氏 名 ( 本 籍 ) 阿部 貴則(栃木県) 学 位 の 種 類 博士(生命科学) 学 位 記 番 号 博 第91号 学位授与の日付 平成27年3月20日 学位授与の要件 学位規則第 5 条第 1 項該当
学 位 論 文 題 目 N-terminal Hydrophobic Amino Acids of Activating Transcription Factor 5 Protein Confer IL-1β-Induced Stabilization
論 文 審 査 委 員 (主査) 高橋 勇二 教授 田中 弘文 教授 柳 茂 教授 浅野 謙一 准教授
論文内容の要旨
IntroductionActivating transcription factor 5 (ATF5) is a stress-response transcription factor of the cAMP response element-binding protein/ATF family and cotains a basic leucine zipper (bZIP) domain. ATF5 regulates processes involved in cellular differentiation, the cell cycle, and apoptosis. Jared et al. reported that ATF5 mRNA is induced in LPS-treated macrophage. This suggests ATF5 involvement in the regulation of immune response. We have shown that ATF5 mRNA is regulated by it’s 5’ UTRα and phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), and ATF5 protein is stabilized after CdCl2 and NaAsO2 exposure. Furthermore, we demonstrated that N-terminal amino acids contribute to the destabilization of the ATF5 protein in steady-state conditions and serve as stabilization domain in the stress response after CdCl2 or NaAsO2 exposure. However, the precise mechanism by which the N-terminal amino acids of ATF5 protein function as a destabilization domain or stress response stabilization domain remains to be elucidated.
Acute phase response (APR) is the earliest stage of the innate immune responses, which is characterized by fever and acute phase response proteins production. APR is triggered by various stimuli such as bacterial infection, tissue damage, or inflammatory cytokines including interleukin 1β (IL-1β), IL-6 and tumor necrosis factor-α secreted from immune cells such as neutrophil granulocytes or macrophages. Secretion of cytokines cause production of acute phase proteins (APPs) in liver. Previously, we have found that ATF5 might supress LPS-indused APP expression levels in the liver and functions as a negative regulator of APR.
In this study, we show that the N-terminal hydrophobic amino acids play an important role in the regulation of ATF5 protein expression in IL-1β-mediated immune response and that ATF5 is a negative regulator for IL-1β-induced expression of SAA1 and SAA2 in HepG2 cells.
Results
ATF5 protein is stabilized by IL-1β treatment
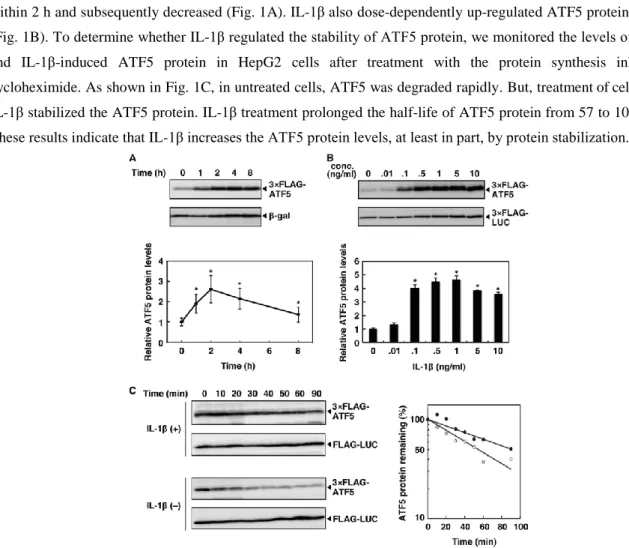
Treatment of HepG2 cells expressing 3×FLAG-ATF5 with IL-1β (0.1 ng/ml) increased ATF5 protein levels within 2 h and subsequently decreased (Fig. 1A). IL-1β also dose-dependently up-regulated ATF5 protein levels (Fig. 1B). To determine whether IL-1β regulated the stability of ATF5 protein, we monitored the levels of basal and IL-1β-induced ATF5 protein in HepG2 cells after treatment with the protein synthesis inhibitor cycloheximide. As shown in Fig. 1C, in untreated cells, ATF5 was degraded rapidly. But, treatment of cells with IL-1β stabilized the ATF5 protein. IL-1β treatment prolonged the half-life of ATF5 protein from 57 to 104 min. These results indicate that IL-1β increases the ATF5 protein levels, at least in part, by protein stabilization.
Figure 1. IL-1β increases ATF5 protein by enhancing it’s stability.
Stabilization of ATF5 by IL- it’s N-terminal hydrophobic amino acids
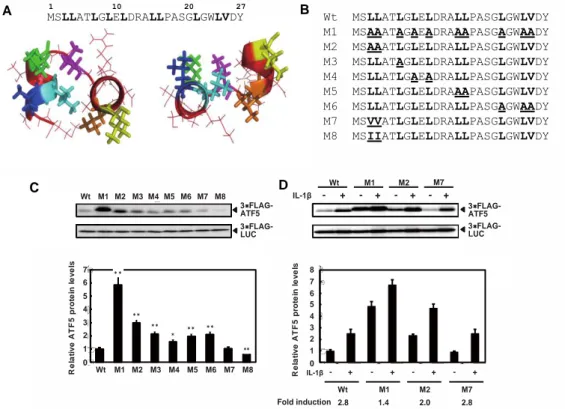
for more hydrophobic amino acids, isoleucine or valine (hydropathy indexes of 4.5 (Ile) and 4.2 (Val)), on expression plasmids (Fig. 2B). The M7 and M8 mutations did not increase ATF5 protein levels (Fig. 2C). We next examined the stress response of the ATF5 point mutants. Protein levels of the ATF5 wild type and ATF5-M1, M2, and M7 increased in the presence of IL-1β or CdCl2 (Fig. 2D). ATF5-M1 and ATF5-M2 increased with IL-1β but to a lesser extent than the ATF5 wild type and ATF5-M7 (Fig. 2D). These results indicated that the N-terminal hydrophobic amino acids is responsible for basal destabilization and for stabilization in response to IL-1β
Figure 2. The N-terminal domain rich in hydrophobic amino acids was responsible for ATF5 protein stabilization.
ATF5 N-terminal region function as a control of protein stabilization
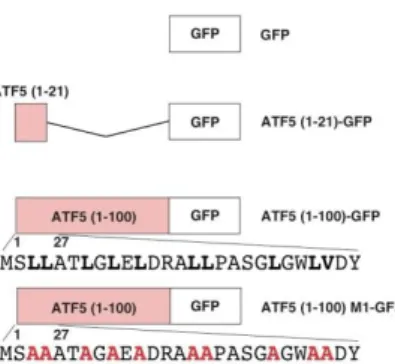
We next confirmed the importance of strong hydrophobic amino acid residues in the N-terminus of ATF5 for basal and inducible protein stability using a transient transfection system to express the ATF5 N-terminal region (amino acids 1-21 or 1-100) fused to the N-terminus of GFP protein in HepG2 cells (Fig. 3). ATF5(1-21)-GFP expression was partially down-regulated compared with GFP alone, whereas ATF5(1-100)-GFP protein expression was severely inhibited. Furthermore, the down-regulation of ATF5(1-100)-GFP was reversed by substituting leucine or valine with alanine in the N-terminus of ATF5. Treatment of HepG2 cells expressing GFP or ATF5(1-100)M1-GFP with IL-1β did not change protein expression levels, whereas cells expressing ATF5(1-100)-GFP showed significantly increased protein levels under stress conditions. These results indicated that the ATF5 N-terminal region fused to N-terminus of GFP conferred instability and stress responsiveness to the chimeric protein. !" #" $" %" &" '" (" )" !" #" $" %" &" '" (" )" *" !" #" $" %" &" '!" '#" Wt M1M2 M3 M4M5 M6 M7M8 Wt M1 M2 M7 - + - + - + - + CdCl2 Wt M1 M2 M7 - + - + - + - + 3 FL
AG-ATF5 3 ATFFL5
AG-3 FL AG-ATF5 R e la tiv e A T F 5 pr ot ei n le ve ls R el at iv e A T F 5 pr ot ei n le ve ls R el at iv e A T F 5 pr ot ei n le ve ls 0 1 2 3 4 5 6 7 0 1 2 3 4 5 6 7 8 0 2 4 6 10 12 8 Wt M1 M2 M7 Foldinduction 1.4 2.0 2.8 - + - + - + - + Wt M1 M2 M7 2.3 1.5 1.6 2.2 - + - + - + - + CdCl2 Foldinduction Wt M1M2 M3 M4M5 M6 M7M8 1 10 20 27 Wt MSLLATLGLELDRALLPASGLGWLVDY・ ・ ・ M1 MSAAATAGAEADRAAAPASGAGWAADY・ ・ ・ M2 MSAAATLGLELDRALLPASGLGWLVDY・ ・ ・ M3 MSLLATAGLELDRALLPASGLGWLVDY・ ・ ・ M4 MSLLATLGAEADRALLPASGLGWLVDY・ ・ ・ M5 MSLLATLGLELDRAAAPASGLGWLVDY・ ・ ・ M6 MSLLATLGLELDRALLPASGAGWAADY・ ・ ・ M7 MSVVATLGLELDRALLPASGLGWLVDY・ ・ ・ M8 MSIIATLGLELDRALLPASGLGWLVDY・ ・ ・ ATF5 : MSLLATLGLELDRALLP Jnet : --HHHHHHHHHHHHH--L3 A 14 L7 L11 L4 L15 G8 D12 A5 L9 R13 T6 E10 R E S I D U E S P O LA R R E S ID U E S NONPOLA R A B C D E 1 5 10 15 Fig.4 3 FL AG-LUC 3 LUCFLAG- 3 LUCFL AG-2.8 !"###$ !%###$ #$ %###$ "###$ &###$ 1 10 20 27 Wt MSLLATLGLELDRALLPASGLGWLVDY・ ・ ・
M1 MSAAATAGAEADRAAAPASGAGWAADY・ ・ ・
M2 MSAAATLGLELDRALLPASGLGWLVDY・ ・ ・ M3 MSLLATAGLELDRALLPASGLGWLVDY・ ・ ・ M4 MSLLATLGAEADRALLPASGLGWLVDY・ ・ ・ M5 MSLLATLGLELDRAAAPASGLGWLVDY・ ・ ・ M6 MSLLATLGLELDRALLPASGAGWAADY・ ・ ・ M7 MSVVATLGLELDRALLPASGLGWLVDY・ ・ ・ M8 MSIIATLGLELDRALLPASGLGWLVDY・ ・ ・ Wt M1 M2 M3 M4 M5 M6 M7 M8 Wt M1 M2 M3 M4 M5 M6 M7 M8 Wt M1 M2 M7 Wt M1 M2 CdCl M7 - + - + - + - + - + - + - + - + Wt M1 M2 M7 Wt M1 M2 M7 - + - + - + - + - + - + - + - + sl e v el ni et or p 5 F T A e vit al e R sl e v el ni et or p 5 F T A e vit al e R sl e v el ni et or p 5 F T A e vit al e R 0 1 2 3 5 4 6 7 0 1 2 4 3 5 6 7 8 0 2 4 6 8 10 12
Fold induction 2.8 1.4 2.0 2.8 Fold induction 2.3 1.5 1.6 2.2 6000 4000 2000 0 -2000 -4000 190 200 210 220 230 240 250 Wavelength (nm) M o la r E ll ip ti c it y ( d e g・ c m 2・ d m o l-1) D E F A B C 1 10 20 27 Wt MSLLATLGLELDRALLPASGLGWLVDY M1 MSAAATAGAEADRAAAPASGAGWAADY M2 MSAAATLGLELDRALLPASGLGWLVDY M3 MSLLATAGLELDRALLPASGLGWLVDY M4 MSLLATLGAEADRALLPASGLGWLVDY M5 MSLLATLGLELDRAAAPASGLGWLVDY M6 MSLLATLGLELDRALLPASGAGWAADY M7 MSVVATLGLELDRALLPASGLGWLVDY M8 MSIIATLGLELDRALLPASGLGWLVDY 2 CdCl2 3 FLAG-ATF5 3 FLAG-LUC 3 FLAG-ATF5 3 FLAG-LUC 3 FLAG-ATF5 3 FLAG-LUC ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ !" #" $" %" &" '" (" )" !" #" $" %" &" '" (" )" *" !" #" $" %" &" '!" '#" Wt M1M2 M3 M4M5 M6 M7M8 Wt M1 M2 M7 - + - + - + - + CdCl2 Wt M1 M2 M7 - + - + - + - + 3 FL
AG-ATF5 3 ATFFL5
AG-3 FL AG-ATF5 R e la tiv e A T F 5 pr ot ei n le ve ls R el at iv e A T F 5 pr ot ei n le ve ls R el at iv e A T F 5 pr ot ei n le ve ls 0 1 2 3 4 5 6 7 0 1 2 3 4 5 6 7 8 0 2 4 6 10 12 8 Wt M1 M2 M7 Foldinduction 1.4 2.0 2.8 - + - + - + - + Wt M1 M2 M7 2.3 1.5 1.6 2.2 - + - + - + - + CdCl2 Foldinduction Wt M1M2 M3 M4M5 M6 M7M8 1 10 20 27 Wt MSLLATLGLELDRALLPASGLGWLVDY・ ・ ・ M1 MSAAATAGAEADRAAAPASGAGWAADY・ ・ ・ M2 MSAAATLGLELDRALLPASGLGWLVDY・ ・ ・ M3 MSLLATAGLELDRALLPASGLGWLVDY・ ・ ・ M4 MSLLATLGAEADRALLPASGLGWLVDY・ ・ ・ M5 MSLLATLGLELDRAAAPASGLGWLVDY・ ・ ・ M6 MSLLATLGLELDRALLPASGAGWAADY・ ・ ・ M7 MSVVATLGLELDRALLPASGLGWLVDY・ ・ ・ M8 MSIIATLGLELDRALLPASGLGWLVDY・ ・ ・ ATF5 : MSLLATLGLELDRALLP Jnet : --HHHHHHHHHHHHH--L3 A 14 L7 L11 L4 L15 G8 D12 A5 L9 R13 T6 E10 R E S I D U E S P O LA R R E S ID U E S NONPOLA R A B C D E 1 5 10 15 Fig.4 3 FL AG-LUC 3 LUCFLAG- 3 LUCFL AG-2.8 !"###$ !%###$ #$ %###$ "###$ &###$ 1 10 20 27 Wt MSLLATLGLELDRALLPASGLGWLVDY・ ・ ・
M1 MSAAATAGAEADRAAAPASGAGWAADY・ ・ ・
M2 MSAAATLGLELDRALLPASGLGWLVDY・ ・ ・ M3 MSLLATAGLELDRALLPASGLGWLVDY・ ・ ・ M4 MSLLATLGAEADRALLPASGLGWLVDY・ ・ ・ M5 MSLLATLGLELDRAAAPASGLGWLVDY・ ・ ・ M6 MSLLATLGLELDRALLPASGAGWAADY・ ・ ・ M7 MSVVATLGLELDRALLPASGLGWLVDY・ ・ ・ M8 MSIIATLGLELDRALLPASGLGWLVDY・ ・ ・ Wt M1 M2 M3 M4 M5 M6 M7 M8 Wt M1 M2 M3 M4 M5 M6 M7 M8 Wt M1 M2 M7 Wt M1 M2 CdCl M7 - + - + - + - + - + - + - + - + Wt M1 M2 M7 Wt M1 M2 M7 - + - + - + - + - + - + - + - + sl e v el ni et or p 5 F T A e vit al e R sl e v el ni et or p 5 F T A e vit al e R sl e v el ni et or p 5 F T A e vit al e R 0 1 2 3 5 4 6 7 0 1 2 4 3 5 6 7 8 0 2 4 6 8 10 12
Fold induction 2.8 1.4 2.0 2.8 Fold induction 2.3 1.5 1.6 2.2 6000 4000 2000 0 -2000 -4000 190 200 210 220 230 240 250 Wavelength (nm) M o la r E ll ip ti c it y ( d e g・ c m 2・ d m o l-1) D E F A B C 1 10 20 27 Wt MSLLATLGLELDRALLPASGLGWLVDY M1 MSAAATAGAEADRAAAPASGAGWAADY M2 MSAAATLGLELDRALLPASGLGWLVDY M3 MSLLATAGLELDRALLPASGLGWLVDY M4 MSLLATLGAEADRALLPASGLGWLVDY M5 MSLLATLGLELDRAAAPASGLGWLVDY M6 MSLLATLGLELDRALLPASGAGWAADY M7 MSVVATLGLELDRALLPASGLGWLVDY M8 MSIIATLGLELDRALLPASGLGWLVDY 2 CdCl2 3 FLAG-ATF5 3 FLAG-LUC 3 FLAG-ATF5 3 FLAG-LUC 3 FLAG-ATF5 3 FLAG-LUC ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ !"###$ !%###$ #$ %###$ "###$ 1 10 20 27 Wt MSLLATLGLELDRALLPASGLGWLVDY・ ・ ・ M1 MSAAATAGAEADRAAAPASGAGWAADY・ ・ ・
M2 MSAAATLGLELDRALLPASGLGWLVDY・ ・ ・ M3 MSLLATAGLELDRALLPASGLGWLVDY・ ・ ・ M4 MSLLATLGAEADRALLPASGLGWLVDY・ ・ ・ M5 MSLLATLGLELDRAAAPASGLGWLVDY・ ・ ・ M6 MSLLATLGLELDRALLPASGAGWAADY・ ・ ・ M7 MSVVATLGLELDRALLPASGLGWLVDY・ ・ ・ M8 MSIIATLGLELDRALLPASGLGWLVDY・ ・ ・ Wt M1 M2 M3 M4 M5 M6 M7 M8 Wt M1 M2 M3 M4 M5 M6 M7 M8 Wt M1 M2 IL-1β M7 Wt CdCl - + - + - + - + - + Wt M1 M2 M7 Wt - + - + - + - + -sl e v el ni et or p 5 F T A e vit al e R sl e v el ni et or p 5 F T A e vit al e R sl e v el ni et or p 5 F T A e vit al e R 0 1 2 3 5 4 6 7 0 1 2 4 3 5 6 7 8 0 2 4 6 8 10 12
Fold induction 2.8 1.4 2.0 2.8 Fold induction 2.3 4000 2000 0 -4000 190 200 D E F 1 10 20 27 Wt MSLLATLGLELDRALLPASGLGWLVDY M1 MSAAATAGAEADRAAAPASGAGWAADY M2 MSAAATLGLELDRALLPASGLGWLVDY M3 MSLLATAGLELDRALLPASGLGWLVDY M4 MSLLATLGAEADRALLPASGLGWLVDY M5 MSLLATLGLELDRAAAPASGLGWLVDY M6 MSLLATLGLELDRALLPASGAGWAADY M7 MSVVATLGLELDRALLPASGLGWLVDY M8 MSIIATLGLELDRALLPASGLGWLVDY IL-1β 2 CdCl2 3 FLAG-ATF5 3 FLAG-LUC 3 FLAG-ATF5 3 FLAG-LUC ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・ ・
Molar Ellipticity (deg
・ cm 2・ dmol -1) -2000 !"###$ !%###$ #$ %###$ "###$ 6000 4000 2000 0 -4000 190 200
Molar Ellipticity (deg
Figure 3. Schematic of ATF5 N-terminal region-EGFP fusions.
ATF5 knockdown boosts IL-1β-induced SAA1 and SAA2 expression
A DNA microarray analysis using mRNAs from the livers of ATF5 knockout mice indicated that SAA1 and SAA2 mRNA expression levels increased with ATF5 gene deficiency. Thorn et al. reported that IL-1β induces SAA1 and SAA2 in HepG2 cells. We examined whether ATF5 knockdown influenced IL-1β-induced SAA1 and SAA2 mRNA expression in HepG2 cells. IL-1β significantly induced SAA1 and SAA2 mRNA 79-fold and 44-fold at 4 h. ATF5 knockdown boosted IL-1β-induction of SAA1 and SAA2 mRNA 141-fold and 94-fold at 4 h. These results support the notion that ATF5 is a negative regulator of IL-1β-induced SAA1 and SAA2 expressions in hepatoma cells, and suggest the importance of ATF5 in inflammation.
Discussion
In this study, we show that IL-1β increases the expression of ATF5 protein in HepG2 hepatoma cells in part by stabilizing the ATF5 protein. The N-terminal domain rich in hydrophobic amino acids predicted to form a hydrophobic network was responsible for destabilization in steady-state conditions and served as an IL-1β response domain. These results show that ATF5 N-terminal hydrophobic amino acid residues interact with a factor that controls protein stabilization via hydrophobic interactions, and it serves as the regulation of ATF5 protein expression. Therefore, we are working to identify the factors that regulate ATF5 stability through interaction ATF5 N-terminal region and reveal the mechanism for control of ATF5 protein stabilization.
Publication
Publication of the doctoral thesis
Abe T., Kojima M., Akanuma S., Iwashita H., Yamazaki T., Okuyama R., Ichikawa K., Umemura M., Nakano H., Takahashi S., Takahashi Y. N-terminal hydrophobic amino acids of activating transcription factor 5 (ATF5) protein confer interleukin 1β (IL-1β) -induced stabilization. J. Biol. Chem., 289:3888-900 (2014)
Other publication