was applied to logarithmically transformed dose concentrations for the standard curve. The differences in means for T4 values between different binding-protein separation methods and age groups were analyzed using an unpaired Student’s t-test with Graph Pad Prism, ver. 5. For different climates in the longitudinal study, the repeated-measures data were analyzed using the generalized least squares (GLS) (Gueorguieva and Krystal 2004; Ugrinowitsch et al. 2004). Bonferroni's multiple comparisons were used to determine the differences in means of T4 values using R software. The level of significance was set at 0.05.
3.3
Results
Standard curve with sodium acetate ethanol methods
The representative dose-response curve for T4 demonstrated linearity between logarithmic dose 0.0078 and 1 ng/tube. The dose-response curve for horse samples at each age paralleled the standard curve (Fig. 3.3)
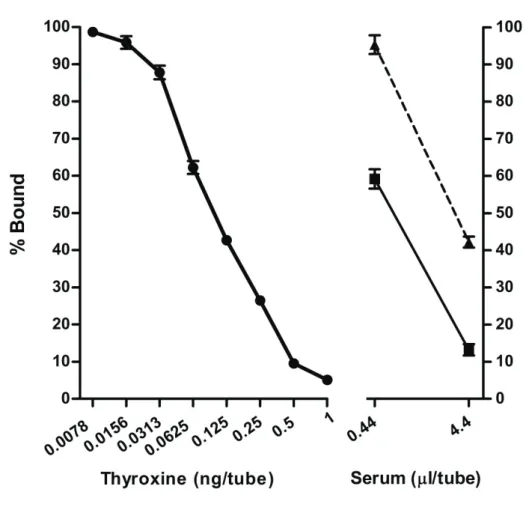
Comparison of acid ethanol and sodium acetate ethanol methods with respect to circulating T4 concentrations
The circulating T4 concentrations of two different separation methods were analyzed in triplicate in the same assay. After extraction, two doses of the extracted samples from both methods were selected, at 4.4 Pl/tube for the higher concentration and 0.44 Pl/tube for the lower concentration. The lower dose of 0.44 Pl/tube was appropriate and closer to 50% binding on the standard curve. The mean concentration of T4 in the 0.44 Pl/tube was not significantly different between acid ethanol and sodium acetate ethanol methods
38
(P=0.438) (Table. 3.1). Also, slopes of both linear regression lines were not significantly different (P=0.614) (Fig. 3.4). The intra-assay coefficient of variance was 5.0%.
RIA with sodium salicylate methods
As a fundamental method for binding protein separation using 2% sodium salicylate in original GGB, standards and sera from adult horses were prepared by mixing with the original GGB to obtain expected dose and were then diluted with modified GGB (without sodium salicylate) to 0.29% and 0.57% sodium salicylate, respectively, for the assay. The representative dose-response curve for T4 showed linearity between the logarithmic dose 0.0195 and 10 ng/tube (Fig. 3.5). The dose- response curves for adult sera (from horses in estrus or diestrus) did not parallel the standard curves. In fact, the percent bound for both estrous and diestrous sera were quite low at every dose, and also lower than for acid ethanol and sodium acetate ethanol methods, when comparing a similar dose (Fig. 3.5). Furthermore, the serial dilution of sodium salicylate containing in the original GGB using in the assay showed that sodium salicylate affected percent bound in dose dependent of manner. However, the percent bound seemed to be quite constant in range from 0.016% to 0.002% of sodium salicylate (Fig. 3.6). In addition, the use of 0.016% sodium salicylate for RIA still presented very low levels of circulating T4 in horse sample (data not shown).
Comparison of circulating T4 concentrations between yearling and adult horses with respect to the sodium acetate ethanol method
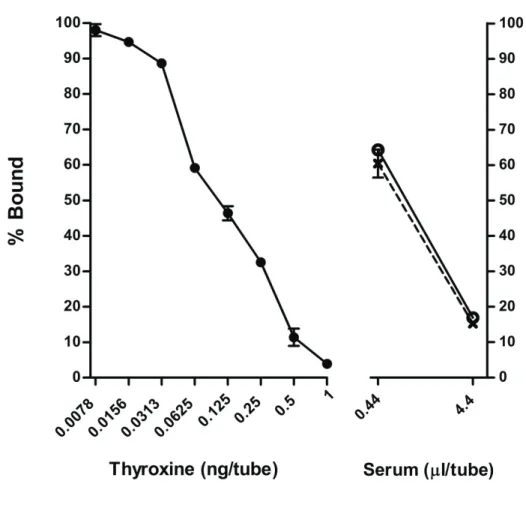
To evaluate whether the novel RIA system in the present study would provide reliable measurements of T4 in horse samples, we determined circulating T4 concentrations after sodium acetate ethanol extraction between different ages of horses (yearlings and adults), in triplicate in the same assay. Yearlings showed a significantly higher mean T4
39
concentration compared to adults at a dose of 0.44 Pl/tube (P=0.013) (Table 3.2). The mean concentration of T4 in yearlings was 6 times as high as in adult horses. Using linear regression analysis, both groups exhibited dose dependency and parallelism, with slopes that were not different (P=0.884) (Fig. 3.3). The intra-assay coefficient of variance was 8.2%.
Comparison of circulating T4 concentrations between Hokkaido and Miyazaki yearlings with respect to the sodium acetate ethanol method
To investigate whether the RIA was an appropriate tool for determination the true value of T4 in horse. Plasma and sera were obtained from growing horses raised in different climatic areas. Hokkaido, in the temperate north, and Miyazaki, in the subtropical south of Japan were selected for this experiment. Samples were then determined in duplicate after sodium acetate ethanol extraction in the same assay. There were no significant differences in circulating T4 concentrations between Hokkaido and Miyazaki male yearlings throughout the experiment (P>0.05). In fact, female Miyazaki yearlings showed significantly lower T4 levels compared with those of Hokkaido only at late January (P=0.007). However, circulating T4 concentrations in both genders of Hokkaido yearlings tended to be greater than those in Miyazaki throughout these periods (Fig. 3.7A and 3.7B). The levels of circulating T4 were also not significantly different among time periods in the Hokkaido and Miyazaki yearlings during the seasonal transition from winter to early spring (P>0.05).
3.4
Discussion
Most of circulating thyroid hormones are bound to plasma proteins. The free forms are small portion in blood. There are differences of T4 binding proteins between species in their quantity and binding affinity (Engelking 2002). These can affect accuracy of detection in
40
T4 concentration. Thereupon, it is essential to remove any binding proteins in serum or plasma before performing immunoassays. In the present study, we adapted two extraction techniques, acid ethanol and sodium acetate ethanol, and evaluated their suitability for determining equine T4 concentrations. The acid ethanol method, which has been commonly used for insulin-like growth factor I (IGF-1) measurement (Daughaday et al. 1980), requires proceeding in several steps that increase protocol time compared with the sodium acetate ethanol method. Therefore, we examined and compared the efficiency of protein extraction between both modified methods. The present study revealed that both extraction procedures had the capability to separate bound proteins from T4, appropriately showing parallelism to dose-response curve for extracted T4 standards. No differences in T4 concentrations between the two techniques indicated that use of the modified sodium acetate ethanol method not only possessed effectiveness in protein extraction similar to that of acid ethanol, but was also simpler, especially in case of large sample quantities. In the present study, GGB was modified by exclusion of sodium salicylate, which has been used as an inhibitor or displacer of thyroid hormone binding to serum protein in thyroid hormone measurements (Tohei et al. 1997). In previous publications, sodium salicylate was capable of binding plasma proteins in humans and other species (McArthur and Smith 1968; Sturman and Smith 1967; Wang et al. 1999), and the application of 2% sodium salicylate was required to separate binding proteins in serum samples. However, our study found that 2% sodium salicylate markedly affected the binding of T4 radioligand to the first antibody. To avoid this effect on binding, I tried to find an appropriate percentage of sodium salicylate which still existed with ability in binding protein separation but had no effect on tracer-antibody binding. This trial found that sodium salicylate should be low as 0.01% (Fig. 3.6). Consequently, the dilution to 0.01% made T4 levels too low to detect in
41
horse serum samples. For this reason, I used the alternative sodium acetate ethanol method instead of sodium salicylate for binding protein separation prior to RIA.
To validate our assay, I investigated parallelism among dose-response curves of yearlings, adults, and T4 standards (Fig. 3.3). As a result, assayed T4 concentrations in yearlings were clearly 6-fold higher than in adult horses, consistent with previous studies showing that young horses had T4 higher than adults (Blackmore and Brobst 1981; Gupta et al. 2002). It is reasonable that yearling horses in the process of development manifest higher metabolism than adults. In addition, we investigated and compared circulating T4 levels in different climatic conditions in yearling horses. Hokkaido in the northern part of Japan is located in a temperate zone such that it had lower temperatures throughout the experimental periods compared with the Miyazaki setting in the subtropical south. In this experiment, there was a tendency for circulating T4 concentrations to be higher in Hokkaido yearlings than in Miyazaki yearlings throughout these periods. This result is consistent with previous research in which horses staying in colder condition tended to have T4 levels higher than horses in warmer climates during the winter (Fazio et al. 2012; Mejdell and Bøe 2005). Our assay was similarly likely to have a sensitivity and specificity that was sufficient for the evaluation of T4 concentrations of physiologic responses at different ages and in different climates.
I believe that total T4 determinations are as important as fT4 for analyses of thyroid gland function, and this is supported by previous reports that fT4 concentrations in plasma were highly correlated with total T4 (Brinkmann et al. 2016; Elliott et al. 2013; Welcker et al. 2013). The value in adult horses in the present study was similar to reference values in several publications using various measurement methods, which reported that total T4 in adult equines were 11-36 ng/ml by CLEIA (Medica et al. 2011), 17-31 ng/ml (Mejdell and Bøe
42
2005); 25.2 ± 11 (winter) and 10.4 ± 6.3 (summer) ng/ml (Brinkmann et al. 2016) by CLIA; 30.72 ± 6.47 ng/ml (Medica et al. 2011); 40.3 ± 2.26 ng/ml (Todini et al. 2010); 24.1-49.47 ng/ml (Fazio et al. 2012); 26.95 ± 1.35 ng/ml (Gupta et al. 2002) by ELISA; 18-30 ng/ml (McBride et al. 1985); 6.2-25.1 ng/ml (Sojka et al. 1993) by RIA; and 4.7-35.74 ng/ml by equilibrium dialysis with RIA (Breuhaus 2006). Interestingly, total T4 levels in yearlings obtained from our RIA, 209.17 ± 15.05 ng/ml, were dramatically different from the reference value of 35 ng/ml (Reed et al. 2004), 68.68 ± 2.0 ng/ml in horses at 6 months to 1 year of age, or from 34.61 ± 0.92 ng/ml in 1-3 year old horses (Gupta et al. 2002). This high T4 levels in yearling horses probably derived from my removal of binding protein using the sodium acetate ethanol method when compared to other techniques (e.g., ELISA and RIA kits) in previous reports (Gupta et al. 2002 and Reed et al. 2004). In conjunction of the present study with previous reports showing that the similarity in adults and difference in yearlings of T4 values might indicate that our immunoassay, compared with other methods, appears to be more physiological and appropriate in revealing the true value of total T4 in equine species.
In conclusion, this is the first report describing a modified sodium acetate ethanol technique that is simpler and more convenient for bound T4 protein extraction, and the first study in which a reasonable radioimmunoassay was established specifically for circulating total T4 measurements in horses. Other physiologic changes in T4 be should be investigated in order to more fully understand thyroid gland function in yearling horses.
43
Fig. 3.3 Radioimmunoassay dose-response curves for total T4 concentrations with sodium
acetate ethanol extraction. T4 standards ( z ) from 0.0078 to 1 ng/tube, yearling plasma ( ) and adult sera ( S ) using 125I-labelled thyroxine as tracer. The X axis shows doses of T4 on a logarithmic scale. Each value is expressed as the mean ± SEM of triplicate measurements. Comparison of percent bound was obtained from yearling plasma and adult sera, and showed that the regressed line paralleled the T4 standard (y= 13.89x ± 74.30 and -13.42x ± 101.2, r2=0.878 and 0.993; P<0.05 and P<0.001, respectively).
44
Fig. 3.4 Radioimmunoassay dose-response curves of total T4 concentrations in yearling
plasma. T4 standards ( z ) from 0.0078 to 1 ng/tube were extracted by acid ethanol method. Samples were extracted by sodium acetate ethanol ( || ) and acid ethanol ( ± ) methods. Each value is expressed as the mean ± SEM in triplicate measurements. Both methods of extraction, sodium acetate ethanol and acid ethanol, showed similar regression lines (y= -11.96x ± 69.55 and -11.41x ± 65.46, r2=0.996 and 0.970; P<0.0001 and P<0.001, respectively).
45
Fig. 3.5 Radioimmunoassay dose-response curves of total T4 concentrations in adult sera
using sodium salicylate for extraction. Estrus ( ) and diestrus ( U ) periods were extracted via the method of bound protein separation with sodium salicylate. Each value is expressed as the mean ± SEM in triplicate and duplicate measurements for standard and serum samples.
46
Fig. 3.6 Radioimmunoassay dose-response curves of total T4 concentrations using sodium
salicylate for binding protein separation. T4 standards ( z ) from 0.0195 to 10 ng/tube and glycine gelatin buffer containing with sodium salicylate (|) from 1% to 0.002% used 125 I-labelled thyroxine as tracer. Each value is expressed as the mean ± SEM in triplicate measurements.
47
Fig. 3.7 Comparison of mean total T4 concentration between Hokkaido ( z, N = 10 ) and Miyazaki ( , N = 10 ) yearlings, both colts (A) and fillies (B) from December to April. Each value is expressed as means ± SEM. * Indicates that the T4 levels differ significantly (P<0.05) between horses in the two climate groups in each period and for each sex.
48
Table 3.1 Comparison of serum total T4 concentration in yearling horses between two
different extraction methods
Dose Mean total T4 concentration in horse serum (ng/ml) (Pl/tube) Sodium acetate ethanol Acid ethanol
0.44 174.37 ± 3.33 197.32 ± 22.27
Data were expressed by mean ± SEM (P>0.05).
Table 3.2 Comparison of serum total T4 concentration between yearling and adult horses
Dose Mean total T4 concentration in horse serum (ng/ml)
(Pl/tube) Yearlings Adults
0.44 209.17 ± 15.05a 34.92 ± 13.69b
Data were expressed by mean ± SEM at doses of 0.44 Pl/tube. (a, b)