FREE
FATTY ACID CONTENTS OF
WASANBON-TO SUGAR
Toshiyuki
MATSUIWasanbon-to sugar is handmade sugar which has been manufactured traditionally in J a p a n by a unique refining procedure The free f a t t y acids with twelve or more carbon atoms were analyzed in cane sugar pressed juice, Shiroshita-to sugar, Wasanbon-to sugar, and Wasanbon-to sugar stored for one year, by the DUNCOMB method, gas chromatography, and GC-MS. The following results were obtained.
Myristic, palmitic, stearic, oleic, linoleic, and arachidic acids were separately determined in Wasanbon-to sugar. There was no significant difference between the products made by manual process and centrifugal one in free f a t t y acid contents Wasanbon-to sugar stored for one year contained less oleic acid and more palmitic acid than fresh sugar From 60 to 90% of total free f a t t y acids was removed during manufacturing Shiroshita-to sugar. Concering total free f a t t y acid contents, there was no significant difference between pressed juice from sugar cane of N : Co 310 (N : Co) and t h a t of Chikusha
@3T@lZ, %fi;?'EO@%sRBET15b
h
a%!?
4oW@'C%
6,W X % ~ ~ U L O R B % ~ % B % E W H ,
137;E ,
?U=&%,
1%.E%
Lf<@Z&EkiRH L,
4 Ya YE,
%'z P .7 b 4r
7 4 .-, GC-MS Tft%L
T ,l
2
7;O%%%%fc,
<
!J x ? Y@, , < / b : 9 Y@, X T , ~YE,
ij- W < Y@, 9 1 ,- jb@, .7? + ~ Y @ & $ ~ S , & @ T E & L ~ : ~
fRilPBk@%&t=%~\,~,
48 L,%&Gft@E
t
4,K . + & % l a I ~ a ~ ~
1$.Ej@
LY:$uz&@ba,
@z&@&
47f. W / I
Y@lX+f~~\fi,
E+
' / @ l h + & ~
fco$f$$tjjBfi@ki,
Q7;@B&+R60%;31b90%@&ShkO &f
%JlBBk@0
5 '6, N : CO 310O&%ff.
t
'uf.EOE%H - t ~ + % E .
f ~ , IntroductionFree amino acid"-a), organic a~idcl-~), carbohydrate'l-a' and mineral component contents(3) in Wasanbon-to sugar were reported in the previous paper(4 5). (compare the review")) Although fair amounts of free f a t t y acids were detected in Wasanbon-to sugar and refinery final molasses extracted with hexane and other organic solvents, t h e development of browning color was observed and acidic fractional-flavor derived from the highest boiling range was increased when Wasanbon-to sugar and refinery final molasses were stored for several years'? Despite of few phenomenon described above, no reports have been published on the substances responsible for t h e unique flavor of this sugar.
In this study, free f a t t y acids were detected and determined by GC-MS and gas chromatogra- phy in pressed juice from sugar cane, Shiroshita-to sugar (pre-refined sugar) and Wasanbon-to sugar. The d a t a obtained were analyzed statistically by t-test") for the purpose of finding out
the differences between paired varieties, factories, and processes Owing t o small amounts of f a t t y acids with eleven or less carbon atoms which showed violent variation among the same lots, the free f a t t y acids with twelve or more carbon atoms were analyzed.
Materials and Methods
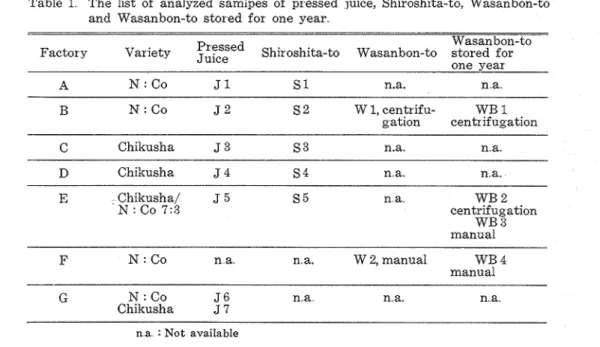
Materzals Wasanbon-to sugur, Shiroshita-to sugar, and sugar cane pressed juice are the 1978 products of Wasanbon-to sugar Refining Co A, Co B, and Co. G, Hiketa, Kagawa P r e f , and similar Sugar Refining Co C, Co D and Co. F, Kamiita, Tolcushima Pref. I n order to compare with Wasanbon-to sugar and t h a t stored for one year, those of Wasanbon-to sugar Co B and Co F were used Five samples from each lot were selected a t random except JG and 57 and each sample was analyzed twice Table 1 shows the sample used in this experiment
Table 1 The list of analyzed samipes of pressed juice, Shiroshita-to, Wasanbon-to and Wasanbon-to stored for one year
Wasanbon-to
Factory Variety
F:P:F~
Shiroshita-to Wasanbon-to stored forone year
B
N
: CoJ
2 S 2 W 1, centrifu- WB 1gation centrifugation
C Chikusha J 3 S 3 n.a.. n.a.
D Chikusha J 4 S 4 n.a.. n.a..
E Chikusha/ J 5 S 5 n a WB 2
N
: Co 7:3 centrifugation WB 3 manual -- -- - F N : Co n a n a. W 2, manual WB 4 manualG N : Co J 6 n..a. n. a,. n,a.
Chikusha J 7
n a : Not available
Separatzon of f r e e f a t t y aczds
1) Extractzon of total lzpzd by FOLCH method(8) Sugar cane pressed juice mas adjusted to pH 8-9 with I N sodium hydroxide, and evaporated to surupy liquid under reduced pressure a t 28-30°C The syrupy liquid was diluted with water, adjusted to pH 4-5 with 1 N phosphoric acid. and extracted with 100 ml of a mixture consisting of 2 parts of chloroform and 1 p a r t of methyl alcohol (approximately ten times of the amount of syrupy solution) for 48 hr by a shaker (120 rpm/min) a t 25-28°C The residue was extracted again with the same solvent by the similar way The total extract was adjusted to pH 8-9, and evaporated under reduced pressure. The extract contained fair amout of sugar, and therefore the extract was washed twice with 40ml of water containing 1 % sodium chloride after adding to 400 ml of the chloroform-methyl alcohol mixed solvent In Shiroshita-to sugar and Wasanbon-to sugar, each sample was diluted with water and treated by the same way described above
2) Extractzon of f a t t y aczds by tlzzn, layer chromatography(TLC).
One dimensional ascending TLC was performed on 20 x 20 cm glass plates coated with a 0 50 mm layer of silicic acid (Kieselgel G, Merck), and the solvent system used was petroleum ether-ethyl ether-acetic acid (80 : 20 : 1, V/V)(8). Color was developed under ultraviolet and/or iodine vapor The main spot of Rf value 0.55 compared to palmitic acid a s the authentic standard was
scraped out, collected and extracted with methyl alcohol-ethyl ether (3 : 1). After evaporating the solvent the fractions of free fatty acids were made up t o 10 ml with methyl alcohol.
Colorimetr.ic d e t e r m i n a t i o n o,f' total f a t t y acid. The aliquot of lOml alcohol solution was
determined from a standard curve according to the procedure described by DUNKOMB(~O).
C o n d ~ t z o n s o f gas c h r o m a t o g r a p h y (GC) The aliquot of 10 ml alcohol solution was methylated
with diazomethane(ll) using the apparatus described by USUI and YOSHITOMI(~~). The free f a t t y acids were analyzed by a Hitachi Model 063 gas chromatograph, fitted with a hydrogen flame ionization detector. The colums were : 2 m x 3 mm i. d stainless steel containing 5 % Carbowax 20 M on 60/80 mesh Chromosorb W (AW-DMCS). The temperature of the column was program. med from 70 to 190°C a t the rate of 5"C/min, with the nitrogen flow rate 30ml/min. The injection port and dector oven temperatures were 270 and 220°C, respectively.
C o n d z t ~ o n s o f GC-MS The GC-MS system consisted of a JGC-20 KP gas chromatograph coupled
with a JEOL JMS-07 mass spectrometer, The gas chromatographic separation was made on a glass column containing 3 % silicon SE-30 on 60/80 mesh Gas Chrome Q (2 m x 3 mm i. d.). The column temperature was programmed from 180 to 270°C a t the rate of 5"C/min with the helium flow r a t e 30 ml/min The spectrometer was operated under the conditions of 70 eV ionization voltage, 3 5 kV ion accelerating voltage,
lop
A of ion current and 280°C of ionization chamber tempeuatur e.Peak a s s z g n m e n t a n d q u a n t z t a t i v e d e t e r m i n a t i o n . Each compound in gas chromatogram was
identified by comparison of retention time with t h a t of authentic compound. Each mass spectrum was also compared with t h a t of the authentic substance or with
t h a t in the literature.. The relative quantity of each peak on the chromatogram was determined by calculating its peak hight t o standard curve on the basis of authentic compound chracterized by GC-MS.
Results and discussion
S e p a r a t i o n a n d i d e n t i f i ' c a t z o n o f f r e e f a t t y a c i d s , F i g 1 shows
the chromatogram of free fatty acids on TLC plate. One of the Rf values of fatty acids including those from C12 was similar t o Rf of palmitate in the solvent system, and therefore the acid was used as the standard. The spot giving Rf value of 0.53 t o 0.. 56 was scrapped out and used for determining total fatty acid contents.
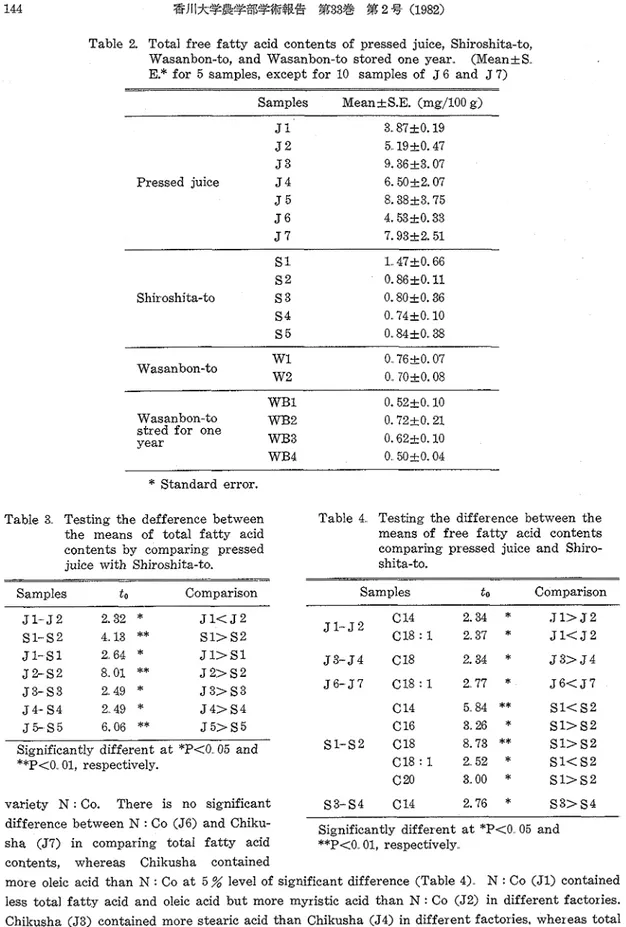
Myristic, palmitic, stearic, oleic, linoleic and arachidic acid were identified on the basis of fragmentation pattern including mass number and based peak such as M+, m/e 74,87, M-43, and M-31(13). Tabie 2 shows the standard error of the mean of total fatty acid contents determined by the Duncomb method. Table 3 shows the result of testing the difference between the means of total fatty acid contents by comparing pressed juice with Shiroshita-to sugar
C o m p a r i s o n o f f a t t y aczds o n pressed juzce f r o m s u g a r cane.
In a previous paper(5), it is concluded t h a t the sugar cane pressed juice from Chikusha variety contained less free amino acids and reducing sugars but higher titratable acidity than did t h a t from
C
0
0
0
6
0
0 Authentic Sample Authentic=Palmitic acid Fig. 1. Thin-layer chromat-ogram of free fatty acids Plate: Silica gel G
Developer : Petrole- um ether-Ethyl e- ther-Acetic acid (80 : 20 : 1)
Table 2. Total free f a t t y acid contents of pressed juice, Shiroshita-to, Wasanbon-to, and Wasanbon-to stored one year.. (MeankS,. E.* for 5 samples, except for 10 samples of J 6 and J 7)
Samples MeankS.E. (mg/100 g) J 1 3.. 87k 0.19 J 2 5.19k0.47 J 3 9.36k3.07 Pressed juice J 4 6.50k2.07 J 5 8.38k3.75 J 6 4.53k0.33 J 7 7.93k2.51 Wasanbonto WB2
stred for one
vear WB3
*
Standard error. Table 3. Testing the defference betweenthe means of total f a t t y acid contents by comparing pressed juice with Shiroshita-to.
Samples t o Comparison
Table 4. Testing t h e difference between the means of free f a t t y acid contents comparing pressed juice and Shiro- shita-to.
Samples t o Comparison
Significantly different a t *P<O. 05 and **P<O.. 01, respectively.
variety N : Co. There is no significant $33- S4 C14 2.76
*
S 3 > S 4difference between N : Co (J6) and Chiku- Significantly different a t *P<O 05 and sha (57) in comparing total f a t t y acid **P<O 01, respectively
contents, whereas Chikusha contained
more oleic acid than N : Co a t 5 % level of significant difference (Table 4) N : Co (Jl) contained less total f a t t y acid and oleic acid b u t more myristic acid than N : Co (52) in different factories. Chikusha (53) contained more stearic acid than Chikusha (54) in different factories, whereas total acid contents had no significant difference
100
50 (%) Ratio
C14 Ci6 Cis Cls=i C18.2 C20 Total
0
Sugar cane pressed J u i c e . S h i r o s h i t a - t o Sugar. Wasanbon- to Sugar.m
Wasanbon-to S u g a r s t o r e d f o r one year.. Fig.. 2.. Average compositions of free f a t t y acidsC o m p a r i s o n o f ' f a t t y a c i d s i n S h i r o s h i t a - Table 5. Testing the difference between the t o s u g a r . Shiroshita-to sugar of S1 contain- means of free f a t t y acid contents comparing Wasanbon-to with t h a t ed more total f a t t y acids than t h a t of S2. stored for one year
Shiroshita-to sugar of S1 contained more
palmitic, stearic, and arachidic acids but less Samples t o Comparison
myristic and oleic acids than t h a t of S2 in C 14 3.73
**
Wl>WB1different factories. Shiroshita-to sugar of W1-WB1 C16 2.60
*
Wl<WBl53 contained more myristic acid than t h a t C18 : 1 3.26
*
Wl>WBlof 54, C 16 2 8 5
*
W2<WB4C o m p a r i s o n o,f , f a t t y a c i d s i n Wasanbon- W2-WB4 C 18 : 1 6.. 41
**
W2>WB4 t o s u g a r . There is no meaningful differenceSignificantly difference a t *P<O. 05 and between W1 and WB1 as well as W2 and **p<o. 01, respectively.
WB4. Significant difference was not ob-
served between the Wasanbon-to sugar of WB3 refined by manual processes and t h a t of WB2 refined by centrifugation In comparison with WB1 and W1, Wasanbon-to sugar of W1 contained more myristic and oleic acids but less stearic acid than that of WB1. The interaction between W2 and WB4 was similar to t h a t between W1 and WB1 except for myristic acid.
C o m p a r zson of f a t t y aczd contents d u r z n g re f z n z n g process. Changes of total f a t t y acid were determined during refining process. From 10 to 40% of total f a t t y acid was removed during condensing process of Shiroshita-to products, and myristic, palmitic and stearic acid contents decreased in all samples. On average about 10% of total f a t t y acid was removed during refining process, Wasanbon-to sugar products, but free fatty acid contents gave no meaningful difference statistically From 60 to 90% of total free fatty acids were removed during manufacturing Shiroshita-to sugar.
Table 6.. Testing the difference between the means of free f a t t y acid contents comparing pressed juice with Shiro- shita-to
Samples t o Comparison
Significantly different a t *P<O. 05 and
**P<O. 01, respectively.
Ackowledgements.
The author wishes t o thank Professor Shozaburo KITAOKA of University of Osaka Prefecture, and Professor Sin'itirB KAWAMURA of Kagawa-Ken Meizen Jounior colledge, and thanks Mr. Yoshi- yuki MIYATAKE of Livelihood Cooperative Association of Izumi city for his technical assistance. He is grateful to Wasanbon-to Sugar Refining Co. Mitani, Co. Yamada, Co. Hattori, Co. Kageyama, Co. and Miura, Co. for gifts of samples
References ( 1 ) MATSUI T. and YAMADA K. : E i y o T o Sho-
k u r y o , 28, 371 (1975).
( 2 ) MATSUI T. : S h o k u h i n Kogyo G a k k a i s h i ,
24, 487 (1977).
( 3 ) MATSUI T. : E i y o T o S y o k u r y o , 30, 223 (1977). ( 4 ) YAMANAKA K. : S h o l c u h i ~ Kogyo Gak-
k a i s h i , 21, 438 (1974).
( 5 ) MATSUI T. : S h o k u h i n Kogyo Gakaishi,
27, 307 (1980).
( 6 ) MATSUI T. and KITAOKA S. : J. N u t r . Sci.
Vitamilzol.. 27, 563 (1981)
( 7 ) SNEDECOR G. W. and COCHRAN W. G. : Sta-
tistical Methods, 91, The Iowa State
University Press, Ames.. Iowa (1978).
( 8 ) FOLCH J. and LEES M.. : J, B i o l , Chem., 191, 801 (1951) ..
k a g a k u K e n k y u h o , P a r t I , ed. by ANDO
E., TERAYAMA H., NISHIZAWA K., and YAMAKAWA T. 84, Asakura Shoten, Tokyo (1967).
(10) DUNCOM W. G.. : B i o c h e m , J., 88,7(1963).
(11) USUI H. and YOSHITOMI K.. : F a t t y A c i d E s t e r s , in M o d e r n G a s C h r o m a t o g r a p h y ,
T h e o r y a n d A p p l i c a t i o n s , ed. by FUNA-
SAKA W. and IKEKAWA H., 6th Ed., Vol.
2,645, Hirokawa Publishing Co., Tokyo (1971).
(12) FIESER L. F. and FIESER M. : R e a g e n t s f o r O r g a n i c S y n t h e s i s . Vo1.1,191, John
Wiley and Sons, Inc., New York (1967). (13) BUDZIKIEWICZ H. DJERASSE C. and WIL-
LIAMS D. : M a s s S p e c t r o m e t r y o f Orga- n i c C o m p o u n d s , 173, Holden-day, San