+30
ῌ ῍
ῌ ῍
+3++/ +3,,+
Hydrogen sulfide concentration in a cavity under snow cover
Osamu M
ATSUBAYA Akita UniversityAbstract
At a snowed area, a cavity is often formed under the snow cover by water flow. If the flowing water dissolves hydrogen sulfide, concentration of hydrogen sulfide in air in the cavity rises, because of degassing of hydrogen sulfide from the water. If a person stands on snow surface upper such a cavity and if ceiling of the cavity collapses under his foot, he breathes hydrogen sulfide and meet with a quite serious accident. Hydrogen sulfide concentration in such a cavity was estimated based on extrapolation of hydrogen sulfide solubility from+atmospheric pressure to much lower pressure by application of the Henry’s law. If hydrogen sulfide concentration of flowing water is in a range of/+/ppm, for instance at+/, the hydrogen sulfide concentration in the cavity reaches a range of+,***
-,***ppm at equilibrium. Such concentration is high enough to kill persons. Time du- ration till the equilibrium was estimated by a model of continuous water flow as the fol- lowing. A constant volume of water is taken into the cavity at a content time interval, and hydrogen sulfide concentration of the water is equilibrated with the air in the cavity, and then the water is taken out the cavity at the same time at the next take-in of constant volume water. The time duration for the equilibration depends on the ratio of the cavity volume and the water volume taken into for a definite time duration, which corresponds the rate of continuous water flow. If the volume of cavity is+**times of the volume of water taken for+hour, for example, after-days concentration in the cavity reaches about 3*ῌof the equilibrium concentration.
Key words : Hydrogen sulfide, Cavity under snow, Degassing, Gas accident
/1 ,**1
+4
!" #
$%&"'( )*( %$+, -./0 12"34," %&" -+5 6 -789:;-5<=>+?, @AB /C+/ppmD+/E +,***C-,***ppm,"
%&" $F+&" #G HIJ +KLM- NB-OPQRSB TTL MMU %,M6HBV O
,L=W XY, H+KLMZ
,TLMZ +"[(N+\LM !
"]^+?,#$B D Z+LM+!+**% -&VH 3*ῌ+
_:`:a: bc34
+
def'g(hi"j $k34l?m &)n o*%&"34*pqrs t+332u vwx+ %*p y,+z{,O?,|} -~ tu .WB f{ /[" 0+?,"!-~
121 !"D bc'3
bc/G'WV4," "56, -/,+Z3.W %XY #3 %1
!" G de7 de 8 ! %7/",7
98:' !" $k34p,"
'3;* ~B+ <I! %?
B$34&= ,? %, !¡"34,**/>+,¢
£?¤@8del?mB % ~ %
B,? 'mB$)/-A0¥ F +,&34G B"34¦§C¨l?mBrs t+332u ©ª «¬A®",¯[+°D, 1d' tu G %?.WB,M-,m±
(¥B,&²E³#´D ²E³ °D,6 BB",S %? )µ¶ %&"
·D K !"-¸6 -#,x+B
,
,+
Z¸6 TF %Z5G+5,¥6!-¹ºH»+?
,@A 6 -+\, TZ6BZ!-ZIJ I/1¼ t,**1u 5
!"
#+ $%&'()(*
% $%&+,++- + ./01 234 56" +/7...gῌl, 8!
*.+-molῌl9 .*7,./gῌl, :;<*.*1molῌl9 )(=>?
@-"
$%& ABCD E ,F GH,SHIG$%&J HSKLMS,K,NOPQR :"
H,S ῍῎ HSSHSK ῌ HSK ῍῎ HSSS,K ῍
#+ C5$%&(8! - NO%TNUVW;X5Y
" -NOZ%TN[(\9 W8! G]^_`a bc8-" defAD E:6" Bῌ_`a HKJ
g)h +**7i)(jkKl+*K1m( no HpHl1J:;<HS[ (+*K1molῌlpWH,S[(HSK[(>qrs:9 S,K[(d!D96tu vw" h $%&x 5pW Bῌ CDE H,SG D9HS yz {o : HpHl.m(J" d|} Z$%&@~H,S:9HSK d+ῌ:"
: x:s )DE Z pW Z%TN_`
D96 :" h $%&)spW{o8
DE Z$%&>?IG$%& HH,SJZ Z vwV !"
,,
) n$%&[(s6+**ppmm(9
¡¢8DE:pW6d sa£ppm¤E" ¥5 )$%&[(
spWa¦ppm" dDE:[(#+C5++agῌl§
¨s=[(" d8 dDE:=[(jkV5c #+ C 5(=[(jk ©ª«:¬!: :" $%&+,++- ( H#+Jh $%&+,=-® ¯6°±:6 +²³R.´ HHenryJµ" ³R.´µ¶ H·
6¸J '+, ¹E69 ABw!"
PlkC ῎
88P$%&+,Cn$%&[(k¹a" ³R.´µ Fig.+ Hydrogen sulfide solubility in water under+atmos- pheric pressure. After Jikken Kagaku Binran (+30+) + +
+30+
)ºT 6
!"#$%&
'()*+,-*.*/ )0) 1#2$ 3456789:
; <=*45*0>0?
6@ABC DE*FGHI*
B=/ )6J Kῌ#P) 1#2$ 3 LMN OP)Q0R*/ S 45CT AUV2$ 345B6 W X<= 6YZ[!\2$ 3 ]^_`6UV2$ 3)\a b!cd 6e/
f, f+g)!c50.Kῌ hij kk li) m6 /n+/nZ[Q 1#2$
345 LMohppmpN #2$ 345 LppmN qrg )OB/ f0.s.0*<=
#2$ 3456iR-+*ppm
Z[ 1#tuvw2$ 345 m6/n+,0**ppm, +/n,,,**ppm*/
<=*45xy9:6; <=*45B/ *z m6]=61#tu 456{ f+0.M0<=m]=6 |!cd6}* ~!cd L45N *6HIB </
-
0.2$ 361#) f,g<=*tu45 S
<C 6e/ 61#ld), 2
$ 36) 10.1#1!ctu*
f-gp 6e/ * 1#l2$ 345 6 lldC 2$ 36) 1 !ctu6 / 1
C, ~2$ 345~d 61#C 6; C ! ctu*/ ¡l¢£C¤ <C ld) , 1#1!ctu*Sp 6e/ ¥<=
jKp 6e/
f-z 1#12$ 3P,Va,l 2$ 345C,
Vw) n¦@ ¡6tuvw§¨)PCPnCn Kῌ<C
Pn©kCn ῍
*/ 2$ 3456145C*0.CnSª} 1#
Fig., Hydrogen sulfide concentration in air equilibrated with water dissolving hydrogen sulfide in a range of ppm concentration.
, ppm
«¬1#2$ 345
/1® L,**1N 7
m
mC*CnVw ῍
m DP !"#$%
DPmRT
Va ῎
R&'(T&)*+n,- ./012 3*Pn+
PnPn+4DP ῏
#ῌ ῍ ῎ ῏56789:;<
Pn῍
῎Pn+4Vw
Va
RTC*῏
ῐ
ῌ
῍῎+4VVwaῌRTk ῏ῐ ῐ#=>?:n+$%@AnBCD1=EF=DG 3* HIJKL
M.& NO:A PQEFRS+ )+/T HM+DGk,,-+ G UV 3*C*+*ppm WVGHIIX+HI Y./Z[2
&=&\Y]? 8^P** _`NH=WVG a&
#ῐ& VwῌVa:bc d: 6\Y] ef(g&hi?
&VaῌVw+** ]ef=j D1k =lm$?1 Ono B p*./mq+mq+*m, 8^/m- WVG&*.*/m-, 8^/*l+ r s UF WVG HIIXt
VwFt ῑ
+ t7&U/*lῌH Y&*.2lῌu+
M.=v?: 3*wxg=yY HI&z<f=&{|B+
$1*HI }-~ wxg 3*ῑCY3*:vG
:&wx=y6:D?+1 wxgCYyHI& VaῌVwp?j
? VaῌVwp??1& =&p?$U UB?
$ UB?t7 &' =&
9 NYYUWkn%] D1
L=&U$% <]o%? ]&$$?
+1 , D1= N' WVG HIIX+HI 'VaῌVw+**
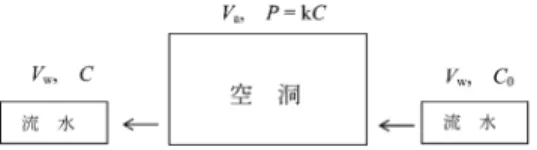
Fig.- A model of degassing from continuous water flow in a cavity, regarding as stepwise take-in and out of a constant volume water at a constant time interval.
VaandVware volumes of the cavity and of the water taken in and out, respec- tively. C*andCare hydrogen sulfide concentrations of the water taken in and out, respectively. Pis partial pressure of hydrogen sulfide in the cavity.
-
!"#$%& VaVw'()*++,-()& C* C'*+./012& P'./034 5637&
)¡¢£
8
ῌ
!"#$%&'()
*+, -."/0123 4 56789 6:
;<" =>?@A7BCD
=>?@AEF9G,H%
I/J@A/I()90"KL I4
.
:;<M7 N=>?7590O
* ND=>
?@AP5QJQ/4 ῎)R
=>?@A7/S+/ppm"KR D=>?@A+,***S-,***
ppm/4 &@AT7UV%WX /@A"K Y5NI/Z
;[T7\] N^_"`a7 bcd] T7Ded% UV
%fgh7J04 NI/Z;
[ iQO* jklmno p ;q'rs%&t50
"KLI4 NI/uvwxyῌz;7K :;7{|}{890/0~ xy;7#_ `a7Q/890&~%04 ;Z'QO*
5/cR/o/04
r ῍ Z +332:+7cT ¡ N¢£¤¥ ¦§¨© +1 +-+ῌ+/..
ª«>©¬ +30+ p.,3, ®\¯° ±²4
,**0³3´0C Cµ¶¨©·¸/3¹·"º
Fig.. An example of the estimation about time variation of hydrogen sulfide concentration in a cavity. The ra- tio of the cavity volume and the volume of water taken in and out is+**, the time interval is+hour, the hydro- gen sulfide concentration of water taken in is+*ppm, and the temperature is+/».
.
!+" #$%+&'()*+
#$+**,- '()*+../%+ .- (+%+*ppm- 0123
%+/4 56
:;<D=>?@A
¸/1¼ ,**1 9