Introduction
There have been significant changes in biota at the global scale, which accompany the glob-al changes in climate (e.g. Perry et glob-al. 2005; Parmesan 2006; Lima et al. 2007). Coastal areas in western Japan have experienced ocean warming related to the recent warming of the Kuroshio Current (Wu et al. 2012), and this has resulted in changes in macroalgal distribution and species composition (Hiraoka et al. 2005; Haraguchi 2006; Tanaka et al. 2012). Kochi Prefecture is located in southwestern Japan, and its coastal area is strongly affected by the Kuroshio Current. The Kochi Prefectural coast
is a transitional region between temperate and subtropical zones; therefore, temperate and tropical algae co-exist in this area (Ohno 1985). The surface seawater temperatures of Tosa Bay reportedly increased by 1.26℃ from 1902 to 2010 (Terazono et al. 2012), and the rate of warming along the Kochi Prefectural coast is higher than average among the Japanese waters (Japan Meteorological Agency 2016, https://www.jma.go. jp/jma/en/NMHS/ccmr/ccmr2015_low.pdf, browsed on Aug. 1, 2017).
Macroalgae could be used as indicator of environmental changes (Tribollet and Vroom 2007), especially regarding temperature. Since macroalgae are immobile, their growth and
Culture and field studies on the temperature related growth rates
of a tropical Sargassum species, Sargassum ilicifolium (Tuner)
C. Agardh in Kochi Prefecture, southwestern Japan
Hiroko HARAGUCHI
1*, Noboru MURASE
2, Zenji IMOTO
3and Kazuo OKUDA
4Abstract : For last three decades, the distribution of temperate Sargassum species has declined, and that of tropical Sargassum species has increased along the coast of Kochi Prefecture, southwestern Japan. However, little is known about the ecological and physi-ological characteristics of tropical Sargassum species in Kochi Prefecture. In the current study we performed culture experiments to understand how the growth of a tropical Sargassum species, S. ilicifolium, is affected by seawater temperature. We also conducted a 31-month field study to understand the seasonality of this species. The culture experi-ments showed that the relative growth rates of S. ilicifolium thalli sharply increased from 11 ℃ to 12 ℃ and gradually increased from 12 ℃ to 15 ℃. From 14 ℃ to 30 ℃, the rela-tive growth rates of the tropical S. ilicifolium were higher than those of temperate spe-cies. The relative growth rates of the germlings were optimal between 25℃ and 30℃, while the germlings at 10 ℃ had almost no growth. The results of the field study indicated that S. ilicifolium formed macroalgal beds steadily every year. We proved that this species has the ability to grow, even during winter along the coast of Kochi Prefecture.
Keywords : Kochi Prefecture, relative growth rate, Sargassum ilicifolium, seasonal growth, seawater temperature
1
Estuary Research Center, Shimane University, 1060 Nishikawatsu-cho, Matsue-shi, Shimane 690-8504, Japan
2
National Fisheries University, 2-7-1 Nagata-Honmachi, Shimonoseki-shi, Yamaguchi 759-6595, Japan
3Usa Marine Biological Institute, Kochi University, 194 Inoshiri, Usa-cho, Tosa-shi, Kochi 781-1164, Japan 4Faculty of Science and Technology, Kochi University, 5-1 Akebono-cho, Kochi-shi, Kochi 780-8520, Japan *
maturation are often controlled by coastal temperatures (e.g. Breeman 1988; Luning and Dieck 1989). Brown macroalgae in the genus Sargassum form luxuriant beds along shallow rocky coasts. These beds function as a nursery for numerous fish and invertebrates (Fuse 1962; Tanaka and Leite 2003), and also a platform of high primary production (Taniguchi and Yamada 1988; Murase et al. 2000). The genus Sargassum in Japan is composed mainly of the two subgenus Bactrophycus and Sargassum (Stiger et al. 2000). The former includes most species in temperate regions of the Japanese coast (Yoshida 1983), and their seasonality or growth characteristics have frequently been studied (e.g. Umezaki 1984; Kimura et al. 1987; Uchida 1993; Murase and Kito 1998; Haraguchi et al. 2005).
Some species of temperate Sargassum, such as S. okamurae Yoshida et T. Konno and S. micracanthum (Kutzing) Endlicher, have de-clined since the 1990s, due to the increase in seawater temperature along the coast of Kochi Prefecture (Hiraoka et al. 2005; Tanaka et al. 2012). Moreover, the period during which the temperate Sargassum species attains the longest thallus or bears reproductive organ has shifted from the past reports in Kochi Prefecture (Ohno 1984; Kimura et al. 1987), and these shifts are related to the temperature-dependent growth characteristic of the temperate Sargassum spe-cies observed in culture experiments (Haraguchi et al. 2009). We have noted that winter seawa-ter temperature in the 1990s was higher than that in 1980s in Kochi Prefecture (Terazono et al. 2012). Meanwhile, the distribution of the tropical species, S. ilicifolium (Tuner) C. Agar-dh, has expanded in Kochi Prefecture (Hiraoka et al. 2005; Tanaka et al. 2012). This species was previously known as‘S. duplicatum Bory' before the taxonomic revision of Mattio et al. (2009). We hypothesized that the elevated seawater temperatures, especially in winter, caused the expansion of the distribution and the increase in growth of the tropical Sargassum species in Kochi Prefecture. In the current study, we con-ducted culture experiments at low temperatures, focusing on winter seawater temperatures, to evaluate the effect of seawater temperature on
the growth characteristics of the tropical Sargassum species, S. ilicifolium. In addition, we clarified the optimal temperature for growth of winter thalli and spring germlings of this species. Furthermore, we performed a 31-month field study to understand the seasonality of S. ilicifolium in Kochi Prefecture.
Materials and Methods
Experiments on the effect of temperature on the growth of Sargassum ilicifolium
1) Developed thalli with main branches
Immature individuals of S. ilicifolium were collected from the field site (3326 N, 13327 E) at Ogisaki, Kochi Prefecture (Fig. 1) in March 2006. After the collection, samples were brought to the laboratory of the Usa Marine Biological Institute, Kochi University, where they were rinsed with sterile water and obvious epiphytes were removed by forceps. As growth points exist at the apices of main branches, apical segments 2 cm in length were taken from the main branches (Matsui et al. 1994). The apical segments of S. ilicifolium were transferred to a flask containing 1 L of 1/2 PESI medium (Tatewaki 1966) and the medium was aerated. The photoperiod was set at 12-h light: 12-h dark to match the photoperiods of the sampling
Fig. 1. Maps showing the study area at Ogisaki in Kochi Prefecture, southwestern Japan.
seasons and the irradiance was set at 100 mol photons m-2 s-1 (measured with a PAR sensor
model LI-192, LI-COR Biosciences, USA) using cool-white fluorescent lamps. In a preliminary culture, apical segments were maintained at 15 ℃ for a week. After that, temperature-gradient culture apparatus (Morita et al 2003) was used to determine the effects of temperature on the growth rates of S. ilicifolium. Segments (five materials per flask) were cultured from 5 ℃ - 30 ℃ with 5 ℃ increments (i.e., 5 ℃, 10 ℃, 15 ℃, 20 ℃, 25 ℃, and 30 ℃), and with a finer resolution of 1 ℃ for temperatures between 10 ℃ and 15 ℃ (i.e., 10 ℃, 11 ℃, 12 ℃, 13 ℃, 14 ℃, and 15 ℃). During the 15-day experiment, the culture me-dia were renewed every three days. The initial wet weight of the segments of S. ilicifolium was 1.3±0.1g (means±SE, n=30). Each segment was identified based on the number of leaves and vesicles, and their position.
Final wet weight was measured, and the rel-ative growth rate per day was calculated for each segment as:
Relative growth rate (% day-1) = ln (final wet
weight/initial wet weight) × 100/T,
where T indicates the number of days of the culture period (Morita et al. 2003). After arcsin transformation, a one-way analysis of variance (ANOVA) followed by a Tukey's multiple-com-parison test was used to examine the effects of temperature on growth.
2) Germling stage
Fertile female and male thalli of S. ilicifolium bearing numerous receptacles were collected from Ogisaki, Kochi Prefecture in July 2006. Collected plants were brought to the laboratory and female and male receptacles were cut from the fronds and shaken together in sterile sea-water until fertilization was confirmed. Then we collected embryos and rinsed them several times in sterile seawater using Pasteur pipettes. The germling experiment was initiated immediately after the isolated embryos extended the rhiz-oids. The embryos were evenly settled on glass slides (15 × 25 mm, density: 15 ind. cm-2) in a
petri dish (90 × 75 mm) filled with 250 ml of 1/2 PESI medium (Tatewaki 1966). Each dish con-tained 10 glass slides, and one dish was set at
each section. The photoperiod and irradiance were similar to those in the frond segment experiments previously mentioned. The germ-lings were cultured in a range of 10℃-35℃ with 5 ℃ increments (i.e., 10 ℃, 15 ℃, 20 ℃, 25 ℃, 30 ℃, and 35 ℃) for eight day. The culture media were renewed every two days. At the beginning of the experiment, mean thallus area of a germling was 0.04 ± 0.00 mm2
(means ± SE, n = 30). Final thallus area was measured for ten materials per petri dish, and the relative growth rates (% day-1)
in thallus area were calculated as in the frond segment experiment. Thallus areas were photo-graphed under a microscope with a digital camera, and each area was measured using image analysis software (LIA32 for Win32). Field study
Field work was carried out by scuba diving in a 50 × 50 m2
area (depth of 1.5-2.5 m) with a dense stand of S. ilicifolium near Ogisaki, Kochi Prefecture (Fig. 1). We measured S. ilicifolium thallus length between the holdfast and the apex of the main branch for 20 longer individuals once or twice a month from May 2005 to November 2007. When we were not able to find 20 individuals, we measured all the individuals found within the area at that time. The pres-ence or abspres-ence of reproductive structures (i.e., receptacles) was also recorded.
Seawater temperatures at the area were re-corded hourly by a data logger (HOBO Water Temp Pro v2, Onset Computer Corporation, USA) inside a bed of S. ilicifolium (depth of 2.0 m).
Results
Experiments on the effect of temperature on the growth of Sargassum ilicifolium
1) Developed thalli with main branches
The optimal growth temperature was 25 ℃, and the growth rate at the temperature condi-tion was 5.7 ± 0.5% day-1 (Fig. 2A). There were
no significant differences between the growth rates at 15 ℃, 20 ℃, and 30 ℃ (3.4 ± 0.3% to 3.9 ± 0.4% day-1). Sargassum ilicifolium had strongly
reduced growth at 10 ℃, and did not survive at 5 ℃.
Between 10 ℃ and 15 ℃, there was a large difference of the relative growth rates (Fig. 2A). Therefore, the growth rate was examined on finer-scaled temperatures within this range (Fig. 2B). S. ilicifolium grew slowly at 10 ℃ and 11 ℃, with the growth rates of 0.5 ± 0.1% day-1 and
1.0 ± 0.2% day-1, respectively. There was a sharp
increase in growth between 11 ℃ and 12 ℃, with the relative growth rate at 12 ℃ (2.1 ± 0.2% day-1)
being more than twice of the growth rate at 10
℃ and 11 ℃. The relative growth rates between 12 ℃ and 15 ℃ gradually increased as the tem-perature was warmed and showed no signifi-cant differences between adjacent temperatures within 1 ℃ interval.
2) Germling stage
The relative growth rates of Sargassum ilicifolium germlings were the largest at 25 ℃ and 30 ℃, ranging from 30.4 ± 1.1% to 32.7 ± 0.6% day-1 (Fig. 3). The growth rate significantly
reduced at 20 ℃ or below. Germlings kept at 10 ℃ exhibited almost no growth with the rela-tive growth rate of 0.6 ± 0.4% day-1. Germlings
did not survive at 35 ℃. Field study
1) Seasonal changes in seawater temperature at Ogisaki
Seawater temperatures measured at Ogisaki, Kochi Prefecture reached minimum values in February 2006 and March 2007, when they were 13.9 ℃ and 14.5 ℃, respectively (Fig. 4). Maxi-mum values were recorded in August 2005,
Fig. 3. Relative growth rates of germlings of Sargassum ilicifolium at 5℃ intervals from 10℃ to 35℃. The culture experiments were performed under a
light intensity of 100 mol photons m-2
s-1 for 8 days. Thick bar indicate the mean of 10 mate-rials and error bars indicate standard error. Bars marked with same letters are not significantly different according to Tukey's multiple com-parison test (p<0.05). No germlings survived at 35 ℃.
Fig. 2. Relative growth rates of Sargassum ilicifolium thalli at 5℃ intervals from 5℃ to 30℃ (A), and 1 ℃ intervals from 10 ℃ to 15 ℃ (B). The culture experiments were performed under a
light intensity of 100 mol photons m-2
s-1 for 15 days. Thick bars indicate the mean of five materials and error bars indicate standard error. Bars marked with same letters are not significantly different according to Tukey's multiple comparison test (p<0.05). No thallus survived at 5 ℃.
August 2006, and September 2007, when they were 28.8 ℃, 30.2 ℃, and 30.1 ℃, respectively. In 2006 and 2007 it exceeded 30 ℃, but not in 2005. Low seawater temperature conditions below 15 ℃ continued from December 2005 to March 2006, while these conditions were rarely observed in 2007.
2) Seasonal changes in mean thallus length of a natural population
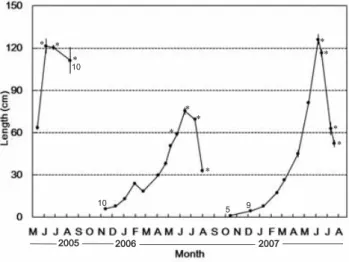
Thalli of Sargassum ilicifolium were 63.6 ± 1.7 cm in length in May 2005 (Fig. 5). Subse-quently, they grew rapidly and had attained a maximum mean thallus length of 121.3 ± 5.3 cm and formed receptacles in June 2005. Most thalli released oospores in July 2005. The thalli that had released oospores began to exhibit senes-cence and completely disappeared by Septem-ber 2005. Length of young thalli was 6.1±0.7 cm long in November 2005, and they steadily grew until February. However, mean thallus length decreased to 18.4 ± 0.4 cm long in March 2006 from 23.9 ± 0.7 cm long in the previous month, due to the feeding activity of seaslugs, Aplysia spp. Subsequently, they grew again with in-creasing seawater temperature, and had recep-tacles early in June 2006. By the end of the same month, this species reached a maximum length of 75.5 ± 2.6 cm, and released oospores. Later,
thalli of S. ilicifolium began to senesce, and completely disappeared by September. Young thalli, which were 1.1 ± 0.2 cm long, were ob-served in October 2006. The thallus length creased slowly until April 2007, and it in-creased rapidly for the following months when seawater temperatures were around 20 ℃. By the middle of June 2007, thalli attained a maxi-mum mean thallus length of 126.1 ± 3.6 cm long and began to produce receptacles. Subsequently, the release of oospores and senescence oc-curred simultaneously. No thalli of a natural population were observed by August 2007. For three years we investigated, S. ilicifolium showed annual life spans.
Discussion
The increase in the tropical Sargassum ilicifolium has been the most conspicuous change in Sargassum beds along the coast of Kochi Prefecture, southwestern Japan since the 1990s (Tanaka et al. 2012). This phenomenon is linked to the increase in winter seawater tempera-tures, which have risen since the 1990s (Terazono et al. 2012). However, little is known about temperature-related growth characteristics
Fig. 4. Seawater temperatures at Ogisaki hourly measured by a data logger from May 2005 to November 2007.
Fig. 5. Seasonal change in mean length of 20 thalli of Sargassum ilicifolium at Ogisaki from May 2005 to November 2007. Bars indicate stand-ard error. The numbers near the black dot shows the number of individuals measured when 20 individuals were not found in the study area. Asterisk (*) shows some or most thalli formed reproductive structure.
of this species (Hwang et al. 2004). We as-sumed that the elevation of the winter seawa-ter temperatures enabled the tropical Sargassum species to grow rapidly in temperate regions like Kochi Prefecture. Thus, we conducted culture experiments, focusing on how winter seawater temperatures affect the growth characteristics of the tropical S. ilicifolium. There was a sharp increase in the relative growth rates of S. ilicifolium between 11 ℃ and 12 ℃, and the growth rate between 12 ℃ and 15 ℃ was 2.1 ± 0.2 % to 3.5±0.2 % day-1(Fig. 2B). Meanwhile, the
growth rates of the two temperate Sargassum species, S. micracanthum and S. okamurae, be-tween 11 ℃ and 20 ℃ were reported 1.2 ± 0.1 % to 2.2 ± 0.1 % day-1and 1.4 ± 0.0 % to 2.6 ± 0.1%
day-1, respectively (Haraguchi et al. 2009). Those
results also show that the growth rates of the tropical S. ilicifolium at 14 ℃ and over are higher than those of the two temperate spe-cies. Winter seawater temperatures along the coast of Kochi Prefecture have increased to 14℃ since the 1990s (Tanaka et al. 2012; Terazono et al. 2012). And the current study showed the minimum seawater temperature of 13.9℃ in 2006 and 14.5 ℃ in 2007. Based on these observa-tions, it seems that the coast of Kochi Prefec-ture has been changing to an environment where the tropical S. ilicifolium can exhibit bet-ter growth than the temperate species, because seawater temperatures rarely drop below 14 ℃. The optimal growth temperature for tropical and temperate species in Kochi Prefecture was 25 ℃ (Fig. 2A, Haraguchi et al. 2009) and the growth rate of the tropical S. ilicifolium at 25 ℃ was approximately double that of temperate species, S. micracanthum and S. okamurae (Haraguchi et al. 2009). Thus, it is likely that tropical species may have a potential to out-grow temperate species in the natural habitat around 25 ℃. The optimal growth temperatures of other tropical Sargassum species ranged be-tween 20 ℃ and 30 ℃ (Hwang et al. 2004), and those for other temperate species were repor-ted at 15 ℃ to 25 ℃ (Haraguchi et al. 2005; Baba 2014). The optimal temperatures for tropical species seem to be slightly higher than those of temperate species.
The current study revealed that the optimal
growth temperature of the germlings of S. ilicifolium was between 25 ℃ and 30 ℃ (Fig. 3). De Wreede (1978) reported that the optimal temperature of Hawaiian Sargassum germlings, a tropical species, was 24 ℃. For several tem-perate species, including S. horneri, S. thunbergii, and S. macrocarpum, optimal temperatures for the germling growth ranged from 20 ℃ to 25 ℃ (Yoshida et al. 1999; Murase 2001; Zhao et al. 2008). These results suggest that there is little difference in optimal temperatures for germ-ling growth between temperate species and tropical species. The Hawaiian Sargassum spe-cies released oospores at 22 - 24 ℃, and these temperatures are almost consistent with the optimal temperatures for germling growth of this species (De Wreede 1976, 1978). For S. ilicifolium in this study, oospores were released when seawater temperatures were higher than 25 ℃ which was in the range of the optimal temperatures for germling growth (Fig. 3-5). The optimal growth temperatures of the germlings of Sargassum species may be influenced by the temperature of the fertilization period.
Tropical S. ilicifolium formed luxuriant com-munity every year from 2005 to 2007 (Fig. 5). Local populations of temperate Sargassum spe-cies along the Japanese coast have a clearly discernible cycle of growth, reproduction, and senescence which is accompanied with the sea-sonal changes in seawater temperature (e.g. Taniguchi and Yamada 1978; Murase and Kito 1998; Tsuda and Akaike 2001). The current study revealed that the S. ilicifolium popula-tions showed almost the same discernible cycle as those in the Japanese temperate species. Maturation of S. ilicifolium in Kochi Prefecture occurred from June to July (Fig. 5). This matu-ration season corresponded with those of S. ilicifolium in Kagoshima and Nagasaki prefec-tures (Shimabukuro et al. 2007; Kiriyama 2009). However, maturation of S. ilicifolium growing in a tropical area occurred in a colder season (Ateweberhan et al. 2005). McCourt (1984) re-ported that the time of peak abundance of the Sargassum populations is in warmer months of the year in temperate regions while it is in colder months of the year in tropical and sub-tropical regions. S. ilicifolium at our sites and
tropical zone (De Wreede 1976; Ateweberhan et al. 2005) matured at nearly equal seawater tem-peratures, though did in different seasons. Thus, seawater temperature is considered to be one of the main factors regulating the maturation of tropical and temperate Sargassum species (e.g. Murase and Kito 1998; Tsuda and Akaike 2001; Haraguchi et al. 2009). S. ilicifolium has expan-ded its distribution along the coast of Kochi Prefecture, southwestern Japan, with the warm-ing of seawater temperature (Hiraoka et al. 2005; Tanaka et al. 2012). In the current study, we demonstrated, using culture experiments at win-ter temperatures in Kochi Prefecture that this species has the ability to rapidly grow during winter. The field study further indicated that S. ilicifolium is able to grow to about one-meter in length every year. Distribution expansion of tropical Sargassum species has also occurred in Kagoshima and Nagasaki prefectures (Tsuchiya et al. 2011; Yatsuya et al. 2011). This is a common phenomenon along the western coast of Japan and it is likely that warming of seawater temperature closely related.
We believe that our results may help to predict northward expansion of tropical spe-cies in the future elevation of seawater temper-ature. However, the grazing pressure by marine animals, especially herbivorous fish, is strongly affected by seawater temperature (Kimura 1994; Kiriyama et al. 2001). Increase in winter seawa-ter temperatures would allow fish herbivory to continue year-round in temperate regions where the fish herbivory is restricted during winter due to lower temperature (Suzuki et al. 2008). The elevation of seawater temperature may result not only in the expansion of tropical Sargassum species, but also in feeding dama-ges on macroalgal beds caused by herbivorous fish. Further studies are needed to clarify the role of herbivores in the distributional change of macroalgal beds along temperate coasts where the elevation of seawater temperature is antici-pated.
Acknowledgments
We would like to deeply thank the referees for fruitful suggestions that helped to improve the
original manuscript and in particular for their suggestions on how to improve terminology and sentences. We wish to extend our deep grati-tude to emeritus Prof. M. Ohno of Kochi Uni-versity. We sincerely thank Dr. G.N. Nishihara of Nagasaki University, Institute for East China Sea Research for valuable comments on the content and for correcting the English of the manuscript. This study was partially suppor-ted by the grant-project of MEXT to Kochi University (2009-2011), Local adaptation to cli-mate change based on Kuroshio Science.
References
Ateweberhan M, Bruggemann JH, Breeman AM. Seasonal dynamics of Sargassum ilicifolium (Phaeophyta) on a shallow reef flat in the southern Red Sea (Eritrea). Mar. Ecol. Prog. Ser. 2005 ; 292 : 159-171.
Baba M. Effects of temperature on the growth and survival of five Sargassaceous species from Niigata Prefecture in laboratory cul-ture. Rep. Mar. Ecol. Res. Inst. 2014 ; 19 : 53-61 (in Japanese with English abstract). Breeman AM. Relative importance of
tempera-ture and other factors in determining geo-graphic boundaries of seaweeds: Experi-mental and phenological evidence. Helgol. Meeresunters. 1988 ; 42 : 199-241.
De Wreede RE. The phenology of three spe-cies of Sargassum (Sargassaceae, Phaeo-phyta) in Hawaii. Phycologia. 1976 ; 15 : 175-183.
De Wreede RE. Growth in varying culture conditions of embryos of three Hawaiian species of Sargassum (Phaeophyta, Sargas-saceae). Phycologia. 1978 ; 17 : 23-31.
Fuse S. The animal community in the
Sargassum belt. Physiol. Ecol. Jpn. 1962 ;11: 23-46 (in Japanese with English abstract). Haraguchi H, Murase N, Mizukami Y, Noda M,
Yoshida G, Terawaki T. The optimal and maximum critical temperatures of nine species of the Sargassaceae in the coastal waters of Yamaguchi Prefecuture, Japan. Jpn. J. Phycol. 2005 ; 53 : 7-13 (in Japanese with English abstract).
Hiraoka M. Species composition of Sargassum beds on the coast of Ogisaki, Kochi, southern Japan. Bull. Mar. Sci. Fish. Kochi Univ. 2006 ; 24:1-9 (in Japanese with English abstract).
Haraguchi H, Hiraoka M, Murase N, Imoto Z, Okuda K. 2009. Field and culture study of the temperature related growth rates of the temperate Sargassum species, Sargassum okamurae Yoshida and S. micracanthum (Kutzing) Endlicher (Fucales, Phaeophyceae) in Tosa Bay, southern Japan. Algal Re-sources. 2009 ; 2 : 27-37.
Hiraoka M, Ura Y, Haraguchi H. Relationship between seaweed beds and seawater tem-perature in the Tosa Bay. Aquabiology 2005 ; 27 : 485-493 (in Japanese with English ab-stract).
Hwang RL, Tsai CC, Lee TS. Assessment of temperature and nutrient limitation on seasonal dynamics among species of Sargassum from a coral reef in southern Taiwan. J. Phycol. 2004 ; 40 : 463-473.
Kimura H. Predation of fishes in the cultured Undaria undarioides. Bull. Wakayama Pref. Mar. Aquacult. Lab. 1994 ; 26 : 12-16 (in Jap-anese).
Kimura T, Orosco CA, Ohno M. Ecological Study of Sargassum okamurae Yoshida et T. Konno in Tosa Bay, Japan. Rep. Usa mar. biol. Inst., Kochi Univ. 1987 ; 9 : 149-167. Kiriyama T, Noda M, Fujii A. Grazing and bite marks on Ecklonia kurome, caused by sev-eral herbivorous fishes. SUISANZOUSHOKU. 2001 ; 149 : 431-438 (in Japanese with English abstract).
Kiriyama T. Study on recent decline of large brown alga population in coastal waters around Nagasaki Prefecture. Bull. Nagasaki Pref. Inst. Fish. 2009 ; 35 : 15-78 (in Japanese with English abstract).
Lima FP, Ribeiro PA, Queiroz, N, Hawkins, SJ, Santos AM. Do distributional shifts of northern and southern species of algae match the warming pattern? Glob. Chang. Biol. 2007 ; 13 : 2592-2604.
Luning K, Dieck IT. Environmental triggers in algal seasonality. Bot. Mar. 1989 ; 32 : 389-397. Matsui T, Ohgai M, Murase N. The effects of
light quality and quantity on germling and thallus growth in Sargassum horneri and S. patens. Nippon Suisan Gakkaishi. 1994 ; 60 : 727-733 (in Japanese with English abstract). Mattio L, Payri CE, Verlaque M. 2009.
Taxo-nomic revision and geographic distribution of the subgenus Sargassum (Fucales, Pha-eophyceae) in the western and central Pa-cific islands based on morphological and molecular analyses. J. Phycol. 2009 ; 45 : 1213-1227.
McCourt RM. Seasonal patterns of abundance, distributions, and phenology in relation to growth strategies of three Sargassum spe-cies. J. Exp. Mar. Biol. Ecol. 1984;74:141-156. Morita T, Kurashima, A, Maegawa, M. Tem-perature requirements for the growth and maturation of the gametophytes of Undaria pinnatifida and U. undarioides (Laminaria-les, Phaeophyceae). Phycol. Res. 2003 ; 51 : 154-160.
Murase N, Kito H. Growth and maturation of Sargassum macrocarpum C. Agardh in Fukawa Bay, the Sea of Japan. Fish. Sci. 1998 ; 64 : 393-396.
Murase N, Kito H, Mizukami Y, Maegawa M. Productivity of a Sargassum macrocarpum (Fucales, Phaeophyta) population in Fukawa Bay, Sea of Japan. Fish. Sci. 2000 ; 66 : 270-277.
Murase N. Ecological study of Sargassum macrocarpum C. Agardh (Fucales, Phaeo-phyta). J.Natl. Fish. Univ. 2001 ; 49 : 131-212 (in Japanese with English abstract).
Ohno M. Seasonal changes of Sargassum spp. in Tosa Bay. Otsuchi Mar. Res. Cent. Rep. 1984 ; 10 : 79-81 (in Japanese with English abstract).
Ohno M. Chapter 18 Tosa Bay: IV Biology. In: The Oceanographic Society of Japan, Coastal Oceanography Research Committee (eds). Coastal Oceanography of Japanese islands. Tokai University Press, Tokyo. 1985 ; 741-742 (in Japanese).
Parmesan C. Ecological and evolutionary re-sponses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006 ; 37 : 637-669. Perry AL, Low PJ, Ellis JR, Reynolds JD.
marine fishes. Science. 2005 ; 308 : 1912-1915. Shimabukuro H, Terada R, Sotobayashi J,
Nishihara GN, Noro T. Phenology of Sargassum duplicatum (Fucales, Phaeo-phyceae) from the southern coast of Satsuma Peninsula, Kagoshima, Japan. Nippon Suisan Gakkaishi. 2007 ; 73 : 454-460 (in Japanese with English abstract).
Stiger V, Horiguchi T, Yoshida T, Coleman AW, Masuda M. Phylogenetic relationships of Sargassum (Sargassaceae, Phaeophyceae) with reference to a taxonomic revision of the dection Phyllocystae based on ITS-2 nrDNA sequences. Phycol. Res. 2000;48:251-260.
Suzuki S, Furuya K, Kawai T, Takeuchi I. Effect of seawater temperature on the productivity of Laminaria japonica in the Uwa Sea, southern Japan. J. Appl. Phycol. 2008 ; 20 : 833-844.
Tanaka K, Taino S, Haraguchi H, Prendergast G, Hiraoka M. Warming off southwestern Japan linked to distributional shifts of subtidal canopy-forming seaweeds. Ecol. Evol. 2012 ; 2 : 2854-2865.
Tanaka MO, Leite FPP. Spatial scaling in the distribution of macrofauna associated with Sargassum stenophyllum (Mertens) Martius: analyses of faunal groups, gammarid life habits, and assemblage structure. J. Exp. Mar. Biol. Ecol. 2003 ; 293 : 1-22.
Taniguchi K, Yamada Y. 1978, Ecological study on Sargassum patens C. Agardh and S. serratifolium C. Agardh in the sublittoral zone at Iida Bay of Noto Peninsula in the Japan Sea. Bull. Jap. Sea Reg. Fish Res. Lab. 1978 ; 29 : 239-253 (in Japanese with English abstract).
Taniguchi K, Yamada H. Annual variation and productivity of the Sargassum horneri pop-ulation in Matsushima Bay on the Pacific Coast of Japan. Bull. Tohoku Reg. Fish. Res. Lab. 1988 ; 50 : 59-65 (in Japanese with English abstract).
Tatewaki M. Fomation of a crustaceous sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia. 1966 ; 6 : 62-66.
Terazono Y, Nakamura Y, Imoto Z, Hiraoka
M. Fish response to expanding tropical Sargassum beds on the temperate coasts of Japan. Mar. Ecol. Prog. Ser. 2012 ; 464 : 209-220.
Tribollet AD, Vroom P. Temporal and spatial comparison of the relative abundance of macroalgae across the Mariana Archipelago between 2003 and 2005. Phycologia. 2007 ; 46 : 187-197.
Tsuchiya Y, Sakaguchi Y, Terada R. Phenology and environmental characteristics of four Sargassum species (Fucales): S. piluliferum, S. patens, S. crispifolium, and S. alternato-pinnatum from Sakurajima, Kagoshima Baty, southern Japan. Jpn. J. Phycol. 2011 ; 59 : 1-8 (in Japanese with English abstract). Tsuda F, Akaike S. Annual life cycle and
productivity of Sargassum confusum popu-lation off the coast of western Shakotan Peninsula in southwestern Hokkaido, Ja-pan. SUISANZOUSHOKU. 2001;49:143-149 (in Japanese with English abstract).
Uchida T. The life cycle of Sargassum horneri (Phaeophyta) in laboratory culture. J. Phy-col. 1993 ; 29 : 231-235.
Umezaki I. Ecological studies of Sargassum horneri (Turner) C. Agardh in Obama Bay, Japan Sea. Nippon Suisan Gakkaishi. 1984 ; 50 : 1193-1200.
Wu L, Cai W, Zhang L, Nakamura H,
Timmermann A, Joyce T, McPhaden MJ, Alexander M, Qiu B, Visbeck M, Chang P, Giese B. Enhanced warming over the global subtropical western boundary currents. Nat. Clim. Change. 2012 ; 2 : 161-166.
Yatsuya K, Kiyomoto S, Yoshimura T. Phenol-ogy of three Sargassum species at Misaki, western coast of Nishi-Sonogi Peninsula, Nagasaki, Japan. Jpn. J. Phycol. 2011 ; 59 : 139-144 (in Japanese with English ab-stract).
Yoshida G, Murase N, Terawaki T. Compari-sons of germling growth abilities under various culture conditions among two Sargassum horneri populations and S. filicinum in Hiroshima Bay. Bull. Fish. Environ. Inland Sea. 1999 ; 1 : 45-54.
Yoshida T. 1983. Japanese species of Sargassum subgenus Bactrophycus
(Phaeo-phyta, Fucales). J. Fac. Sci. Hokkaido Univ. Ser. V (Botany). 1983 ; 13 : 99-246.
Zhao Z, Zhao F, Yao J, Lu J, Ang PO, Duan D. 2008. Early development of germlings of Sargassum thunbergii (Fucales, Phaeophyta) under laboratory conditions. J. Appl. Phy-col. 2008 ; 20 : 925-931.
Received 19 December 2017 Accepted 23 July 2018