Title
Separation of Inorganic Anions by Liquid Chromatography with
Crown Ether as Eluent Additive( 本文(Fulltext) )
Author(s)
TAKEUCHI, Toyohide; LIM, Lee Wah
Citation
[Analytical sciences : the international journal of the Japan
Society for Analytical Chemistry] vol.[27] no.[10]
p.[1019]-[1023]
Issue Date
2011-10-10
Rights
The Japan Society for Analytical Chemistry (公益社団法人日本
分析化学会)
Version
出版社版 (publisher version) postprint
URL
http://hdl.handle.net/20.500.12099/49213
Introduction
Crown ethers, e.g., 18-crown-6-ether (18C6), have been employed as an eluent additive in ion chromatography in order to improve the selectivity of cations in ion chromatography.1–4 For example, the addition of 18C6 in the eluent apparently increases the retention of potassium ion to improve the resolution. This is because the association constant of complexation between 18C6 and potassium ion is larger than that for the other cations. Macrocyclic ligands such as crown ethers and cryptands are highly selective in binding metal and other cations and have been employed in a variety of separation technologies, including solvent extraction, membranes and chromatography.2 Typically, crown ethers have been adsorbed, covalently bonded or polymerized on particulate substrates. In some instances, excellent separations among alkali and other metal ions have been achieved.1,2
The unique ability of macrocyclic ligands, such as crown ethers and cryptands, to selectively complex alkali metal cations can be used as the basis for chromatographic separations of anions.5 It was reported that alkylated macrocycles such as n-tetradecyl-18C6 or n-decylcryptands permanently coated onto a reversed-phase column formed positively charged anion-exchange sites when they combined with eluent cations and that anion retention increased with increasing eluent strength and organic modifier content using 18C6 as the eluent additive.5
Poly(oxyethylene) (POE) chemically-bonded stationary phases allowed us to separate anions in ion-exchange mode, where the eluent cations fixed on the oxygen atoms of several POE chains by ion-dipole interaction worked as the anion-exchange sites.6,7 It is expected that the cavity sizes for the eluent cation formed by the surrounding several brush-type
POE chains are variable and the effect of the eluent cation on the retention of analyte anion would not be very apparent.6
The present work examines the eluent conditions for the separation of inorganic anions using crown ethers as the eluent additive in capillary liquid chromatography.
Experimental
Reagents and materialsReagents employed were of guaranteed reagent grade and were obtained from Wako Pure Chemical Industries (Osaka, Japan), unless otherwise noted. 15-Crown-5-ether (15C5) and 12-crown-4-ether (12C4) were obtained from Alfa Aeser (Ward Hill, MA). Purified water was produced in the laboratory by using an RFS432PA water distillation system (Advantec, Tokyo, Japan). All solutions used in this work were prepared using the purified water. Develosil C30-UG-5 (C30; the mean particle diameter, 5 μm; Nomura Chemical, Seto, Japan) was used as the stationary phase. The packing material was packed into the fused-silica tubing with 0.53 mm i.d., as previously reported.8 Apparatus
The chromatographic measurements were carried out by using a capillary LC system constructed by an L.TEX-8301 Micro Feeder (L.TEX Corp., Tokyo, Japan) equipped with an MS-GAN 050 gas-tight syringe (0.5 ml; Ito, Fuji, Japan) as a pump, a Model M435 microinjection valve with an injection volume of 0.15 μl (Upchurch Scientific, Oak Harbor, WA) as an injector, a 0.53-mm i.d. × 100 mm microcolumn, and a UV-970 UV detector (JASCO, Tokyo, Japan). The UV detector was operated at 210 or 240 nm. A capillary flow cell (75 μm; JASCO) was equipped with a UV detector. The data were acquired by a Chromatopac C-R4A data processor (Shimadzu, Kyoto, Japan). The inlet pressure was monitored by an L.TEX-8150 Pressure Sensor (L.TEX).
2011 © The Japan Society for Analytical Chemistry
† To whom correspondence should be addressed. E-mail: take-t@gifu-u.ac.jp
Separation of Inorganic Anions by Liquid Chromatography with
Crown Ether as Eluent Additive
Toyohide T
AKEUCHI†and Lee Wah L
IMDepartment of Chemistry, Faculty of Engineering, Gifu University, 1-1 Yanagido, Gifu 501–1193, Japan
Inorganic anions were separated on a reversed-phase stationary phase dynamically modified with crown ether as a selector in capillary ion chromatography. The eluent contained crown ether, acetonitrile and a salt. Free and cation-trapped crown ether molecules in the eluent were adsorbed on a hydrophobic stationary phase such as triacontyl-functionalized silica (C30). The eluent cations trapped on crown ether worked as the ion-exchange sites, where the eluent anions and the analyte anions were competing for electrostatic interaction. The sizes of crown ether and the salt cation affected the retention of analyte anions. The concentrations of acetonitrile and crown ether as well as the eluent anion also affected the retention of analyte anions. An aqueous solution containing 18-crown-6-ether, potassium salt and acetonitrile achieved larger retention for analyte anions. Effects of the eluent conditions on the retention of analyte anions were examined in detail. (Received August 20, 2011; Accepted August 27, 2011; Published October 10, 2011)
Results and Discussion
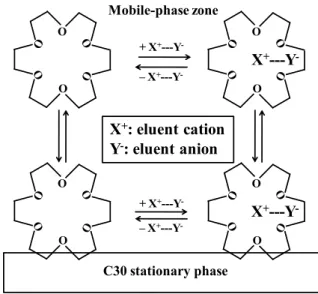
Mechanism of retention of anions18C6 is often used as the eluent additive in ion chromatography in order to improve the resolution of the cations, where the retention of potassium ion is especially enhanced by the addition of 18C6. This is because 18C6 is adsorbed on the stationary phase and potassium ions are favorably retained on the adsorbed 18C6, leading to the increase in the retention. It is expected that 18C6 will be adsorbed on the C30 stationary phase by hydrophobic interaction when the eluent contains 18C6. Free 18C6 and K+-incorporated 18C6 are present in the eluent, and both species could be adsorbed on the hydrophobic stationary phase. Potassium ions trapped on 18C6 then attract anions by electrostatic interaction, and analyte anions compete for the trapped potassium ions with the eluent anions, viz., an ion-exchange mechanism is involved in the retention of anions.
There are two different routes for 18C6 to adsorb on the C30 stationary phase: adsorption of free 18C6 followed by tapping of potassium ion or adsorption of K+-incorporated 18C6, as illustrated in Fig. 1. The retention of anions in the present system should be influenced by the concentrations of an organic modifier, 18C6 and eluent salt as well as by the crown ether size, and these parameters were examined in the following. Effect of acetonitrile
It is expected that the larger the amounts of 18C6 adsorbed on the stationary phase, the larger the retention of analyte anions will be. Since the organic modifier can control the amount of 18C6 adsorbed on the stationary phase, it can then control the retention of analyte anions. Figure 2 demonstrates ion-exchange separation of iodate, nitrate, iodide and thiocyanate on a C30 column using 10 mM potassium chloride containing 0.1% 18C6 and 2 – 10% (v/v) acetonitrile as the eluent. It can be seen that the analyte retention increases with decreasing acetonitrile concentration in the eluent. When the eluent contained no acetonitrile, the retention of analyte anions was not stable and gradually decreased. This may be because the surface of the packing material is hydrophobic and the eluent was gradually excluded from the pores.9 Therefore, 2 or 3% acetonitrile was contained in the eluent in the following experiments. In addition, bromate, nitrite and bromide eluted in this order
between iodate and nitrate, but these three anions eluted very close and they could not be separated from each other.
Effect of 18C6 concentration
The concentration of 18C6 in the eluent on the retention of analyte anions was examined up to 0.3% using 10 mM potassium chloride containing 3% acetonitrile as the eluent. The retention time of anions increased with increasing 18C6 concentration, as shown in Fig. 3. The increase in the retention time is due to the increase in the amount of adsorbed 18C6 on the C30 stationary phase. The increase in the retention time of anions was not significant at the concentrations higher than 0.17% (w/v).
Effect of eluent cation and anion
The association constant for complexation between 18C6 and metal cations is different for different cation, as shown in C30 stationary phase O O O O O O + X+---Y -O O
X
+: eluent cation
Y
-: eluent anion
Mobile-phase zoneX
+---Y
-X
+---Y
-+ X+---Y -– X+---Y -– X+---Y-Fig. 1 Retention mechanism of 18C6 and ions.
0 5 10 15 Time / min 1 2 3 4 2% 3% 6% 10% [CH3CN] 1 2 3 4 1 2 3 4 1 2 3 4
Fig. 2 Separation of inorganic anions on a C30 column using eluents containing different concentrations of acetonitrile. Column, Develosil C30-UG-5 (100 × 0.53 mm); eluent, 10 mM potassium chloride containing 0.1% (w/v) 18C6 and different concentrations of acetonitrile; flow rate, 8.0 μl/min; wavelength of UV detection, 210 nm; analytes, 0.2 mM each of iodate (1), nitrate (2), iodide (3) and thiocyanate (4); injection volume, 0.15 μl.
0 0.1 0.2 0.3 0 5 10 15 SCN -I -NO3 -IO3 -[18C6], % (w/v) R et en tio n tim e / m in
Fig. 3 Effect of 18C6 concentration on the retention of analyte anions. Eluent, 10 mM potassium chloride containing 3% (v/v) acetonitrile and 18C6; other operating conditions as in Fig. 2.
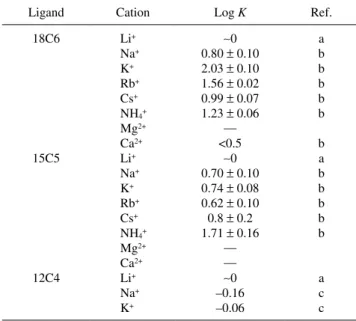
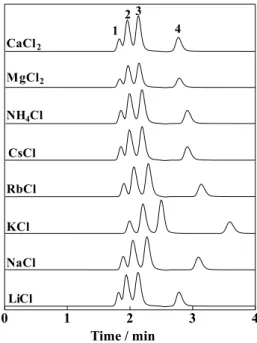
Table 1.10–13 Therefore, the eluent cation could affect the retention of anions in the present separation system. Figure 4 demonstrates the separation of anions on a C30 column using 10 mM chloride of various salts containing 3% acetonitrile and 0.1% 18C6 as the eluent. It is seen that potassium chloride gives the largest retention, followed by rubidium chloride, ammonium chloride, cesium chloride, and sodium chloride. Lithium chloride, magnesium chloride and calcium chloride gave nearly the same smaller retention time. The elution order was the same for all of the eluents examined, iodate, nitrate,
iodide and thiocyanate in this order. It should be noted that the larger the association constant between 18C6 and the metal cation is, the larger is the retention observed in Fig. 4, although the association constants are not given for magnesium and calcium ions. It can be concluded that the larger association constant leads to the larger amounts of the eluent cation trapped on the stationary phase via 18C6, leading to the increase in the cation-exchanged site and the retention of analyte anions.
The eluent anion also affected the retention of analyte anions, because analyte anions were retained in the ion-exchange mode. Figure 5 demonstrates the separation of anions on the C30 column using an aqueous solution of 10 mM potassium salt containing 0.1% (w/v) 18C6 and 3% (v/v) as the eluent. It can be seen that potassium dihydrogenphosphate gave the largest retention, followed by potassium chloride, potassium sulfate and potassium perchlorate.
Effect of salt concentration
The effect of eluent concentration on the retention of anions on the C30 column was examined using aqueous solutions of potassium chloride containing 0.1% 18C6 (w/v) and 3% (v/v) acetonitrile as the eluent. Separations of anions were carried out by using different concentrations of potassium chloride (5 – 60 mM). Figure 6 illustrates the retention time as the function of the potassium chloride concentration. As the concentration of potassium ion in the eluent increases, the amounts of trapped potassium ion on 18C6 increase, leading to an increase in the retention of analyte anions. Contrarily, as the concentration of chloride in the eluent increases, the elution strength increases, leading to a decrease in the retention of analyte anions. Both effects are competing, and the maximum retention times were observed at 10 – 20 mM, depending on the analyte.
Effect of crown ether size
Table 1 indicates that the size of crown ether affects the stability of the complex with metal cations. This means that the size of crown ether affects the retention of anions when crown ether is used as the eluent additive. Figure 7 demonstrates the separation of anions on the C30 column using 10 mM chloride of various salts containing 3% acetonitrile and 0.1% 15C5 as the
0 5 10 15 Time / min 1 2 3 4 LiCl NaCl KCl RbCl CsCl NH4Cl MgCl 2 CaCl 2
Fig. 4 Effect of eluent cation on the retention of analyte anions. Eluent, 10 mM aqueous chloride solution containing 0.1% 18C6 (w/v) and 3% (v/v) acetonitrile; other operating conditions as in Fig. 2.
Table 1 Association constants for 1:1 complexation between crown ether and metal ion
Ligand Cation Log K Ref.
18C6 15C5 12C4 Li+ Na+ K+ Rb+ Cs+ NH4+ Mg2+ Ca2+ Li+ Na+ K+ Rb+ Cs+ NH4+ Mg2+ Ca2+ Li+ Na+ K+ ~0 0.80 ± 0.10 2.03 ± 0.10 1.56 ± 0.02 0.99 ± 0.07 1.23 ± 0.06 — <0.5 ~0 0.70 ± 0.10 0.74 ± 0.08 0.62 ± 0.10 0.8 ± 0.2 1.71 ± 0.16 — — ~0 –0.16 –0.06 a b b b b b b a b b b b b a c c a. Ref. 11 (27 ± 1°C). b. Ref. 10 (25°C). c. Ref. 13 (25.0°C). 0 5 10 15 Time / min 1 2 3 4 KCl KClO4 KH2PO4 K2SO4 1 2 3 4 1 2 3 4 1 2 3 4
Fig. 5 Effect of eluent anion on the retention of analyte anions. Eluent, 10 mM potassium aqueous solution containing 0.1% 18C6 (w/v) and 3% (v/v) acetonitrile; other operating conditions as in Fig. 2.
eluent. It is seen that the anions are separated, and the elution order was the same for all of the eluents examined: iodate, nitrate, iodide and thiocyanate in this order. However, the retention times of the anions are smaller than those observed for 18C6. It is expected that the retention of the anions is smaller for 15C5 because the association constants between cations and 15C5 are smaller than those for 18C6 except for NH4+, as shown in Table 1. However, the retention of the analyte anions is smaller than expected from the association constants. It may be therefore expected that the adsorption of both 15C5 and cation-trapped 15C5 on the C30 stationary phase is weaker than 18C6 and cation-trapped 18C6.
In addition, it should be noted that the addition of 15C5 in the eluent enhanced the retention of analyte anions, as demonstrated in Fig. 8, where 20 mM potassium chloride aqueous solution
containing 2% (v/v) acetonitrile with or without 15C5 is used as the eluent. It is clear that the retention of the anions increased by the addition of 15C5 in the eluent and that the analyte anions are retained on the C30 stationary phase even if crown ether is not contained in the eluent. In the latter case, the anions are retained by hydrophobic interaction.14
Figure 9 demonstrates the separation of anions on the C30 column using 12C4 as the eluent additive, where aqueous solutions of 10 mM chloride of various salts containing 3% (v/v) acetonitrile and 0.1% (w/v) 12C4 as the eluent. It is seen that the anions are separated, but the retention values of the
0 1 2 3 4 Time / min 1 2 3 4 LiCl NaCl KCl RbCl CsCl NH4Cl MgCl 2 CaCl 2
Fig. 7 Separation of anions on the C30 column using 15C5 as the eluent additive. Eluent, 10 mM chloride aqueous solution containing 0.1% (w/v) 15C5 and 3% (v/v) acetonitrile; other operating conditions as in Fig. 2. 0 2 4 6 1 23 4
B
A
1 2 3 4Fig. 8 The separation of anions using eluents with or without 15C5. Eluents, 20 mM potassium chloride aqueous solution containing 2% (v/v) acetonitrile and 0.3% (w/v) 15C5 (A) or 20 mM potassium chloride aqueous solution containing 2% (v/v) acetonitrile (B); other operating conditions as in Fig. 2.
0 20 40 60 0 5 10 15 SCN -I -NO3 -IO3 -[KCl] / mM R et en tio n tim e / m in
Fig. 6 The retention time as the function of the potassium chloride concentration. Eluent, potassium chloride aqueous solution containing 0.1% (w/v) 18C6 and 3% (v/v) acetonitrile; other operating conditions as in Fig. 2. 0 1 2 3 4 Time / min 12 3 4 LiCl NaCl KCl RbCl CsCl NH4Cl MgCl 2 CaCl 2
Fig. 9 Separation of anions on the C30 column using 12C4 as the eluent additive. Eluent, 10 mM chloride aqueous solution containing 0.1% (w/v) 12C4 and 3% (v/v) acetonitrile; other operating conditions as in Fig. 2.
analyte anions are smaller than those of 18C6 and 15C5. It should be noted that the retention of the analyte anions is slightly larger than that observed in Fig. 8B, where no crown ether is contained.
Indirect detection
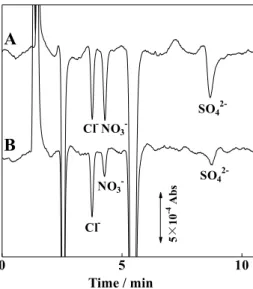
Figure 10 demonstrates indirect detection of chloride, nitrate and sulfate using 1 mM potassium iodide aqueous solution containing 0.1% (w/v) 18C6 and 3% (v/v) acetonitrile as the eluent, where the wavelength of UV detection is set at 240 nm, considering the background level (0.367 absorbance) and its linearity. The possibility of indirect detection supports the conclusion that the analyte anions replace the eluent anions trapped on the 18C6.
Conclusion
The addition of a crown ether such as 18C6 in the eluent allowed the separation of anions on a hydrophobic stationary phase. The concentrations of crown ether, salt and acetonitrile as well as the eluent cation and anion could control the retention of anions. The present separation system will be an alternative to ion chromatography with common ion exchangers.
References
1. P. R. Haddad and P. E. Jackson, “Ion Chromatography: Principles and Applications (J. Chromatogr. Library Vol. 46)”, 1990, Elsevier, Amsterdam-London-New York-Tokyo, 233.
2. J. D. Lamb and R. G. Smith, J. Chromatogr., A, 1991, 546, 73. 3. M. W. Läubi and B. Campus, J. Chromatogr., A, 1995, 706,
103.
4. K. Ohta, K. Kusumoto, Y. Takao, A. Towata, S. Kawakami, M. Ohashi, and T. Takeuchi, Chromatographia, 2003, 58, 171. 5. J. D. Lamb, R. G. Smith, and J. Jagodzinski, J. Chromatogr.,
A, 1993, 640, 33.
6. T. Takeuchi, B. Oktavia, and L. W. Lim, Anal. Bioanal. Chem., 2009, 393, 1267.
7. T. Takeuchi and L. W. Lim, Anal. Sci., 2010, 26, 937. 8. T. Takeuchi and D. Ishii, J. Chromatogr., 1981, 213, 25. 9. T. Enami and N. Nagae, Am. Lab., 2004, October, 20. 10. R. M. Izatt, R. E. Terry, B. L. Haymore, L. D. Hansen, N.
K. Dalley, A. G. Avondet, and J. J. Christensen, J. Am. Chem. Soc., 1976, 98, 7620.
11. A. J. Smetana and A. I. Popov, J. Solution Chem., 1980, 9, 183.
12. R. M. Izatt, J. S. Bradshaw, S. A. Nielsen, J. D. Lamb, and J. J. Christensen, Chem. Rev., 1985, 85, 271.
13. L. C. Manege, T. Takayanagi, M. Oshima, and S. Motomizu, Analyst, 2000, 125, 1928.
14. T. Takeuchi, B. Jiang, and L. W. Lim, Anal. Bioanal. Chem., in press. 0 5 10 Time / min Cl-NO 3 -SO4 2-Cl -NO3- SO4
2-A
B
5× 10 -4 A bsFig. 10 Indirect detection of chloride, nitrate and sulfate using 0.1% (w/v) 18C6, 3% (v/v) acetonitrile and 1 mM potassium iodide as the eluent. Wavelength of UV detection, 240 nm; samples, 0.1 mM each (A) or tap water (B); other operating conditions as in Fig. 2.