Development of Functional Mesocrystalline
Materials and Ferroelectric Perovskites
Wenxiong Zhang
March 2019
1
Table of Contents
Chapter I General Introduction ...3
1.1 Overview on mesocrystals ... 4
1.1.1 Structural and formation principles of Mesocrystals ... 4
1.1.2 Characteristic properties of mesocrystals ... 7
1.1.3 Recent advances and future outlook in mesocrystal materials ... 10
1.2 Metal oxides and complex compounds mesocrystals ... 13
1.2.1 TiO2 mesocrystals ... 13
1.2.2 CaCO3 mesocrystals ... 16
1.2.3 SrTiO3 mesocrystals ... 17
1.2.4 Ferroelectric perovskite mesocrystals ... 20
1.3 Ferroelectric perovskites... 20
1.3.1 Inorganic metal oxide ferroelectric perovskites ... 20
1.3.2 Halide perovskites ... 26
1.4 Lattice strain engineering ... 32
1.5 Topochemical synthesis ... 37
1.5.1 Approach of topochemical synthesis ... 37
1.5.2 Soft chemical process for mesocrystalline nanocomposites ... 40
1.6 Purpose of present study ... 41
1.7 Reference ... 44
Chapter II ...56
Anomalous Piezoelectric Response of Ferroelectric Mesocrystalline BaTiO3/Bi0.5Na0.5TiO3 Nanocomposites Designed by Strain Engineering ...56
2.1 Introduction ... 56
2.2 Experimental ... 59
2.2.1 Sample Preparation ... 59
2.2.2 Physical Properties Analysis ... 60
2.3 Results and discussion ... 62
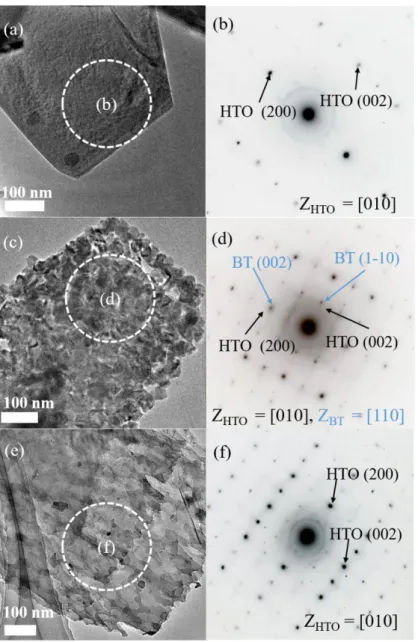
2.3.1 Synthesis of mesocrystalline BT/HTO nanocomposite ... 62
2
2.3.3 Formation reaction mechanism of mesocrystalline BT/BNT nanocomposite 71 2.3.4 Ferroelectric and piezoelectric responses of mesocrystalline BT/BNT
nanocomposite ... 73
2.3.5 Dielectric responses of mesocrystalline BT/BNT nanocomposite ... 84
2.4 Conclusion ... 89
2.5 References ... 89
Chapter Ⅲ ...94
Ferroelectric Mesocrystalline BaTiO3/BaBi4Ti4O15 Nanocomposite: Formation Mechanism, Nanostructure, and Anomalous Ferroelectric Response ...94
3.1 Introduction ... 94
3.2 Experimental ... 97
3.2.1 Sample Preparation ... 97
3.2.2 Physical Properties Analysis ... 98
3.3 Results and discussion ... 100
3.3.1 Synthesis of BT/BBT nanocomposites and BBT mesocrystals ... 100
3.3.2 Nanostructural analysis for BT/BBT nanocomposite ... 107
3.3.3 Formation mechanism of BT/BBT nanocomposite ... 108
3.3.4 Ferroelectric, dielectric and piezoelectric responses of BT/BBT nanocomposite ... 113
3.4 Conclusions ... 123
3.5 References ... 124
Chapter Ⅳ ...129
Compelling Evidences for Antiferroelectric to Ferroelectric Transition of MAPbI3-xClx Perovskite in Perovskite Solar Cells...129
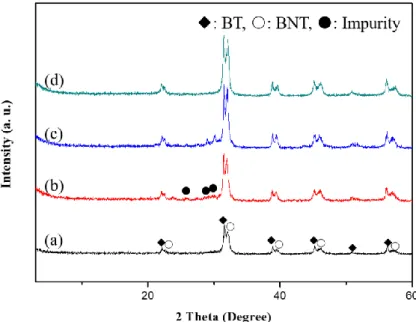
Chapter V Summary ...131
Publications ...137
Publications in Journals ... 137
Publications in Conferences ... 138
3
Chapter I General Introduction
The study of nanocrystals has become a major interdisciplinary research area and drawn increasing attentions in the past decades because nanocrystals can be used as building units to fabricate hierarchically structured solid materials.1 Solids organized by nanocrystals open up the opportunities of fabricating new materials and devices, which not only exhibit the properties from individual nanocrystals but also exhibit collective properties produced via their interactions.2, 3 In these solids, assemblies constructed
from crystallographically oriented nanocrystals have single-crystal-like structures and much higher porosity than conventional single crystals. Subsequently, the assemblies have been defined as Mesocrystal, a new class of material, which is a polycrystal constructed from oriented nanocrystals or microcrystals.3-5 In particular, for the material chemistry, mesocrystals can offer unique new opportunities for the design of materials, and be applied to catalysis, sensing, and energy storage and conversion.6-8 Hence, they have attracted considerable attention of physicists and chemists in recent years, and become a hot research field. However, the understanding of mesocrystals is still very limited, such as the preparation approaches, the formation mechanisms, microstructures, and characteristics, as well as the developments for the various types and high-performance applications.6
On the other hand, the metal oxide perovskites and perovskite-related halides are important materials as they possess a number of interesting properties, such as ferroelectric; piezoelectric; electron-acceptor behavior; a large optical transmission domain; high resistivity; antiferromagnetic; exceptional magnetic; photoluminescent properties; anionic conductivity over a wide temperature range.9 Some halide
4
perovskites have the semiconducting property, which has been widely applied to photovoltaic areas.10 Still, the ferroelectricity is also possible in this kind of halide perovskites, hence, it has quite interesting and debatable behaviors.11, 12 Therefore, figuring out the connection between the semiconductor and ferroelectric behavior in the halide perovskite materials is significant.13
In this chapter, structural and formation principles, characteristic properties, the applications of conventional mesocrystals, recent advances and future outlook in mesocrystalline materials are described. Furthermore, the structure, characteristic properties and the application of the perovskite materials have also been presented. In addition, the purposes of this dissertation are also clarified.
1.1 Overview on mesocrystals
1.1.1 Structural and formation principles of Mesocrystals
Mesocrystal, as an abbreviation for mesoscopically structured crystalline materials, represents a structure composed of nanocrystals aligned in a crystallographic pattern but separated by porosity or a second phase. The first observation of mesocrystal traces back to 1969 when a porous intermediate structure of BaSO4 was reported by Petres and
co-workers.14 In 2003, Cölfen et al. established some principles and concepts that
illustrate the mesoscale self-assembly of nanocrystals contributing another mechanism for growing the single crystals.3 In such mesoscale self-assembly (Fig. 1.1, the red route), the crystal growth by the ordered aggregation results in organized crystals with iso-oriented directions, which is totally different from the classical crystallization process (Fig. 1.1, the blue route). For the classical crystallization, including the
5
nucleation and the growth processes, the crystallized primary nanoparticles grow via ion-by-ion (or molecule-by-molecule) attachment to fulfill the formation of the single crystal. Until 2005, Cölfen et al. created the word mesocrystal as a replacement for the iso-oriented crystals and proposed that mesocrystals are kinetically metastable intermediates, in which the primary units can be still identified.4 Hence, it raises a challenge to the well-known classification of solids as amorphous, polycrystalline, or single crystalline. Moreover, mesocrystals show much higher crystallinity than polycrystalline materials, and in some case even exhibit many characteristic properties of a conventional single crystal.3, 15, 16 In 2010, Song et al. contributed a more precise definition that mesocrystals are superstructured crystals consisting of mesoscopically scaled (1-1000 nm) crystalline subunits that are aligned in the same crystallographic direction.17
Fig. 1.1 Schematic presentation of classical crystallization (blue route) via ion-by-ion addition
versus single crystal formation (red route) by a mesocrystal intermediate made of nanoparticles.
6
other nanostructured materials have been investigated for several decades, the systematic research on mesocrystals only started about 10 years ago. Yet, very little is known for mesocrystal formation processes, which are fundamental for the understanding of the fabrication of mesocrystal structure and the control of its synthesis process.6 Up to now, a large number of mesocrystals have been successfully synthesized, whereas the growth mechanism of mesocrystal is still a big problem due to the large range of involved time and length scale during the growth of mesocrystals.5 Recently, Sturm and Cölfen demonstrated seven scenarios of different mesocrystal formation pathways, as shown in Fig. 1.2: (a) alignment by organic matrix; (b) alignment by physical forces; (c) crystalline bridges, epitaxial growth, and secondary nucleation; (d) alignment by spatial constraints; (e) alignment by oriented attachment; (f) alignment by face-selective molecules and (g) topotactic (epitaxial) solid phase transformation.6 Each of these mechanisms involves different driving forces of chemical and physical origin directing the formation of the mesocrystalline materials, in which (a) to (f) can be classified to self-assembled materials (red route in Fig. 1.1) and (g) is obtained from topochemical process (purple route in Fig. 1.1). The topotactic transformation in solids is well-known and has been quite extensively studied for many classes of solid-state materials. In other words, if the initial material is single crystalline, the transformation induces the formation of a new phase in a specific crystallographic orientation, as shown in Fig. 1.3. It can be obviously seen that the obtained mesocrystals still maintain the morphologies of the original precursors after one or multi-step transformation, suggesting that the morphologies of the mesocrystals are inherited from the original precursors. Usually, this reaction occurs via accompanying the ion exchange, intercalation, de-intercalation, and topochemical micro/nanocrystal conversion.6, 18
7
Fig. 1.2 Simplified schematic illustration of main formation pathways of mesocrystals. (a)
alignment by organic matrix; (b) alignment by physical forces; (c) crystalline bridges, epitaxial growth and secondary nucleation; (d) alignment by spatial constraints; (e) alignment by oriented attachment; (f) alignment by face selective molecules and (g) topotactic (epitaxial) solid phase transformation. Reproduced with permission.6 Copyright 2017, MDPI.
Fig. 1.3 Various dimensional schematic diagrams for 1 and/or n-step in-situ topotactic
conversion reaction for formations of mesocrystals from original precursors.
1.1.2 Characteristic properties of mesocrystals
It is well-known that the nanocrystal building units with structural multiplicity and nanoscale size can provide additional opportunities for self-assembly.5 A variety of
8
self-assemblies or topochemical bridge connections for the formations of the mesocrystals can offer new possibilities for superstructure formations, giving rise to the mesocrystals presenting some different characteristic properties. The mesocrystals from functional materials are highly attractive due to the emergent properties of mesocrystalline materials, such as single-crystal-like behavior, high crystallinity, high porosity and inner connection bridged by organic components and/or inorganic nanocrystals.19, 20 Hence, the mesocrystals can exhibit the following characteristic properties.
Firstly, the mesocrystal is a polycrystal constructed from iso-oriented nanocrystals, which exhibits a single crystal behavior in X-ray scattering and electron diffraction. For instance, the octahedron-shaped magnetite (Fe3O4) mesocrystal shows a
single-crystal-like SAED pattern (Fig. 1.4(e)).21 In addition, all the nanocrystals for the construction of the mesocrystal exhibit the same direction of the interplanar spacing. These are due to that the mesocrystals are constructed from nanocrystals, and each nanocrystal is crystal-axis-oriented with each other.
9
magnetic field. (c) HRTEM image of one single nanoparticle in the assembly (solid red triangle: (111) plane at the top; dashed red triangle: (111) plane at the bottom); solid blue triangles: interparticle spaces. (d) Model of the magnetite octahedra viewed along the [111] zone axis. (e) Fast Fourier transformation (FFT) pattern and (f) selected area electron diffraction (SAED) pattern of the 2D mesocrystals shown in (b). Reproduced with permission.21Copyright 2010,
American Chemical Society.
Secondly, the mesocrystals show some special properties of well-aligned and oriented crystalline assembly, which is unrivalled for the amorphous, polycrystalline, and single-crystalline materials. Namely, some of desired properties can be satisfied by using the mesocrystalline superstructure rather than using the same material in amorphous, polycrystalline aggregate, and single-crystalline. As the case in Fig. 1.4, magnetic mesocrystals with non-spherical shapes demonstrate more appealing anisotropic magnetic properties.22
Thirdly, because of the primary crystallites sharing a common crystallographic orientation, the mechanical properties of the mesocrystals are unusual.23 They can
exhibit higher ductility and toughness than the corresponding single crystalline materials, and almost all the mesocrystals exhibit the fracture surfaces like amorphous glasses, but unlike the single crystals.24
Finally, the mesocrystal simultaneously present over two kinds of functional properties and prevail on the materials having a large improvement in some application areas. For instance, mechanical toughness and dielectric dissipation, or optical and magnetic properties can be combined in one system. It is said that these properties would never be mixed on this nanoscale.5 They combine the high crystallinity with
10
small crystal size, high surface area and high porosity of the mesocrystal as well as good handling because the size range of mesocrystal is in the nanometer to micrometer. The existence of the superlattice structure is also the main reason of high attraction due to the emergent properties of mesocrystals. A self-assembly process for mesocrystals does not occur by an ion-by-ion manner, however, ionic strength and species of the solution are still important variables in controlling crystallization to form mesocrystals. In particular for surfactant phases and microemulsion involved crystallization processes, the phase equilibrium and physical characteristics of the product can strongly depend on ionic strength and species, especially if the cationic lyotropic phases are applied.17
1.1.3 Recent advances and future outlook in mesocrystal materials
Recently, the researches on mesocrystals are drawing much attention, not only by fundamental research but also by its applications, and an increasing number of the mesocrystals preparations for applications to a wide range of the functional materials have been reported.21, 25, 26 In addition, the development of the techniques used for the characterization of the mesocrystals will further allow for the observation of the mechanisms of mesocrystal formation, and understanding of their structural principles. The aforementioned techniques mainly include the in-situ techniques like AFM, TEM, and High-Resolution TEM or other in-situ microscopic and diffraction techniques, namely SAXS/WAXS, Grazing-Incidence Small-Angle Scattering (GISAXS)/Grazing-Incidence Wide-Angle Scattering (GIWAXS).
In the research area of the formation mechanism, the carbonates mesocrystals were studied at the earliest and most. The initial demonstration for the formation of mesocrystal was originated from the carbonates chemistry, which is a fundamental
11
source of the mesocrystal theory today.27 However, one of the earliest referring
indications of the mesocrystal intermediates was not derived from CaCO3 but the
BaSO4 crystal with the porous structure.28 After exploration of some intermediate metal
oxides mesocrystals, the mesocrystals with even higher definition were reported for CaCO3 made in silica gels in 1986.29 Therefore, CaCO3 mesocrystals are increasingly
studied and developed via controlling the polymorph and morphology. However, very little is known that all the carbonate mesocrystals are involved with the high-performance applications. Subsequently, the study on the metal oxides mesocrystals not only becomes attracting but also becomes the hottest. Especially in ZnO and TiO2 mesocrystals, the preparation approaches, the formation mechanisms,
microstructures, and the high-performance applications have been getting increased attention. At present, the ZnO and TiO2 mesocrystals have been used to catalysis,
sensing, and energy storage and conversion.23, 26 The perovskite metal oxides mesocrystals have been also developed unsubstantially, they are likely to widely apply to the high-performance electro-optic field, ferroelectric materials, and other functional composite materials. Very recently, Hu et al. have developed the platelike mesocrystalline BaTiO3/SrTiO3 and BaTiO3/CaTiO3 nanocomposite via the
topochemical process with highly elevated dielectric, ferroelectric and piezoelectric responses.30, 31
Although an increasing number of mesocrystals have been developed, the formation mechanisms of the mesocrystals are still limited and the mesocrystals are still a new study field for the solid materials. For example, the metal oxide and carbonate mesocrystals are predominantly investigated, and they are still understood limitedly, furthermore, their formation mechanisms are still very difficult to be clear. In addition,
12
the varieties of the mesocrystals are not enough, the application studies of the other mesocrystals are rarely found out. In the following, the carbonates, metal oxides, and perovskite mesocrystals are briefly introduced and summarized, respectively. The goal is to further understand the approach, formation mechanism, and functional application of the mesocrystals, and is ready for the development and application of new kinds of functional mesocrystals.
The above contents already reveal the tremendous potential of mesocrystals. Hence, application-driven research will be increasing, and more applications will be reported without a doubt. In the near future, the new mesocrystal applications will be investigated. In the meantime, the development of the aforementioned in-situ techniques will be achieved for the observation of the formation mechanisms and understanding of the structuration of the mesocrystals in much more detail than it was possible before. In addition, given that a large number of optimizations of mesocrystal formation processes are done by trial and error, so that the introduction of new methodological methods for characterization and interpretation of the mesocrystal structures is extremely needed. Up to now, mesocrystals have been applied to Li-ion batteries, catalysts or sensors, and solar cells like quantum dot based solar cell etc.25, 32, 33 Also, for plasmonic materials, the two different surface plasmon resonance bands for metal nanorods can lead to interesting directional couplings in metal nanoparticle mesocrystals, and therefore, further exploitation of metal nanoparticle mesocrystals can be anticipated in the future. Furthermore, mesocrystals from magnetic nanoparticles where coupling of the magnetic fields can also be anticipated. Besides, an untouched area in the mesocrystal research is mesocrystals of organic nanocrystals, which have a great potential in the pharmaceutical formulations.6 Therefore, a large number of exciting developments can be foreseen in
13 the field of mesocrystals.
1.2 Metal oxides and complex compounds mesocrystals
1.2.1 TiO2 mesocrystals
As described above, the mesocrystal materials exhibit specific properties in comparison to its single crystal, which have wide application in sensing, catalysis, energy storage, and conversion etc. As far as we know, TiO2 (titanium dioxide) is
among the most widely investigated metal oxides materials for its functional properties and many promising applications in environment, energy, photocatalysts, and sensing areas.25, 26, 34, 35 Crystal structures for TiO2, including TiO2 (B), TiO2 (Ⅱ), brookite,
anatase, rutile etc. have been widely studied. Among them, the rutile is a thermodynamic stable phase and others are metastable phases. In addition, anatase is the most stable phase in the metastable phases.
TiO2 nanocrystals have been increasing investigated and developed into the functional
materials. In addition, the effectiveness of TiO2 in practical applications varied
considerably with its specific surface area and mesoporosity,36 compositions,37 crystallinity38, 39 and, importantly, the morphology and texture of the material.40 Well-defined structural TiO2 materials obtained from the controlled synthesis with, such
as single crystals, ordered mesoporous thin films, nanotubes, and spherical particles, has attracted much attention in recent decade. For instance, the anatase single crystals containing high percentage of reactive facets have been fabricated via solvothermal process with adding the fluorine species. Thermally stable mesoporous TiO2 thin films
with uniformly distributed pores can be fabricated via an evaporation-induced self-assembly process in the presence of various block copolymers.41 TiO
14
with different aspect ratios can be synthesized via anodization of titanium foil with variable potential and electrolyte composition.42 Furthermore, 3D interconnected TiO2
networks with high crystallinity and controllable macropores have been successfully obtained from preformed templates via a sol-gel nanocasting process.43
Excellent candidates for the applications in sensing, photocatalysis, solar cells and lithium-ion batteries have been manifested for nanoporous TiO2 materials with large
surface areas. It is noted that anatase TiO2 mesocrystals were first reported by Ye et al.
via the topotactic conversion from NH4TiOF3 mesocrystals, which were fabricated with
the assistance of non-ionic surfactants.44 In such transformation process, the NH4TiOF3
mesocrystal serves as a crystallographically matched template for the subsequent growth of the TiO2 mesocrystals. Furthermore, the direct mesoscale assembly of TiO2
mesocrystals and their photocatalytic properties have been attracted much attentions. In the meantime, solid templates and organic additives were gradually introduced during these mesoscale transformation processes.
Liu et al. have demonstrated that the rutile TiO2 hollow spheres mesocrystals can be
synthesized through the hydrothermal reaction process by using L-serine and N,N′-dicyclohexylcarbodiimide (DCC) as biologic additives.45 Subsequently, Zhang et
al. have illustrated the formation of the photocatalytically active rutile TiO2
mesocrystals without the help of surfactants or additives, which exhibited the BET specific surface area of only 16 m2/g. To explore the additive-free approaches for TiO2
mesocrystals with high porosity and high crystallinity, Ye et al. have reported the first additive-free fabrication of nanoporous anatase TiO2 mesocrystal by using tetrabutyl
titanate as the titanium source and acetic acid as the solvent, as shown in Fig. 1.5.46 In this formation process, organic titanium firstly reacts with organic acid by a
15
hydrolytic/nonhydrolytic condensation reaction to from amorphous fiber-like titanium acetate complex precursors with Ti-O-Ti bonds. After two times of continuing condensation processes, the other crystalline spherical-like precursors come into being at the expense of the amorphous precursor. Subsequently, the crystalline spherical-like precursors gradually release soluble titanium including the nucleation and growth of anatase nanocrystals. Finally, the formed anatase nanocrystals gather along the [001] direction, accompanying with the entrapment of in situ produced butyl acetate, giving rise to the formation of the spindle shaped anatase mesocrystals elongated along the [001] direction. The acetic acid molecules played multiple key roles during the nonhydrolytic processing of the [001]-oriented anatase mesocrystals. The obtained anatase mesocrystals with nanoporous exhibits remarkable crystalline stability and improved performance as anode materials for lithium-ion batteries. In this regard, TiO2
16
Fig. 1.5 Schematic illustration of the formation mechanism of nanoporous anatase TiO2
mesocrystals without additives. Reproduced with permission.46 Copyright 2010, American
Chemical Society.
1.2.2 CaCO3 mesocrystals
CaCO3 (calcium carbonate), as one of the most abundant natural existing minerals in
such as the sedimentary rocks47 and the biological skeletons and tissues,3 has been drawn much attention. There are three kinds of crystalline polymorphs for CaCO3,
including: calcite (dominated phase at lower temperature), aragonite (dominated phase at higher temperature), and vaterite (at higher supersaturation).48 There are some new methods for the development of the CaCO3 with the controlling morphologies and
crystallization. Besides, the various inorganic or organic–inorganic composite materials, superstructure materials, and improved functional materials can be fabricated accordingly.49, 50 A number of studies on CaCO
3 materials have effectively promoted the
development of the mesocrystal chemistry and provided theoretical basis for the mesocrystal chemistry. A similar gel-sol reaction or a block copolymerization reaction has been widely used to prepare CaCO3 mesocrystals.
By using the self-assemble in a polyacrylamide gel, a typical calcite mesocrystal with a pseudo-octahedron morphology formed from rhombohedral nanocrystals has been fabricated (Fig. 1.6).51 It is obvious that the calcite mesocrystal was obtained by a precipitation process from an aqueous solution containing Ca2+ and sulfide. It can be clearly seen that crystallographic block with rough surfaces are constructed from well-oriented nanocrystalline building units. According to the TEM image (Fig. 1.6b), the tightly connected nanocrystals and the organic matrix between the individual
17
nanocrystal interspaces can be observed. The inserted SAED pattern reveals a single-crystal-like crystallographic block, which means the formation of a mesocrystal structure. In addition, the calcite mesocrystal can be also fabricated by adding the CO2
vapor diffusion into a Ca(OH)2 solution. Since the CO2 vapor diffusion approach has the
advantage of avoiding the interference of the extraneous ions, minimizing ionic strength and approaching a pH close to biological conditions at the end of the crystallization reaction, this approach is beneficial to the growth of calcite mesocrystals. As a result, the vapor diffusion approach gives a better understanding of the driving forces for the oriented and/or self-assembling of nanocrystals to mesocrystals.
Fig. 1.6 (a) SEM and (b) TEM images of calcite (CaCO3) aggregate with characteristic
pseudo-octahedral morphology obtained from polyacrylamide gel. (Inserted in (b): SAED pattern of calcite mesocrystal). Reproduced with permission.51 Copyright 2003, American
Mineralogical Society.
1.2.3 SrTiO3 mesocrystals
18
attention in the field of solar energy conversion systems and electromagnetic devices.52
In regard to further cutting down human energy usage and controlling environmental pollution, heterogeneous solid photocatalysts have been anticipated to show promising solar-to-chemical energy conversion from water since 1972.
For the development of more active ST-based photocatalysts, it is crucial to seek a versatile route for the structure and property design. It may be expected that the selectivity and efficiency of the photocatalytic reactions of ST with tailored crystal facets and morphologies can be achieved for making its mesocrystals. However, there remain challenges that are relevant to the fabrication of the organized assembly of such structure-controlled nanoparticles up to the micrometer scale. Only a few of ST mesocrystals have been reported. Calderone et al. reported the formation of the ST mesocrystal with cubic morphology by precipitation approach from a suspension of hydrolyzed TiOCl2 aqueous gel.53 The obtained ST mesocrystal was formed via an
epitaxial self-assembly of nanocrystals with a size of 4-5 nm. Furthermore, the formation process is a non-additive spontaneous process. In addition, Kalyani et al. demonstrated that ST mesocrystals with a [010] zone axis along the direction of the wire-like morphology can be fabricated via an oriented topochemical conversion approach. In this formation process, the H2Ti3O7 nanowire precursor was firstly
thermally reacted to form the anatase nanowire. The solvothermal and hydrothermal treatments of the anatase nanowire in Sr(OH)2 solutions were carried out to form the
mesocrystalline ST. The formation of mesocrystals is achieved from a topochemical reaction.
Very recently, TiO2 mesocrystals, which consist of nanocrystal building blocks, have
19
their efficient charge transfer and separation along the neighboring nanoparticles. The epitaxial intergrowth of ST from TiO2 has drawn much attention due to the scientific
and technology importance of oxide interface engineering. Zhang et al. reported that the ST mesocrystals with enhanced photocatalytic activity were produced by topotactic epitaxial transformation from anatase TiO2 mesocrystals via a facile hydrothermal
process (Fig. 1.7).54 Compared with the conventional disordered system, the material
exhibits the three-fold photocatalytic efficiency (Fig. 1. 7(c)) for the hydro evolution reaction of water splitting in alkaline aqueous solution because of the ordered mesocrystalline structure (Fig. 1.7 (d-g))
Fig. 1.7 (a) Schematic presentation of the formation of SrTiO3 mesocrystals via topotactic
epitaxial transformation of TiO2. (b) Structural model of interface between SrTiO3 and TiO2,
suggesting the epitaxial intergrowth of both phases. (c) Simplified scheme of a SrTiO3
mesocrystal showing the photogeneration of electrons and holes and anisotropic electron transport form the inside to the outside. (d) SEM image of typical SrTiO3 mesocrystals, (e)
zoomed image inside the red frame marked in (d). (f) TEM image and SAED pattern of SrTiO3
mesocrystals. (g) High resolution TEM image of the area marked with red-square in (f). Reproduced with permission.54 Copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA,
20
Weinheim.
1.2.4 Ferroelectric perovskite mesocrystals
Ferroelectric materials have been paid much attention to scientific and technological
fields due to their wide-applications in sensors, actuators, and energy harvesters.55, 56 The ferroelectric mesocrystals with specific morphology can be fabricated via the bottom-up self-assembly or the topochemical process.57, 58 In our previous research, the BaTiO3 mesocrystals with platelike morphology have been fabricated by a hydrothermal
soft chemical process from the H1.07Ti1.73O4 (HTO) precursor with a lepidocrocite-like
layered structure.58 Such perovskite mesocrystals with complex chemical compositions are very difficult to be prepared via the conventional methods. In addition, the platelike Ba1−xCaxTiO3, Ba0.5(Bi0.5K0.5)0.5TiO3 and Bi0.5Na0.5TiO3 mesocrystals have also been
developed from the same HTO precursor via the topochemical process.59-61 Furthermore, all of these platelike mesocrystals are promising for making the oriented ceramics with improved dielectric and ferroelectric properties.62 Very recently, KNbO3 mesocrystals
with different morphologies have been prepared via the self-assembly without polymer additives by our group.57
1.3 Ferroelectric perovskites
1.3.1 Inorganic metal oxide ferroelectric perovskites
Perovskite is a calcium titanium oxide mineral (calcium titanate, CaTiO3). The
mineral was discovered in the Ural Mountains of Russia by Gustav in 1839 and is named after Russian mineralogist Lev Perovski. Its name is lent to the class of
21
compounds which have the same type of crystal structure as CaTiO3, known as the
perovskite structure. Generally, perovskites are materials described by the formula ABX3, where X is an anion and A and B are cations of different sizes (A being larger
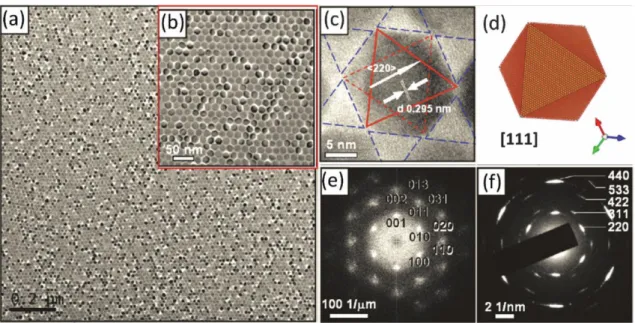
than B), and the crystal structure is described in Fig. 1.8(a). Namely, the BX6
octahedrons connect with each other via corner-sharing, forming the 3 dimension space. The structure was first described by Victor Goldschmidt in 1926, in his work on tolerance factors.63 Generally, the phase stability of the perovskite materials can be confirmed through the tolerance factor t, which is defined as below:
t = γA+ γX √2(γ𝐵+ γ𝑋)
Where γA, γB and γX represent for the ionic radii of the A-site cation, B-site cation and
X-site anion in the perovskite ABX3 structure, respectively. A t value between 0.8 and
1.0 is favorable for perovskite structure with rhombohedral or orthorhombic structure, and larger (>1) one tends to form the tetragonal structure, whereas the smaller (<0.8) values of tolerance factor usually result in non-perovskite structures.64, 65
22
Fig. 1.8 (a) ABX3 perovskite structure and (b) P-E hysteresis loop of the ferroelectric perovskite
materials. (c) Piezoelectric effect and inverse piezoelectric effect of ferroelectric perovskite materials.
Ferroelectrics are a section of a much larger class of substances called pyroelectrics. A pyroelectric has the property that the single crystal with no surface changes is polar; polarity is masked by the twining or by surface charges and is only revealed by the heating treatment. A ferroelectric has an additional property that the polarization direction can be reversed by applying the external electric-field, therefore it exhibits hysteresis as shown in Fig. 1.8(b).66 Since the discovery of ferroelectricity in single-crystal materials (Rochelle salt) in 1921 and its subsequent extension into the realm of polycrystalline ceramics (BaTiO3) during the early to mid-1940s, there has
23
been a continuous succession of new materials and technology developments that have led to a significant number of industrial and commercial applications.67 All the ferroelectric materials exhibit an invariably piezoelectric nature, which have piezoelectric and inverse piezoelectric effects as shown in Fig. 1.8(c). Up to now, the inorganic metal oxide perovskite ferroelectric materials, such as Pb(Zr1-xTix)O3, BaTiO3,
Bi0.5Na0.5TiO3 etc., have been widely applied to actuators, sensors and energy
harvesters.55, 56, 68
Pb(Zr1-xTix)O3 perovskite ferroelectric. Lead zirconate titanate, namely
Pb(Zr1-xTix)O3 (PZT), was discovered in 1955 by B. Jaffe et al.,69 which has much more
excellent piezoelectric performance than that of BaTiO3 ceramics.55 PZT and related
perovskite compositions have been the mainstream for the high performance actuators and transducers, owing to their excellent dielectric, piezoelectric, and electromechanical coupling coefficients.69
PZT is a solid solution constructed from PbZrO3 (PZ) and PbTiO3 (PT) where the x =
0.48 of PT, and its ceramic lies near a morphotropic phase boundary (MPB) separating the tetragonal and rhombohedral phases. The MPB can usually exhibit anomalously high dielectric and piezoelectric responses.70-72 The enhancements of the piezoelectric and dielectric responses are associated with lattice strains derived from the lattice mismatches between the two different crystalline symmetries with slightly different lattice constants at their interface.72 Dopants are always used to improve the property of the PZT materials. With the adding of different kinds of dopants, the PZT material is categorized into two classes of “soft” and “hard” PZT, respectively, which can be applied to different areas.55
24
and materials due to the concerns regarding its poisonousness. The replacement of the lead-based piezoelectric ceramics represented by PZT has fueled the excitement and spurned wide-spread scientific activity to find the alternatives in the last decade due to the publication by Saito et al..70 Since PZTs contain more than 60 weight percent lead, the work of Saito et al.70 got attention for identifying a mixture of morphotropic and polymorphic phase transition region in a (K, Na)NbO3-based system (KNN) with
PZT-like values of piezoelectric coefficients in the texture ceramics. Finally, they achieved a comparable piezoelectric response with PZTs.
The lead-free piezoelectric materials can be categorized into two general groups: one competes for the same application as PZT and another excels in properties that are outside the range where PZT can be used. As for the first group (in competition with PZT), it contains KNN, Bi0.5Na0.5TiO3 (BNT) and (Ba,Ca)(Zr,Ti)O3 (BCZT) based
ceramics. These materials are inferior to PZT in some sense but are superior in another. The second group includes materials with properties with which PZT cannot compete. Examples include: BaTiO3, LiNbO3 (single crystal), Bi-based layered compounds with
Aurivillius structure, and other high temperature piezoelectric materials.73
(K, Na)NbO3 (KNN) perovskite ferroelectric. KNN-based ceramics have attracted
considerable attention due to the high Curie temperature, large piezoelectric response and strong ferroelectricity. These ceramics are supposed to be suitable alternatives for lead-based PZT.74 Reported in 1955, a morphotropic phase boundary (MPB) separating two orthorhombic ferroelectric phases was identified at x = 0.5 ((K1-xNax)NbO3).
Inherent to MPBs, a maximum in remnant polarization (Pr) and minimum in coercive
field (Ec) were reported.75 The KNN-based ceramics obtained by solid state reaction
25
the lead-based PZT systems76 due to that the phase stability of KN is limited to 1040 oC
and KNN is limited to 1140 oC.77 Until now, the high density KNN ceramics can be only synthesized by hot pressing techniques.78 It is important to note that KNN material prepared by spark plasma sintering reported significantly higher dielectric and piezoelectric properties than those synthesized by hot pressing, solid state and molten salt reaction process, with εr = 700 and d33 = 148 pC/N.79, 80 It is indicated that the
enhancement is the result of extrinsic contributions to the polarizability associated with submicron grain sizes, similar to that found in fine grain BaTiO3 ceramics.81
Bi0.5Na0.5TiO3 perovskite ferroelectric. Bi0.5Na0.5TiO3 (bismuth sodium titanate, or
BNT) is a well-known lead-free perovskite ferroelectric material.It has a perovskite structure with a rhombohedral R3c space group (a = 38.91 nm; α= 89.6o) at room
temperature.82 In the perovskite structure with ABO3 formula, where one half of A-site
is filled with Na+ and the other half with Bi3+, and the B-site is filled with Ti4+. The BNT has also been regarded as one of the most promising candidate materials for the development of lead-free piezoelectric materials due to its relatively large remnant polarization (Pr = 38 μC/cm2) and high Curie temperature (Tc = 320 oC).83 However, a
large coercive field and a high conductivity of the un-doped BNT result in difficult poling, which then directed the exploration of BNT toward BNT-based materials, such as Bi0.5Na0.5TiO3/BaTiO3 (BNT/BT), Bi0.5Na0.5TiO3/Bi0.5K0.5TiO3 (BNT/BKT),
Bi0.5Na0.5TiO3/BiAlO3 (BNT/BA), Bi0.5Na0.5TiO3/SrTiO3 (BNT/ST) etc., and the
piezoelectric properties along with the electrical resistivity have been greatly improved.84-86 Processing and properties of the binary BNT-based ceramics have been extensively reported.87, 88
26
BaTiO3 (barium titanate, or BT), which exhibits a tetragonal symmetry of perovskite
structure at room temperature having large piezoelectricity and excellent dielectric properties,89 which make it the most important compound applied in the composition of ceramic capacitors, especially for the manufacture of multilayer ceramic capacitors (MLCC).90, 91 Since the performance of the BT ceramics significantly depends on the microstructure of the calcined body, much attention has been focused on the synthesis of BT nanoparticles. In recent years, wet-chemistry synthesis techniques, including sonochemical synthesis,92 sol-gel,93 hydrothermal,58 solvothermal94 and chemical coprecipitation,95 have been investigated to prepare BT nanoparticles. However, it is still a challenge to synthesize well dispersive BT nanoparticles with controlled morphology. In addition, the molten salt method has also been used to prepare many ceramic materials.96 Above the melting point of the chosen salt, the molten salt forms a liquid phase to act as a solvent for reactant dissolution, diffusion and precipitation. To achieve the competition to the lead-based ferroelectric materials, two kinds of effective approaches, i.e. domain engineering and oriented engineering, have been widely used to improve the piezoelectric performance for the ferroelectric materials.97-99 The domain
engineering is to reduce the domain size of the ferroelectric materials, while the oriented engineering is to optimize their orientation direction. The piezoelectric response of BaTiO3 (BT) can be improved from 200 to 788 pC/N using the combined domain and
oriented engineering, the value is larger than that of 500 pC/N for the commercially available PZTs.89
1.3.2 Halide perovskites
27
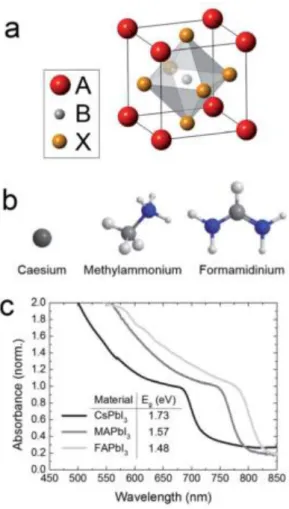
photovoltaics have demonstrated this class of materials to be excellent semiconductors for optoelectronic devices.9, 100, 101 For halide perovskite, perovskite compounds based on metal halides adopt the ABX3 perovskite structure, which has existed for more than a
century. This structure consists of a network of corner-sharing BX6 (B = Pb(II), Sn(II),
Ge(II); X = Cl, Br, I) octahedra, while the A cation is selected to balance the total charge and it can even be a Cs+ or a small organic ions (CH
3NH3+ or MA, HC (NH2)2+ or FA).
MAPbX3 perovskites. Examples of insulating, semiconducting, and superconducting
perovskite structured materials are known; they represent a unique system class across solid-state chemistry and condensed matter physics.9 Particularly, they have phase transitions with accessible monoclinic, trigonal, orthorhombic, tetragonal and cubic polymorphs relying on the rotation and tilting of the BX6 octahedra in the lattice.
Stimuli, like temperature, electric field and pressure, can give rise to the reversible phase transitions. In fact, the Cl-, Br- and I- with different ionic radii have been widely applied to establish the MAPbX3 (X: Cl, Br, I) perovskite structures, and it has been
found that a smaller ion radius for X- tends to form the cubic phase, such as MAPbCl3
and MAPbBr3, while the MAPbI3 exhibits a tetragonal structure.102 MAPbI3, with a
bandgap of about 1.5-1.6 eV and a light absorption spectrum up to a wavelength of 800 nm (Fig. 1.9), has been extensively used as a light harvester in solar cells.103 In addition, the ferroelectricity of the MAPbI3, has been confirmed by some researches, in which the
spontaneous polarization of the ferroelectric property gives rise to a more efficient charge-separation, then improve the power conversion efficiencies (PCEs) of perovskite solar cells (PSCs).12, 104-110 On the other hand, the MAPbCl3 and MAPbBr3 exhibit
paraelectric property due to their room temperature cubic structure. But, the MAPbBr3
28
potential, whereas the low current density impedes its further improvement of the PCE.111 In order to fulfill a high open circuit voltage in a solar cell, it is necessary to combine a suitable energy band structure of the constituent materials with good charge transfer kinetics. Therefore, the MAPbI-based (MAPbI3-xBrx, or MAPbI3-xClx)
perovskites have been widely studied.112-115 The advantages of utilizing a perovskite material as the main ingredient are ascribed to its large absorption coefficients, high carrier mobility, ambipolar transporting properties, and low-cost solution-based fabrication process.116
Chronologically, Miyasaka et al. demonstrated the formation of the MAPbBr3 and
MAPbI3 in dye-sensitized solar cells (DSSCs) and the efficiency of 2.6 % and 3.8 %,
respectively in 2006 and 2009. Subsequently, Park et al. reported an efficiency of 6.5 % by creating a cell architecture similar to the extremely thin absorber (ETA) DSSC. In 2012, Park et al. replaced the liquid-based hole transport layer with solid-state spiro-MeOTAD and immersed perovskite into the TiO2 scaffold because of the
corrosion problems for the liquid electrolytes, in the meantime an efficiency of 9.7 % was fulfilled. Since then, the researches on the perovskite solar cells show the explosive growth.10, 117, 118 In 2015, Park reported the MAPbI3 PSCs with a PCE of 19 %.119 Many
studies and investigations on the performances of perovskite solar cells are still ongoing in the effort to surpass this PCE of 20%, as well as to establish a stable performance for PSCs, in order to eliminate costly silicon PV cells.120
29
Fig. 1.9 Tuning perovskite bandgap by replacing the A cation. (a), The ABX3 perovskite crystal
structure. (b) The atomic structure of the three A site cations explored. (c) UV-Vis spectra for the APbI3 perovskites formed, where A is either methylammonium (MA), formamidinium (FA) or
caesium (Cs). Reproduced with permission.121 Copyright 2014, Royal Society of Chemical.
FAPbI3 perovskites. The chemical modification of the X site anions for MAPbI3
(substitution of I for Br or Cl) has been achieved and was shown to increase the bandgap while modulating the PCEs of PSCs. Similarly, the substitution of the MA+ with a longer chain C2H5NH3+ was reported and gave rise to a larger band gap with a
lower PCE (2.4 %). Therefore, the exploration of the alternative perovskites with attractive bandgaps is necessary, which can be formed into efficient solar cells.
30
It is known that the optimal bandgap for a single-junction solar cell is between 1.1 and 1.4 eV, currently beyond the range of the MAPbI3 system. Koh et al. have
demonstrated the introduction of a new metal-halide perovskite based on FAPbI3,which
displays a favorable bandgapand exhibits a broader absorption compared to MAPbI3.122
This is because that the FA cation has shown a slightly larger ionic radius than the MA group, which is expected to result in an increase in perovskite tolerance factor (t).65 An
increase in the tolerance factor t while maintaining the FAPbI3 perovskite structure
generally leads to an increase in symmetry, with an expected reduction in electronic bandgap (1.47 eV) (Fig. 1.9). This bandgap for FAPbI3 is closer to the optimum value of
∼1.4 eV and presents FAPbI3 as an appealing candidate for photovoltaic applications, as
it displays an extended absorption of light compared to the MAPbI3 analogue.123 Eperon
et al. have reported that the short-circuit currents of FAPbI3 PSCs achieved > 23
mA/cm2, giving rise to PCE of up to 14.2 %.121 Hence, FAPbI
3 perovskite is promising
as a new candidate for this class of solar cell.
However, the photovoltaic performance of FAPbI3 has been reported lower than that
of MAPbI3.121 In addition, a black perovskite polymorph (α-phase: stable at temperature
above 160 oC) was discovered to transformed into a yellow FAPbI3 polymorph (δ-phase:
non-perovskite) in an ambient humid atmosphere.122 Given the suitable bandgap that is
lower than that in MAPbI3, the performance of FAPbI3 solar cells can be considerably
improved by stabilizing the perovskite structure of the FAPbI3 phase. Based on this,
Pellet et al. reported an improved PCE by using mixed cation lead iodide perovskites by gradually substituting MA with FA cations, which increases the absorption range by red shift, allowing for a higher current density, but the performance was still dominated by MAPbI3 rather than FAPbI3.124 Therefore, Jeon et al. proposed a strategy for extending
31
the absorption range of solar light by replacing MAPbI3 with FAPbI3 in the combined
composition of FAPbI3 and MAPbBr3.103 Consequently, the (FAPbI3)0.85(MAPbBr3)0.15
perovskite with a trigonal structure at room temperature was fabricated, confirming that the co-substitution of MA to FA and I to Br can efficiently stabilize the perovskite phase. Furthermore, the fabricated FAPbI3-based PSCs exhibited a maximum PCE greater than
20%.125
CsPbX3 perovskite. Although all top-performing photovoltaic cells used MA or FA
as the A cation, it is attracting that whether an inorganic A cation would also form light absorbers with comparable properties to the MA ones. Studies on the all-inorganic halide perovskites have revealed that these materials have great potential in optoelectronic applications.126, 127 To find out whether the organic nature or anisotropic
geometry of the A cation is essential for the high performance of the hybrid PSCs, the CsPbBr3 was chosen because, unlike the CsPbI3 compound, this compound occurs at
standard temperature and pressure in the perovskite structure and exhibits very good charge transport properties.128 Beal et al. reported that by replacing the volatile MA cation with cesium, it is possible to synthesize a mixed halide absorber material with improved optical and thermal stability, a stabilized PCE of 6.5%, and a bandgap of 1.9 eV.129
Although the PCE of PSCs has a tremendous potential to exceed the silicon solar cells (25 %), conclusive charge separation mechanism is still missing for PSCs because of the lack of the fundamental understanding of the structure-property-performance relationship of the perovskite properties, which hampers the optimization and development of high-performance PSCs. Up to now, a traditional p-n junction charge separation has been employed to PSCs, however, there are some mysterious behaviors
32
like the strong current-voltage (J-V) hysteresis, yet remains unexplained reasonably.13, 130 And the J-V response of the PSCs could lead to unfaithful estimation of the solar cell
device efficiency, where the reverse scan and forward scan exhibit the overestimated and underestimated PCEs.131 Therefore, some possible theories have been proposed to explain the origin of the hysteresis in PSCs, involving ferroelectricity,106 vacancy-assisted ionic migration,132 charge carrier trapping,133 and capacitive effect.13
1.4 Lattice strain engineering
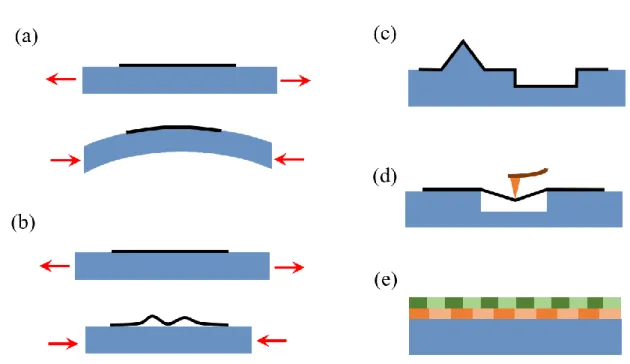
Two-dimension (2D) materials have been paid much attention in the past decade due to their extraordinary properties and great potential in a wide range of applications. Strain engineering is regarded as a powerful tool to modulate the properties of 2D materials because of its direct impact on lattice structure, and thus alters electronic structure.135 There are five possible methods, like deforming a substrate,136 creating wrinkles,137 employing a pre-patterned substrates,138 deforming a suspended membrane139 and lattice mismatch,140 which have been reported in the literatures, as shown in Fig. 1.10. Among these methods, the lattice mismatch has been widely applied to ferroelectric 2D thin film materials for enhancing their dielectric and ferroelectric responses.140-143
33
Fig. 1.10 Schematic presentation of methods to introduce strains to 2D materials. (a) Deforming
a substrate; (b) Creating wrinkles; (c) Employing a pre-patterned substrate; (d) Deforming a suspended membrane. (e) Lattice mismatch.
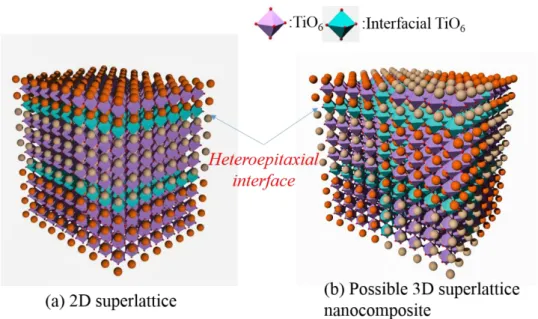
Enormous strains can appear in thin film materials when one material is epitaxially grew on another, originating from differences in the lattice constants of crystals and the different thermal expansion behaviors between the film and the underlying substrate or the defects formed during the film deposition. Therefore, some properties of thin film can be found remarkable different from the corresponding unstrained bulk materials. In the meantime, the suitable strains are needed for the enhancement of properties of a chosen material in thin film form, namely lattice strain engineering. It has been widely applied to 2D thin film materials with superlattice structure, correspondingly causing the 2D heteroepitaxial interface, as shown in Fig. 1.11(a).
34
Fig. 1.11 Schematic illustrations of local environments for (a) the 2D heteroepitaxial interface in
the superlattice nanocomposite obtained by the MBE approach and (b) the possible 3D heteroepitaxial interface in the mesocrystalline nanocomposite.
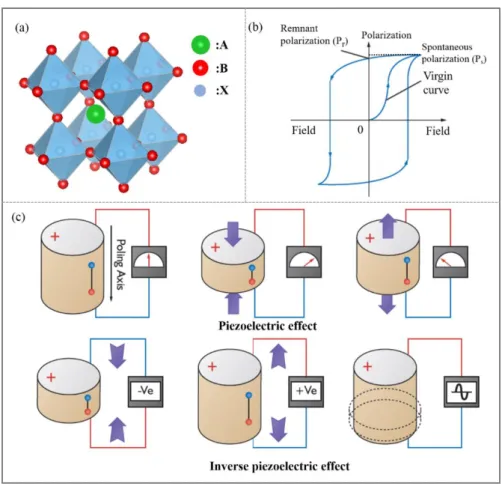
Heteroepitaxial growth has been established as a powerful technique to create single-crystalline thin films or artificial low-dimensional quantum systems. Furthermore, it gives the possibility to combine crystalline materials, which can be very different in symmetry, bonding and lattice constant, respectively. The atomic arrangement at the interface is the most responsible for the specific epitaxial alignment, and also for the development of the resulting microstructure. Residual strains as well as extended defects affect the desired physical properties and, thus, strongly decide the quality of functionality of heterosystem. Hence, it is possible to synthesize heterosystems with tailored electrical, optical or mechanical properties.144 For the ferroelectric materials, the spontaneous polarization direction around the heteroepitaxial interface can be changed to the other directions due to the lattice distortion originated from different lattice constants (Fig. 1.12). Therefore, a sloping spontaneous polarization structure can
35
be imported into the interfaces of the
Fig. 1.12 Schematic diagrams of imported heteroepitaxial interfaces from (a)
ferro-tetragonal-ferro-trigonal composite with polarization direction along [001] and [111] respectively, (b) ferro-tetragonal-ferro-orthorhombic composite with polarization direction along [001] and [110] respectively, (c)ferro-tetragonal-para-cubic composite.
different substances, which results in producing the polarization reversal sustaining.145 The artificial interfaces such as in artificial superlattices can produce an obviously significant enhancement of piezoelectric and dielectric constants.140, 141, 143, 146, 147
As mentioned above, the replacement of lead-based Pb(Zr1−xTix)O3 (PZT)
ferroelectric materials has become quite significant for the environmental protection. However, the reported lead-free materials exhibit quit lower piezoelectric performance than that PZTs. Although, the piezoelectric response of BaTiO3 (BT) can be improved
from 200 to 788 pC/N using the both domain engineering and oriented engineering, the value is larger than that of 500 pC/N for the commercially available PZTs.89 However, the piezoelectric application of BT is restrained in a temperature range of below 130 °C
36
of its Curie temperature (Tc). Therefore, the elevated Tc is also important for the
development of high performance lead-free ferroelectric materials. Haeni et al. first reported the improvement of Curie temperature (Tc) in SrTiO3 thin film by introducing
the substrate-induced epitaxial strain, in the meantime Choi et al. demonstrated the same manner in BaTiO3 thin film.140, 141 However, the increase of Tc by means of
substrate control is limited to film materials with only tens of nanometers thick, whereas many ferroelectric devices need much thicker films. Therefore, a BaTiO3/Sm2O3
composite thick film has been reported with a highly improved Tc of BaTiO3, where the
BaTiO3 lattice bears a tensile strain with +2.35 % lattice mismatch at the BaTiO3/Sm2O3
interface by Harrington et al.143
At present, the most studies on the superlattices of ferroelectric materials are in regard to 2D BaTiO3/SrTiO3 superlattice structure prepared by the
molecular-beam-epitaxy (MBE) process.141 But the MBE process is inefficient and high cost, and the industrialization production is difficult to achieve by MBE process.142 Furthermore, 3D superlattice structures (Fig. 1.11(b)) which can achieve a high density interface are difficult to be fabricated via the MBE process.30, 31, 148 Hence, the
exploration of a new approach to develop the superior superlattice composite materials is expected. The development of the ferroelectric mesocrystalline nanocomposites with the 3D superlattice structure by a facile low cost process is a significant subject and will attract much attention in the ferroelectric materials field.149 In our former research, the mesocrystalline ferroelectric BaTiO3/SrTiO3 (BT/ST) and BaTiO3/CaTiO3
nanocomposites constructed from the different nanocrystals with same crystal-axis direction exhibit an both elevated dielectric and ferroelectric responses via the introduction of the strains originated from the lattice mismatches between BT and ST or
37
BT and CT, which have different lattice constants.30, 31
1.5 Topochemical synthesis
Topochemical synthesis is a quite useful and classical approach for the fabrication of the targeted particles with the desired morphologies.54, 150 Compared with other reactions, the topochemical conversion reaction can be regarded as special phase transformations of the parent crystals into the daughter crystals and is driven by the crystal structures rather than by the chemical nature of the reactants. Therefore, the crystallographic directions of parent and daughter crystals have some certain topological correspondences. Some mesocrystals can be prepared by the topochemical syntheses method as described above. The conventional synthesis approaches, such as hydrothermal/solvothermal process, molten salt process, and solid-state reaction process etc., can be employed for the topochemical synthesis.
1.5.1 Approach of topochemical synthesis
The hydrothermal/solvothermal process is a liquid chemical reaction process under a higher pressure than 1 atm and a higher temperature than boiling point of the solvent used. When an aqueous solution is used as the solvent, it is called hydrothermal process. When other solvents rather than aqueous solvent, such as organic solvents, organic and aqueous mixed solvents, are used, it is called solvothermal process. These processes are widely applied to prepare the ceramics powders. The basic mechanism of crystal nucleation and growth under the hydrothermal and solvothermal conditions is the dissolution-deposition reactions. The particle size is controlled by the crystal growth rate, reaction time, and reaction temperature. The particle morphology is dependent on the crystal growth direction or the non-classical self-assemble direction that is not easy
38
to be controlled in the normal cases. The advantages of the hydrothermal/solvothermal process are preparations of the products with a controllable morphology, a controllable crystal facet, a uniform size distribution, a small crystal size at a relatively low temperature. The hydrothermal/solvothermal process is a potential method for the topochemical synthesis.
Because of the advancements in modern technology, the study of molten salt synthesis has achieved considerable progress, and lots of molten salts have been used for this process. The molten salt process is usually carried out in a low-temperature molten salt as a reaction medium, and the molten salt can also act as a reagent.151 Salt melts have a long history as a solvent in research as well as in industry due to their low toxicity, low cost, low vapor pressure, abundant availability, high heat capacity, large electrochemical window, and high ionic conductivity. The crystal growth occurs easily in the salt melt medium, the product particles usually have its original crystal morphology, uniform and large particle size. The precursor host particles can react easily with the guest ion or molecule species in the molten-salt via host-guest mechanism to obtain the desired composition and morphology of the products. Therefore, the molten salt process is suitable for the topochemical synthesis.
For the normal solid-solid reaction process, the ball-milled precursor powders with desired compositions should be annealed at high temperatures. This reaction occurs simply via solid-state diffusion at a high temperature. The obtained products usually have the characteristics of isometric morphology such as cubic or spherical, large particle size, and compositional inhomogeneity.152 Nonetheless, the solid-state process can still be used for the topochemical synthesis.153
39
1.5.2 Layered protonated titanate HTO as precursor for topochemical synthesis
Fig. 1.13 Schematic diagrams of HTO (H1.07Ti1.74O4·H2O) crystal with lepidocrocite structure. (a)
[100] zone axis structure and (b) three-dimensional (3D) structure.
Layered titanates with variety 2D structures have recently been drawn much attention because of their interesting interlayer chemistry. One of the most studied layered titanate is lepidocrocite (γ–FeOOH)-type protonated titanate, which has a composition of H1.07Ti1.73O4·H2O (HTO) and exhibits excellent ion-exchange/intercalation
reactivities, and can be readily exfoliated/delaminated into its structure unit single sheets with a distinctive 2D morphology and a thickness of about 1 nm.58, 154 In the HTO crystal structure, the TiO6 octahedrons are combined with each other via angle and
edge-sharing to form a 2D TiO6 octahedral sheet, as illustrated in Fig. 1.13. The host
sheets are stacked with a basal spacing of about 8.82 Å in a body-centered orthorhombic system (a = 3.7831 Å, b = 17.6413 Å, c = 2.9941 Å), accommodation H2O and H3O+
40
between the octahedral sheets (Fig. 1.13). Approximately 52% of the interlayer sites are occupied by H3O+ and remaining by H2O. The positive charge of H3O+ is balanced with
minus one of the host TiO6 octahedral sheets arising from the Ti site vacancies.
In our previous works, we have used the HTO crystal as a precursor to prepare the various perovskite titanate mesocrystals and the TiO2 platelike mesocrystals,18 and
furthermore as a template to fabricate oriented ceramics by a reactive template grain growth method.62 Very recently, the phase transition mechanism of the HTO crystal to anatase TiO2 under supercritical water has been reported.18 These results suggest that
the HTO crystal is an excellent precursor for the preparations of the titanate mesocrystals and titanium oxides mesocrystals by the topochemical conversion reaction mechanisms.
1.5.2 Soft chemical process for mesocrystalline nanocomposites
The solvothermal soft chemical process is a useful and unique method for preparation and design of functional inorganic materials.18, 31, 57, 148, 155 Advantages of the hydrothermal/solvothermal process are suitable for the soft chemical synthesis, especially in effectively maintaining the precursor morphologies in the synthesis process. The solvothermal soft chemical process typically comprises two steps: the first step is the preparation of a framework precursor with layered structure and insertion of structural directing-agents (template ions or molecules) into its interlayer space by a soft chemical reaction; the second step is the structural transformation of the structural directing-agent- inserted precursor into a desired structure by a solvothermal reaction. The crystal structure of the product can be controlled by the structural directing-agent used, and the product particle morphology is dependent on the precursor morphology
41
used. This process has been utilized for the synthesis and design of metal oxides mesocrystals and organic-inorganic nanocomposites with controlled structure, morphology, and chemical composition.20, 54, 156 As described above, the platelike mesocrystalline nanocomposites perovskite mesocrystals, such as BaTiO3/SrTiO3 and
BaTiO3/CaTiO3 nanocomposites, have been successfully fabricated by using the facile
two-step solvothermal soft chemical process, which give rise to highly elevated dielectric and ferroelectric properties.30, 31
1.6 Purpose of present study
As described above, up to now, studies on the mesocrystals have been reported mainly on the syntheses and formation mechanisms; however, the understandings on mesocrystal properties, formation mechanisms, and especially the potential application possibilities are still not enough. Further development of the functional mesocrystals, investigations of the mesocrystal performances and the formation mechanisms are necessary in current nanomaterial research fields. The development of the ferroelectric perovskite mesocrystals has drawn much attention since the potential improvements on dielectric, ferroelectric and piezoelectric responses of the mesocrystalline materials. In addition, the approaches, like oriented engineering, domain engineering and nanocomposition engineering, can be applied to improve the piezoelectric property of the ferroelectric materials by using the mesocrystalline materials. However, simultaneously elevated Curie temperature and piezoelectric responses have not yet been reported, in which the application temperature ranges of the ferroelectric materials are restrained by their Curie temperatures.
42
temperature of the ferroelectric materials by introduction of 2D heteroepitaxial interface. But for bulk ferroelectric ceramic materials, the strain engineering for simultaneous improvement of both piezoelectric response and Curie temperature has not been reported, as far as we know, since the 3D heteroepitaxial interface is very difficult to be introduced into bulk materials. Therefore, in the present study, the 3D heteroepitaxial interface is challenged to introduce into the functional mesocrystalline nanocomposites through a two-step soft chemical process via the in situ topochemical conversion mechanism. In the meantime, both piezoelectric response and Curie temperature of the mesocrystalline ferroelectric nanocomposites can be highly improved.
On the other hand, the perovskite compounds possess a number of interesting properties, such as electron-acceptor behavior; a large optical transmission domain; antiferromagnetic; exceptional magnetic; piezoelectric; photoluminescent properties; anionic conductivity over a wide temperature range. The semiconducting properties of perovskite-related halides have been widely applied to photovoltaic areas. Still, the ferroelectricity is also possible in perovskite-related halides, which would exhibit quite interesting and debatable behaviors in the PSCs. Therefore, figuring out the semiconductor and ferroelectric behaviors in the perovskite-related halides is significant.
In Chapter II, the ferroelectric mesocrystalline BaTiO3/Bi0.5Na0.5TiO3 (BT/BNT)
nanocomposite synthesized from a layered titanate H1.07Ti1.73O4 (HTO) by an ingenious
two-step topochemical process is introduced. The BT/BNT nanocomposite is constructed from well-aligned BT and BNT nanocrystals with the same crystal-axis orientation. The BT/BNT heteroepitaxial interface in the nanocomposite is promising for the enhanced piezoelectric performance by using the lattice strain engineering,
![Fig. 1.12 Schematic diagrams of imported heteroepitaxial interfaces from (a) ferro-tetragonal-ferro-trigonal composite with polarization direction along [001] and [111]](https://thumb-ap.123doks.com/thumbv2/123deta/5737989.1020702/36.892.129.769.136.556/schematic-diagrams-heteroepitaxial-interfaces-tetragonal-composite-polarization-direction.webp)