Original Article
Wide expression of ZEB1 in sarcomatous component of spindle cell carcinoma of the esophagus
Takuro Nakazawa,1Sumihito Nobusawa,1Hayato Ikota,1Hiroyuki Kuwano,2Izumi Takeyoshi3and Hideaki Yokoo1
Departments of1Human Pathology,2General Surgical Science and 3Thoracic and Visceral Organ Surgery, Gunma University Graduate School of Medicine, Maebashi, Japan
The pathogenesis of sarcomatous component in spindle cell carcinoma (SpCC) of the esophagus is unclear. To investigate the involvement of epithelial-mesenchymal tran- sition (EMT) in sarcomatous differentiation, we performed immunohistochemistry for Slug, Twist, ZEB1, and ZEB2, transcription factors associated with EMT and E-cadherin, in 14 cases of SpCC of the esophagus. In order to verify the neoplastic nature of sarcomatous components,TP53muta- tion status and protein expression were examined in each case. Nuclear ZEB1 expression was extensive in the sarco- matous component, greater than invasive front of carci- noma components (P < 0.0001). Membranous E-cadherin expression was mostly lost in sarcomatous cells in all cases (P <0.0001). The p53 expression pattern was almost con- cordant between the two areas in all cases.TP53mutation analysis revealed that seven cases harbored identical muta- tions in both components. One case had mutations only in the sarcomatous component. It is noteworthy that none of them harbored mutation in exon 5, unlike conventional esophageal squamous cell carcinoma. These findings show that ZEB1 are widely expressed in the sarcomatous area of SpCC of the esophagus, suggesting the involvement of EMT. The avoidance of exon 5 in terms of TP53mutation may also be a feature of the tumor.
Key words: epithelial-mesenchymal transition, esophagus, spindle cell carcinoma,TP53, ZEB1
Spindle cell carcinoma (SpCC) of the esophagus is a rare malignancy consisting of both carcinoma and sarcomatous
components, accounting for approximately 0.5–2.8% of esophageal carcinomas.1It usually grows as a large intralu- minal, polypoid mass. The body of the polypoid mass is mostly formed by sarcomatous spindle cells, and in situ and/or invasive squamous cell carcinoma components sur- round the base. This characteristic tumor has also been referred to as carcinosarcoma, sarcomatoid carcinoma, pseudosarcomatous squamous cell carcinoma, polypoid car- cinoma, metaplastic carcinoma, squamous cell carcinoma with a spindle-cell component, or carcinoma with mesenchy- mal component. The various terms of use reflect the uncer- tain origin of this malignancy, and the histogenesis of the epithelial and sarcomatoid elements of SpCC of the esopha- gus has been a matter of controversy. Previous studies con- sidered the sarcomatous component to be reactive mesenchyme,2,3 concomitant sarcoma,4–7 or a variant of poorly differentiated squamous cell carcinoma.8,9Despite the presence of these two distinct histological components, recent studies have shown that the sarcomatous component is neoplastic, having transformed from squamous cell carcinoma.10–12From a biological point of view, it has been suggested that the transdifferentiation of epithelial cells to malignant spindle cells can be caused through the process of epithelial–mesenchymal transition (EMT). Indeed, there is some evidence indicating that EMT plays an important role in the pathogenesis of SpCC/carcinosarcoma in various sites throughout the body.13–15
Analyses of SpCC/carcinosarcoma of several organs have been reported in terms of EMT; however, to our knowledge, no previous studies have mentioned whether EMT- associated transcription factors play a role in the morphogen- esis of SpCC of the esophagus. In this study, we investigated this attractive and enigmatic manifestation by employing esophageal SpCC cases, focusing on insight into the immunophenotypic and molecular genetic correlations of the dual components using EMT-related markers andTP53as a hallmark of clonality.
Correspondence: Takuro Nakazawa, MD, Department of Human Pathology, Gunma University Graduate School of Medicine, 3-39-22, Showa-machi, Maebashi, Gunma 371-8511, Japan. Email:
ntakuro3@yahoo.co.jp
Received 14 July 2015. Accepted for publication 26 September 2015.
© 2015 Japanese Society of Pathology and Wiley Publishing Asia Pty Ltd
Pathology International2015 doi:10.1111/pin.12354
MATERIALS AND METHODS Patients and tumor samples
Fourteen patients diagnosed with SpCC of the esophagus between 2000 and 2013 at Gunma University Hospital and affiliated institutes were included in this study. Archival par- affin blocks from 14 SpCC of the esophagus were collected from the participating institutions, and serial 4-μm-thick par- affin sections were prepared. The male-to-female ratio was 13:1, and the age range at tumor resection was 55–80 years, with a mean age of 68 years. Only one was a recurrent case, which had undergone chemotherapy. The clinical features are given in Table 1.
Tumors were reviewed by light microscopy with the criteria established for SpCC of the esophagus according to the World Health Organization classification system.16 The Tumor–Node–Metastases (TNM) Classification of Malignant Tumor (7th edition) was used for pathological staging.17
Ethics statement
All clinical samples were procured from the Pathology Archive of Gunma University and its affiliated hospital and analyzed according to a protocol approved by the Medical Ethics Committee of Gunma University (based on the prin- ciples detailed in the Declaration of Helsinki). All patient infor- mation associated with this study was obtained in a de-identified format.
Immunohistochemistry
Paraffin sections were dewaxed in xylene and rehydrated through a graded series of ethanol. Endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen perox- ide for 10 min. After pretreatments performed according to
the manufacturer’s instructions, sections were incubated at 4°C overnight with primary antibodies. For coloration, a com- mercially available biotin-streptavidin immunoperoxidase kit (Histofine, Nichirei, Tokyo, Japan) and diaminobenzidine were employed. Antibodies were as follows: rabbit monoclo- nal to Slug (1:50 dilution, 9585; Cell Signaling, Boston, MA, USA), mouse monoclonal to Twist (1:100, ab508887; Abcam, Cambridge, UK), rabbit polyclonal to ZEB1 (1:1000, HPA027524; Atlas Antibodies, Stockholm, Sweden), rabbit polyclonal to ZEB2 (1:200, HPA027524; Atlas Antibodies), mouse monoclonal to p53 (1:50; Leica Biosystems, New- castle, UK), and mouse monoclonal to E-cadherin (1:50;
Leica Biosystems).
Immunoreactivity to each antibody was evaluated in carci- noma and sarcomatous components within the polypoid or main tumor region separately. When antibodies to EMT- related transcription factors showed statistical significance, we added the comparative evaluation between invasive front of carcinoma components and sarcomatous areas. Staining was defined as nuclear (Slug, Twist, ZEB1, ZEB2, p53) or membranous (E-cadherin) immunoreactivity in neoplastic cells. The percentage of neoplastic cells with immunoreac- tivity for Slug, Twist, ZEB1, and ZEB2 was counted in 5–10 fields per tumor area by light microscopy at×400 magnifica- tion and scored as follows:−, 0% positive cells;+, 1–10%;++, 11–50%;+++, 51–80%; and++++,>80%. The staining of p53 was evaluated by light microscopy using a three-tiered system (1+, 2+, and 3+), corresponding to the intensity of the nuclear staining. The percentage of tumor cell staining was also recorded.
TP53mutation analysis
To characterize the neoplastic nature of sarcomatous com- ponents, the TP53 mutation status in both carcinomatous
Table 1 Clinicopathological data of spindle cell carcinoma of the esophagus
Case Age Sex Location
Macroscopic
pattern TNM stage
1 71 M Ut-Mt Polypoid T2N1M0 Stage IIB
2 73 M Mt Polypoid T2N2M0 Stage IIIA
3 80 M Lt Polypoid T1bN0M0 Stage IA
4 71 M Mt-Lt Polypoid T1aN0M0 Stage IA
5 76 M Ut Polypoid T1bN0M0 Stage IA
6 55 M Mt Polypoid T1bN3M0 Stage IIIC
7 69 M Lt Polypoid T1bN0M0 Stage IA
8 67 M Mt Polypoid T1bN0M0 Stage IA
9 59 M Lt-Ae Ulcerated state T3N1M1 Stage IV
10 63 M Lt Polypoid T1bN1M0 Stage IIB
11 72 M Lt Polypoid T3N1M0 Stage IIIA
12 67 M Lt, Ae, Mt Polypoid T3N1M0 Stage IIIA
13 56 M Ut Polypoid T1bN0M0 Stage IA
14 73 F Mt Polypoid T1bN0M0 Stage IA
Ae, abdominal esophagus; F, female; Lt, lower thoracic; M, male; Mt, middle thoracic; Ut, upper thoracic.
and sarcomatous areas was examined by DNA sequencing.
We selected 13 cases in which sarcomatous and epithelial tumor areas were sufficiently large. Phenotypically different tumor components were microdissected separately and genomic DNA was extracted as previously described,18and amplified by polymerase chain reaction (PCR) using four pairs of primer sets for exons 5 through 8 of theTP53gene.19 The PCR products were then sequenced on a 3130xl Ana- lyzer (Applied Biosystems, Foster City, CA, USA) with Big Dye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems) according to standard procedures.
Statistical analysis
Pvalues were calculated using Mann–WhitneyUtest or by one-way analysis of variance followed by Bonferroni’s post hoctest. We declared differences to be statistically significant at the traditional threshold of P = 0.05. GraphPad Prism Software Version 6.0 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses.
RESULTS Pathological findings
The tumors were located in the middle and lower esophagus in 11 cases, and in the upper esophagus in three cases. The tumors of 13 cases presented as a large pedunculated polyp (Fig. 1a,b), whereas one case displayed an ulcerated lesion, which had undergone neo-adjuvant chemotherapy. The depth of tumor invasion was variable. Nine cases were con- fined up to the submucosal layer. Two had infiltrated into the muscularis propia and the remaining three cases showed tumor infiltration to the adventitia. Lymph node metastasis was noted in seven cases, two of which contained a sarco- matous component in the metastatic lesions. One case with metastasis in the liver was observed (Table 1).
Histologically, all of the tumors showed characteristic fea- tures. They were composed of a mixture of carcinoma and malignant sarcomatoid elements, with the latter forming the bulk of the tumor. The malignant epithelial components ranged from in situ (Fig. 1c) or minimally invasive into the submucosa. The sarcomatous element was composed of undifferentiated, spindle-shaped cells. These cells were occasionally embedded in edematous, loose-textured stroma (Fig. 1d). In most cases, the areas of transition between the two elements were identified (Fig. 1e). Some cases showed osseous and/or cartilaginous matrix produc- tion (Fig. 1f).
Immunohistochemistry to Slug, Twist, ZEB1, ZEB2, and E-cadherin
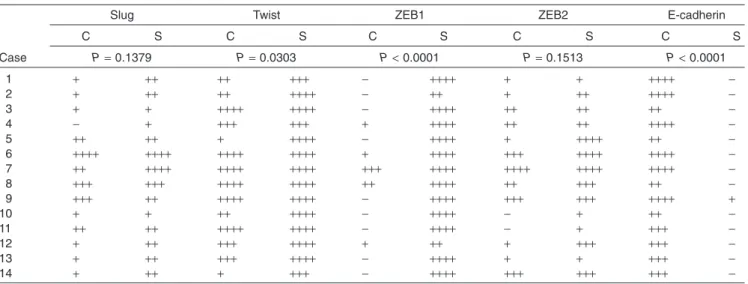
All results are shown in Tables 2 and 3. There was a ten- dency for Slug expression to be more widely observed in sarcomatous rather than in carcinoma components (Fig. 2a).
In the sarcomatous area, Slug-positive neoplastic cells were distributed throughout the region, while in the carcinoma area, Slug positivity was more widely observed at the basal layer of thein situcomponent and scattered invasive carci- noma nests. However, there was no significant correlation between carcinoma and sarcomatous components regarding Slug expression (P=0.1379).
As for Twist expression, a difference between the two components was observed. There were four cases (cases 2, 5, 10, and 14) that showed apparently wide immunoreactivity in sarcomatous components compared with that in carci- noma areas (Fig. 2b). The distribution patterns of Twist- positive neoplastic cells among the two areas were similar to Figure 1 Gross appearance and microscopic findings of spindle cell carcinoma of the esophagus. (a) The large polypoid mass pro- trudes into the lumen (case 5). (b) The cut surface of the tumor shows coalescence of white and tan-colored areas accompanied by hemorrhage and necrosis. Scale bar = 1 cm (case 5). (c) Intraepithelial malignant cells. Architectural disarray, loss of polarity, and cellular atypia are evident (case 13). (d) Undifferentiated and spindle-shaped cell proliferation in the sarcomatous components (case 6). (e) Characteristic biphasic pattern of spindle cell carcinoma of the esophagus. Some malignant epithelial cells show transforma- tion into sarcomatous cells (case 5). (f) Eosinophilic osseous matrix in the sarcomatous components (case 5).
those of Slug. Moreover, Twist showed significantly wider positivity in the sarcomatous components than in the carci- noma areas (P=0.0303), unlike Slug.
In terms of ZEB1 expression, an obvious difference was observed in almost all cases (P<0.0001) (Fig. 2c). Twelve cases showed ZEB1 expression in>80% neoplastic cells in the sarcomatous component and these ZEB1-positive neo- plastic cells were distributed uniformly in the sarcomatous area. In contrast, of these 12 cases, neoplastic cells in the carcinoma components were completely negative for ZEB1 in eight cases (Table 2). Of 14 cases, there were two cases that showed 11–50% ZEB1-positive cells in the sarcomatous components, and these two cases showed negative or 1–10% ZEB1-positive cells in the carcinoma components.
In terms of ZEB2 expression, there was a tendency for more positive neoplastic cells to be observed in the sarco- matous components (Fig. 2d). ZEB2-positive neoplastic cells were distributed uniformly throughout the sarcomatous area, while in the carcinoma area, ZEB2-positive malignant cells were observed more widely in thein situcomponent than in carcinoma nests scattered in the sarcomotous component.
However, there was no significant difference between the two areas (P=0.1513).
As described above, both ZEB1 and Twist expression showed statistical difference between carcinoma and sarco- matous areas within the main polypoid region. Then we focused on the invasive front, and demonstrated that ZEB1 expression of the sarcomatous component was still higher than the invasive front (P<0.0001), while Twist did not reach statistical difference (P=0.3762) (Table 3).
With regard to membranous E-cadherin expression, an apparent difference was observed in all cases between car- cinoma and sarcomatous components (P<0.0001) (Fig. 2e).
E-cadherin expression was widely observed in the carcinoma
areas in most cases, and six cases had>80% positive cells in carcinoma components. However, neoplastic cells in sar- comatous components were largely negative for E-cadherin.
Bonferroni’s correction revealed that ZEB1 showed nar- rower reactivity than Twist (P < 0.0001) in the carcinoma components. Furthermore, ZEB1 showed wider reactivity than Slug and ZEB2 in the sarcomatous components (Slug:
P=0.0007, ZEB2:P=0.0156).
Immunohistochemistry of p53 andTP53mutation analysis
The results are summarized in Table 4. Immunohistochemi- cal study was carried out in all 14 cases. Moderate to strong nuclear p53 expression was detected in five cases, and the sarcomatoid and epithelial tumor components showed almost concordant p53 expression patterns (Fig. 2f,g). There were eight cases that had no p53 staining. Case 8 showed strong p53 staining in a lower percentage of tumor cells in sarcomatous components than in malignant epithelial ones.
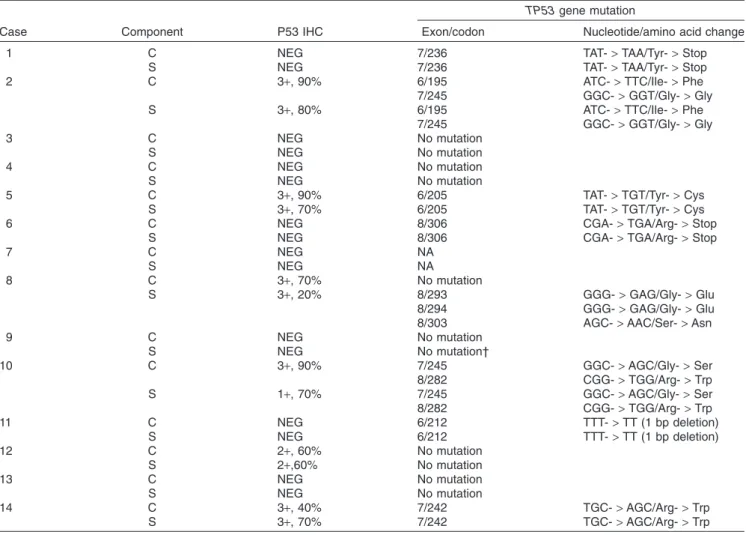
Sequencing analysis was performed in 13 cases. Case 7 was excluded because it did not have sufficient tumor size for analysis. In case 9, mutation analysis was successful only in exon 5 in the sarcomatous area, probably because of the poor quality of the specimen.TP53mutations were identified in 8 of 13 cases. Of these, seven cases possessed an iden- tical mutation in the carcinoma and sarcomatous compo- nents (cases 1, 2, 5, 6, 10, 11, and 14), while case 8 had mutations only in the sarcomatous component. Representa- tiveTP53mutations found in both carcinoma and sarcoma- tous areas are illustrated in Fig. 3. Two cases had dual mutations: case 2 in exons 6 and 7, and case 10 in exons 7 and 8. Case 8 showed triple mutations in exon 8 only in the sarcomatous component.
Table 2 Immunohistochemical study of molecules associated with epithelial-mesenchymal transition
Case
Slug Twist ZEB1 ZEB2 E-cadherin
C S C S C S C S C S
P=0.1379 P=0.0303 P<0.0001 P=0.1513 P<0.0001
1 + ++ ++ +++ − ++++ + + ++++ −
2 + ++ ++ ++++ − ++ + ++ ++++ −
3 + + ++++ ++++ − ++++ ++ ++ ++ −
4 − + +++ +++ + ++++ ++ ++ ++++ −
5 ++ ++ + ++++ − ++++ + ++++ ++ −
6 ++++ ++++ ++++ ++++ + ++++ +++ ++++ ++++ −
7 ++ ++++ ++++ ++++ +++ ++++ ++++ ++++ ++++ −
8 +++ +++ ++++ ++++ ++ ++++ ++ +++ ++ −
9 +++ ++ ++++ ++++ − ++++ +++ +++ ++++ +
10 + + ++ ++++ − ++++ − + ++ −
11 ++ ++ ++++ ++++ − ++++ − + +++ −
12 + ++ +++ ++++ + ++ + +++ +++ −
13 + ++ +++ ++++ − ++++ + + +++ −
14 + ++ + +++ − ++++ +++ +++ +++ −
C, carcinoma; S, sarcomatous;−, 0% positive cells;+, 1–10%;++, 11–50%;+++, 51–80%;++++,>80%.
In general, p53 immunopositivity impliesTP53mutation. Of 13 cases whose TP53 mutation was analyzed, nine cases showed concordant results between TP53 mutation status and p53 immmunoreactivity, while four cases (cases 1, 8, 11, and 12) showed discordance (Table 4).
DISCUSSION
The purpose of this study was to investigate the relation- ship between EMT-associated transcription factors and
pathogenesis of the sarcomatous component in SpCC of the esophagus. We uncovered that ZEB1 were more widely expressed in the sarcomatous components than in any carcinoma areas in our series, suggesting that EMT is associated with the pathogenesis of SpCC of the esopha- gus. In addition, TP53 mutation status and p53 protein expression in both the carcinoma and the sarcomatoid components showed relatively high concordance. These findings indicate the monoclonal origin of both components and guarantee that the spindle cell component is neoplastic.
Figure 2 Immunohistochemistry for epithelial-mesenchymal transition-asso- ciated transcription factors and p53. (a) Slug is almost equally and modestly expressed in the carcinoma and sarcoma- tous components (case 11). (b) Twist is predominantly expressed in the sarco- matous area (case 5). (c) ZEB1 immunostaining is limited in the sarcoma- tous area throughout the tumor (case 5).
Almost all cases showed the staining pattern. (d) ZEB2 expression is compa- rable in each area (case 9). (e) Membra- nous E-cadherin expression is evident in the carcinoma area (case 6). This expres- sion pattern was consistent in all cases. (f) Malignant squamous cells show immuno- reactivity for p53 (case 6). (g) Malignant spindle-shaped sarcomatous cells show immunoreactivity for p53 (case 6). The strong p53 expression in spindle-shaped sarcomatous cells suggests that these cells are neoplastic.
EMT is a phenomenon in which epithelial cells lose the epithelial phenotype and gain the mesenchymal one. Several factors have been described as major EMT-related factors, including Snail, Twist, and the ZEB family.20 In the present study, all EMT-related factors were expressed in carcinoma components to some extent (Table 2). Some reports show that EMT-related factors are also expressed in early step of carcinogenesis, even before the invasion.21,22On the other hand, Twist and ZEB1 (the ZEB family contains two family members: ZEB1 and ZEB2, also known as δEF1 and SIP1, respectively) were widely expressed in the sarcoma- tous components and showed a statistically significant differ- ence, concordant with previous reports about SpCC/
carcinosarcoma.13,14,23 We also performed immunohisto- chemistry for E-cadherin, whose loss of expression is con- sidered to be a key step in EMT.24E-cadherin showed nega- tive reactivity for sarcomatous components in almost all cases (Table 2). Regarding cross-regulation among EMT- inducing transcription factors, recent studies have indicated that ZEB factors are downstream of the Snail and Twist families in the EMT interactome.20 The lower hierarchical position of ZEB1 in the EMT process suggests that this important EMT-inducing transcription factor may be involved in the later step of pathogenesis of SpCC of the esophagus.
In other words, after the development of the carcinoma com- ponent, ZEB1 may trigger the crucial step of carcinoma com- ponents being transformed into sarcomatous ones. We suppose that this is the reason why ZEB1 showed a very significant difference between the two areas, and may be the major factor in the morphogenesis of SpCC of the esophagus.
The concept of EMT is currently considered relevant in a wide range of areas in tumor biology, including tumor budding
at the invasive front,21,22,25,26 de-differentiation,27and sarco- matous change.13–15EMT is a marker of aggressiveness and poor prognosis in most tumours. In contrast, “SpCC of the esophagus “ is often a superficial tumour, showing equivalent prognosis as classical SCC28,29despite its high expresson of EMT-associated transcription factors, which seems to be a distinct feature. Regarding ZEB1, some studies have shown the positive correlation of ZEB1 expression and malignant potential of squamous cell carcinoma of the esophagus,25,30 while another has shown that ZEB1 expression is closely related to sarcomatous morphology of pleomorphic carci- noma of the lung, and less effective in tumor invasion of non-small cell lung carcinoma.21The latter finding is in line with our observation that ZEB1 was preferably expressed in the sarcomatous element rather than the invasive front (Table 3). The sarcomatous change does not always mean an acquisition of malignant potential, which may be the reason why the prognosis is not significantly different between conventional squamous cell carcinoma and SpCC of the esophagus. The role of ZEB1 may not be uniform in each tumor and intratumoral location, and the diverse role will be a subject of further studies.
We additionally analyzed p53 protein expression andTP53 mutation status in both carcinoma and sarcomatous compo- nents. There was one case that hadTP53mutation only in the sarcomatous components (case 8). The possession of mutations in sarcomatous areas along with the wild-type TP53 gene in carcinoma has also been pointed out in a previous report of SpCC of the esophagus,31 as well as lung,32 kidney,33 and uterus.34 With these previous lines of evidence, theTP53mutation status of this case implies that the sarcomatous component originates from carcinoma because it is reasonable to consider the acquisition ofTP53 mutation during transformation in this direction, but not vice versa.
Previous studies have reported thatTP53mutations were frequently detected in codons 175, 176 (exon 5), 248 (exon 7), 273, and 282 (exon 8) in esophageal squamous cell carcinoma,35,36whereas our SpCC cases had no mutations in these codons. Although there are several reports of studies involvingTP53mutation analysis in SpCC of the esophagus, no mutations have been observed in exon 5.10,31,37,38 In line with these previous reports, our SpCC cases did not harbor mutation in exon 5. Of note, the avoidance of exon 5 in terms of TP53 mutation has also been observed in SpCC/
carcinosarcoma of the upper respiratory tract, whose ana- tomical position is adjacent to the esophagus,39 whereas it has been detected in SpCC/carcinosarcoma of other organs, such as skin,40lung,41kidney,33urinary bladder,42breast,43,44 and female genital tract.34,45The reason for this is unclear, but these findings suggest that the avoidance of exon 5 inTP53 mutation may be a feature of SpCC/carcinosarcoma of the upper aerodigestive tract, including the esophagus. In Table 3 Immunohistochemical study of molecules associated with
epithelial-mesenchymal transition
Case
Twist ZEB1
F S F S
P=0.3762 P<0.0001
1 ++++ +++ + ++++
2 ++++ ++++ − ++
3 ++++ ++++ − ++++
4 +++ +++ ++ ++++
5 +++ ++++ + ++++
6 ++++ ++++ ++ ++++
7 ++++ ++++ +++ ++++
8 ++++ ++++ ++++ ++++
9 ++++ ++++ + ++++
10 ++ ++++ − ++++
11 ++++ ++++ − ++++
12 ++++ ++++ + ++
13 +++ ++++ ++ ++++
14 + +++ − ++++
−, 0% positive cells;+, 1–10%;++, 11–50%;+++, 51–80%;++++,>80%.
F, front of carcinoma; S, sarcomatous.
addition, conventional squamous cell carcinomas of the esophagus are known to exhibit male predominance,46 and this is more pronounced in SpCC, as shown in our cases and previous reports.11,28,47The reason for this is unknown, but a
similar tendency is also observed in SpCC/carcinosarcoma of the urinary organs.33,42
In conclusion, we have shown that ZEB1 are widely expressed in the sarcomatous component in SpCC of the esophagus. Moreover, our p53 expression andTP53muta- tion analyses confirmed the monoclonality and detected the avoidance of exon 5. These findings suggest that ZEB1 may play an important role in the pathogenesis of SpCC of the esophagus through EMT. The distinct pattern ofTP53muta- tion may also be a feature of the tumor.
ACKNOWLEDGMENTS
We are grateful to Drs. Masafumi Kurosumi, Kazuha Sakamoto, and Hideaki Ito for generously providing tissues, and appreciate the assistance of the staff at the pathology and surgical divisions (Gunma University Hospital, Saitama Prefectural Cancer Center Hospital, Maebashi Red Cross Hospital, Fukaya Red Cross Hospital).
Table 4 TP53mutations and p53 expression in spindle cell carcinoma of the esophagus
Case Component P53 IHC
TP53gene mutation
Exon/codon Nucleotide/amino acid change
1 C NEG 7/236 TAT->TAA/Tyr->Stop
S NEG 7/236 TAT->TAA/Tyr->Stop
2 C 3+, 90% 6/195 ATC->TTC/Ile->Phe
7/245 GGC->GGT/Gly->Gly
S 3+, 80% 6/195 ATC->TTC/Ile->Phe
7/245 GGC->GGT/Gly->Gly
3 C NEG No mutation
S NEG No mutation
4 C NEG No mutation
S NEG No mutation
5 C 3+, 90% 6/205 TAT->TGT/Tyr->Cys
S 3+, 70% 6/205 TAT->TGT/Tyr->Cys
6 C NEG 8/306 CGA->TGA/Arg->Stop
S NEG 8/306 CGA->TGA/Arg->Stop
7 C NEG NA
S NEG NA
8 C 3+, 70% No mutation
S 3+, 20% 8/293 GGG->GAG/Gly->Glu
8/294 GGG->GAG/Gly->Glu
8/303 AGC->AAC/Ser->Asn
9 C NEG No mutation
S NEG No mutation†
10 C 3+, 90% 7/245 GGC->AGC/Gly->Ser
8/282 CGG->TGG/Arg->Trp
S 1+, 70% 7/245 GGC->AGC/Gly->Ser
8/282 CGG->TGG/Arg->Trp
11 C NEG 6/212 TTT->TT (1 bp deletion)
S NEG 6/212 TTT->TT (1 bp deletion)
12 C 2+, 60% No mutation
S 2+,60% No mutation
13 C NEG No mutation
S NEG No mutation
14 C 3+, 40% 7/242 TGC->AGC/Arg->Trp
S 3+, 70% 7/242 TGC->AGC/Arg->Trp
†Only exon 5 was analyzed.
C, carcinoma component; IHC, immunohistochemistry; NA, not available; NEG, negative; S, sarcomatous component.
Figure 3 TP53sequencing of a representative case (case 6). An identical point mutation between carcinoma and sarcomatous com- ponents is shown at codon 306 (CGA->TGA, Arg->stop) of exon 8. This finding robustly indicates the monoclonality.
DISCLOSURE STATEMENT None declared.
REFERENCES
1 Madan AK, Long AE, Weldon CB, Jaffe BM. Esophageal carci- nosarcoma.J Gastrointest Surg2001;5: 414–7.
2 Enrile F, DeJesus PO, Bakst AA, Baluyot R. Pseudosarcoma of the esophagus.Cancer1973;31: 1197–202.
3 Takubo K, Tsuchiya S, Nakagawa H, Futatsuki K, Ishibashi I, Hirata F. Pseudosarcoma of the esophagus.Hum Pathol1982;
13: 503–5.
4 Martin MR, Kahn LB. So-called pseudosarcoma of the esopha- gus: Nodal metastases of the spindle cell element.Arch Pathol Lab Med1977;101: 604–9.
5 Talbert JL, Cantrell JR. Clinical and Pathologic characteristics of carcinosarcoma of the esophagus.J Thorac Cardiovasc Surg 1963;45: 1–12.
6 Linder J, Stein RB, Roggli VL et al. Polypoid tumor of the esophagus.Hum Pathol1987;18: 692–700.
7 Nakagawa S, Nishimaki T, Suzuki T, Yokoyama N, Kuwabara S, Hatakeyama K. Histogenetic heterogeneity in carcinosarcoma of the esophagus: Report of a case with immunohistochemical and molecular analyses.Dig Dis Sci1999;44: 905–9.
8 Battifora H. Spindle cell carcinoma: Ultrastructural evidence of squamous origin and collagen production by the tumor cells.
Cancer1976;37: 2275–82.
9 Ooi A, Kawahara E, Okada Y et al. Carcinosarcoma of the esophagus. An immunohistochemical and electron microscopic study.Acta Pathol Jpn1986;36: 151–9.
10 Kashiwabara K, Sano T, Oyama Tet al. A case of esophageal sarcomatoid carcinoma with molecular evidence of a monoclo- nal origin.Pathol Res Pract2001;197: 41–6.
11 Handra-Luca A, Terris B, Couvelard A, Molas G, Degott C, Flejou JF. Spindle cell squamous carcinoma of the oesophagus:
An analysis of 17 cases, with new immunohistochemical evidence for a clonal origin. Histopathology 2001; 39:
125–32.
12 Matsumoto T, Fujii H, Arakawa Aet al. Loss of heterozygosity analysis shows monoclonal evolution with frequent genetic pro- gression and divergence in esophageal carcinosarcoma.Hum Pathol2004;35: 322–7.
13 Kojc N, Zidar N, Gale Net al. Transcription factors Snail, Slug, Twist, and SIP1 in spindle cell carcinoma of the head and neck.
Virchows Arch2009;454: 549–55.
14 Castilla MÁ, Moreno-Bueno G, Romero-Pérez Let al. Micro- RNA signature of the epithelial-mesenchymal transition in endo- metrial carcinosarcoma.J Pathol2011;223: 72–80.
15 Conant JL, Peng Z, Evans MF, Naud S, Cooper K. Sarcomatoid renal cell carcinoma is an example of epithelial-mesenchymal transition.J Clin Pathol2011;64: 1088–92.
16 Montgomery E, Field JK, Boffetta P, Daigo Y, Shimizu M, Shimoda T. Squamous cell carcinoma of the oesophagus. In:
Bosman FT, Carnerio F, Hruban RH, Theise ND, eds. WHO Classification of Tumors of the Digestive System, 4th edn.
Lyon: International Agency for Research on Cancer, 2010;
18–24.
17 Sobin L, Gospodarowicz M, Wittekind C.TNM Classification of Malignant Tumors, 7 th edn. New York: Wiley, 2009.
18 Ohgaki H, Dessen P, Jourde Bet al. Genetic pathways to glio- blastoma: A population-based study. Cancer Res 2004; 64:
6892–9.
19 Kim YH, Nobusawa S, Mittelbronn Met al. Molecular classifica- tion of low-grade diffuse gliomas.Am J Pathol2010;177: 2708–
14.
20 Sánchez-Tilló E, Liu Y, de Barrios Oet al. EMT-activating tran- scription factors in cancer: Beyond EMT and tumor invasive- ness.Cell Mol Life Sci2012;69: 3429–56.
21 Uchikado Y, Natsugoe S, Okumura Het al. Slug expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma.Clin Cancer Res2005;11: 1174–80.
22 Sasaki K, Natsugoe S, Ishigami Set al. Significance of Twist expression and its association with E-cadherin in esophageal squamous cell carcinoma.J Exp Clin Cancer Res 2009;28:
158.
23 Matsubara D, Kishaba Y, Yoshimoto T et al. Immunohisto- chemical analysis of the expression of E-cadherin and ZEB1 in non-small cell lung cancer.Pathol Int2014;64: 560–8.
24 Thiery JP. Epithelial-mesenchymal transitions in development and pathogenesis.Curr Opin Cell Biol2003;15: 740–46.
25 Ohashi S, Natsuizaka M, Naganuma S et al. A NOTCH3- mediated squamous cell differentiation program limits expan- sion of EMT-competent cells that express the ZEB transcription factors.Cancer Res2011;71: 6836–47.
26 Kahlert C, Lahes S, Radhakrishnan Pet al. Overexpression of ZEB2 at the invasion front of colorectal cancer is an indepen- dent prognostic marker and regulates tumor invasion in vitro.
Clin Cancer Res2011;17: 7654–63.
27 Rosivatz E, Becker I, Specht Ket al. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer.Am J Pathol2002;161: 1881–91.
28 Sano A, Sakurai S, Kato Het al. Clinicopathological and immu- nohistochemical characteristics of esophageal carcinosarcoma.
Anticancer Res2009;29: 3375–80.
29 Iyomasa S, Kato H, Tachimori Y, Watanabe H, Yamaguchi H, Itabashi M. Carcinosarcoma of the esophagus: A twenty-case study.Jpn J Clin Oncol1990;20: 99–106.
30 Yokobori T, Suzuki S, Tanaka Net al. MiR-150 is associated with poor prognosis in esophageal squamous cell carcinoma via targeting the EMT inducer ZEB1.Cancer Sci2013;104: 48–54.
31 Iwaya T, Maesawa C, Uesugi Net al. True carcinosarcoma of the esophagus.Dis Esophagus2006;19: 48–52.
32 Kawano T, Takeshima Y, Inai K. Alteration of the p53 gene of lung carcinomas with sarcomatous transformation (spindle cell carcinoma): Analysis of four cases.Pathol Int1996;46: 38–45.
33 Oda H, Nakatsuru Y, Ishikawa T. Mutations of thep53gene and p53 protein overexpression are associated with sarcomatoid transformation in renal cell carcinomas.Cancer Res1995;55:
658–62.
34 Jin Z, Ogata S, Tamura Get al. Carcinosarcomas (malignant mullerian mixed tumors) of the uterus and ovary: A genetic study with special reference to histogenesis. Int J Gynecol Pathol 2003;22: 368–73.
35 Egashira A, Morita M, Kakeji Y et al. p53 gene mutations in esophageal squamous cell carcinoma and their relevance to etiology and pathogenesis: Results in Japan and comparisons with other countries.Cancer Sci2007;98: 1152–6.
36 Petitjean A, Mathe E, Kato Set al. Impact of mutant p53 func- tional properties onTP53mutation patterns and tumor pheno- type: Lessons from recent developments in the IARC TP53 database.Hum Mutat2007;28: 622–9.
37 Amatya VJ, Takeshima Y, Kaneko M, Inai K. Esophageal car- cinosarcoma with basaloid squamous carcinoma and rhabdo- myosarcoma components withTP53mutation.Pathol Int2004;
54: 803–9.
38 Iwaya T, Maesawa C, Tamura Get al. Esophageal carcinosar- coma: A genetic analysis.Gastroenterology1997;113: 973–7.
39 Ansari-Lari MA, Hoque MO, Califano J, Westra WH. Immuno- histochemical p53 expression patterns in sarcomatoid carcino- mas of the upper respiratory tract.Am J Surg Pathol2002;26:
1024–31.
40 Paniz-Mondolfi A, Singh R, Jour Get al. Cutaneous carcinosar- coma: Further insights into its mutational landscape through massive parallel genome sequencing.Virchows Arch2014;465:
339–50.
41 Holst VA, Finkelstein S, Colby TV, Myers JL, Yousem SA. p53 and K-ras mutational genotyping in pulmonary carcinosarcoma, spindle cell carcinoma, and pulmonary blastoma: Implications for histogenesis.Am J Surg Pathol1997;21: 801–11.
42 Armstrong AB, Wang M, Eble JNet al.TP53mutational analysis supports monoclonal origin of biphasic sarcomatoid urothelial carcinoma (carcinosarcoma) of the urinary bladder.Mod Pathol 2009;22: 113–8.
43 Lien HC, Lin CW, Mao TL, Kuo SH, Hsiao CH, Huang CS. p53 overexpression and mutation in metaplastic carcinoma of the breast: Genetic evidence for a monoclonal origin of both the
carcinomatous and the heterogeneous sarcomatous compo- nents.J Pathol2004;204: 131–9.
44 Lee JS, Kim YB, Min KW. Metaplastic mammary carcinoma with osteoclast-like giant cells: Identical point mutation of p53 gene only identified in both the intraductal and sarcomatous compo- nents.Virchows Arch2004;444: 194–7.
45 Kounelis S, Jones MW, Paradaki H, Bakker A, Swalsky P, Finkelstein SD. Carcinosarcomas (Malignant Mixed Mullerian Tumors) of the female genital tract: Comparative molecular analysis of epithelial and mesenchymal components. Hum Pathol1998;29: 82–7.
46 Cook MB, Dawsey SM, Freedman NDet al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev2009;18: 1174–82.
47 Lauwers GY, Grant LD, Scott GV, Carr NJ, Sobin LH. Spindle cell squamous carcinoma of the esophagus: Analysis of ploidy and tumor proliferative activity in a series of 13 cases. Hum Pathol1998;29: 863–8.