第 54 卷 第 5 期
2019 年 10 月
JOURNAL OF SOUTHWEST JIAOTONG UNIVERSITY
Vol.54 No.5 Oct. 2019
ISSN: 0258-2724 DOI:10.35741/issn.0258-2724.54.5.10
Engineering
USING GUAR GUM AS A CO
-
FRIENDLY INHIBITOR TO CONTROL
CORROSION IN CONTAINERS IN BASRAH REFINERY
Akram A. Al-Asadia , Abdulrazzaq S. Abdullahb , Layla Balasem Almalikea Corrosion Eng. Lab., Chemical & Petrochemical Techniques Engineering Department,
Basra Engineering Technical College, Southern Technical University, Basra/Iraq. akramalasadi@stu.edu.iq
b Corrosion Eng. Lab., Chemical & Petrochemical Techniques Engineering Department,
Basra Engineering Technical College, Southern Technical University, Basra/Iraq abdalmaliky@stu.edu.iq
c Polymer Chemistry Lab., Chemical & Petrochemical Techniques Engineering Department,
Basra Engineering Technical College, Southern Technical University, Basra/Iraq
Abstract
Corrosion is responsible for numerous damages mainly in the industrial scope. The best way to fight this damage is prevention. A corrosion inhibitor is one of the most well-known and useful methods to avoid or mitigate destruction/degradation on the surface of metals. Low cost, environmental friendliness and non-toxicity are some of the best features in the guar gum inhibitor used in this research. The study shows that the potential of the metal is shifted positively when the inhibitor concentration is increased. Low current density was also observed due to the increase in the inhibitor concentration. Therefore, a low corrosion rate means low current density and more positive potential of the metal. Different concentrations of the inhibitor are used and the optimum concentration that showed a low corrosion rate is 2.5 g/L. The efficiency of the optimum concentration is 65%. The result states that the guar gum is absorbed on the surface of the metal and gives an unchanging chelate five-membered-ring with ferrous cation Fe+2.
Keywords:corrosion, mild steel, green inhibitor, natural inhibitor, guar gum
摘要 : 腐蚀主要在工业范围内造成许多损害。对抗这种损害的最佳方法是预防。腐蚀抑制剂是避免或减轻 金属表面破坏/降解的最著名和最有用的方法之一。低成本,环境友好和无毒是该研究中使用的瓜尔豆胶抑 制剂的一些最佳功能。研究表明,当抑制剂浓度增加时,金属的电位正向移动。由于抑制剂浓度的增加, 还观察到低电流密度。因此,低腐蚀速率意味着低电流密度和更高的金属正电势。使用不同浓度的抑制剂 ,并且显示出低腐蚀速率的最佳浓度为 2.5 g / L。最佳浓度的效率为 65%。结果表明瓜尔豆胶被吸收在金 属表面,并与亚铁阳离子 Fe + 2 形成不变的螯合五元环。 关键词: 腐蚀,低碳钢,生绿色抑制剂,天然抑制剂,瓜尔胶
I.
I
NTRODUCTIONThe most important engineering material used these days throughout the world is mild steel. A long-standing struggle of scholars and engineers is to combat metal corrosion. One of the utmost
applicable helpful actions in this respect is the applying of suitable inorganic and organic corrosion inhibitors. Nowadays, inorganic inhibitors having phosphate, oxygen atoms,
heavy metals, and chromate are used widely to prevent such corrosion. Some of these organic inhibitors are environmentally damaging and poisonous. Consequently, many researchers have put considerable effort into obtaining suitable, environmentally friendly inhibitors in various corrosive mediums. A study reported that natural products used as a green inhibitor, such as extract of plants, biopolymers, proteins, and amino acids show good resistance to corrosion [1-9].
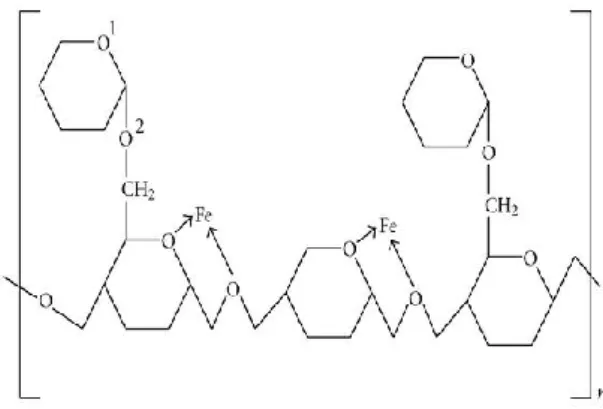
Many studies are conducted using some macromolecules, natural polymers, known as green inhibitors [10]. Figure 1 shows the structure of complex of guar gum and iron.
Figure 1. Structure of guar gum
Guar gum also called guaran is a compound of the polysaccharide having repetitive heterocyclic-pyrane moiety in its structure. Guar gum is considered as a good inhibitor because of its structure [11], [12]. It was investigated as a corrosion inhibitor for mild steel in 1M sulfuric
acid solutions for 1 day [12]. This study indicated that enhancing the guar gum concentration resulted in the increased efficiency of the inhibition process.
The target of this research is to investigate the inhibition influence of guar gum as a natural polymer on carbon steel in water solution collected from a tank-2204 placed in Basrah Crude Oil Refinery. In order to simulate the tank-2204 field condition, many experiments were conducted at room temperature. Guar gum, which is a natural polymer, was used as corrosion inhibitor in the 1M acetic acid in 2240 tank water for 20 hours.
II.
M
ATERIALSA
NDM
ETHODSA. Materials
Table 1 exhibits the chemical composition of carbon steel (0.18 % C) 1cm2 used in this study. This chemical composition was analyzed in the laboratory of the Basrah University, Mechanical Engineering Department.
B. Inhibitor electrolyte solution
Guar gum solution was made through dissolving 2.5 g of guar gum in 1 L of tank-2204 water, which parameters are shown in Table 2. The tank-2204 water analysis is provided by the Al-Shaiba Crude Oil Refinery in Basrah. Analytical dilution of the stock solution was utilized to prepare the concentrations of 0.1 g/L, 0.5 g/L, and 1.5 g/L.
Table 1.
Analysis of the chemical composition of carbon steel
Element C Si Mn P S Cr Cu Fe
Wt% 0.18 0.13 0.44 0.013 0.019 0.12 0.24 Remainder
Table 2.
Analysis of chemical- physical parameters of tank-2204 water
Parameters PH Conductivity Us-cm-1 CaCO3 mg/L Ca+2 mg/L Mg+ mg/L CL- mg/L Na+ mg/L Value 7.9 4490 1058 232 116 998 638 Parameters HCO3 mg/L Suspended solid mg/L SiO3 mg/L C.O.D mg/L B.O.D mg/L Turbidity mg/L T.D.S mg/L Value 214 4 6 21 9.7 5.8 3027 C. Sample preparation
The mild steel specimen was polished using sand paper 100, 180, 220, and 400 grits, respectively. Then, the samples were rinsed with
water in order to evade any probable alteration in the mild steel microstructure. After that, they were rinsed with alcohol such as isopropanol in order to avoid any contamination.
D. Electrochemical measurements
A counter electrode (CE) which is platinum rod, a working electrode (WE) which is a carbon steel specimen and reference electrode (RE) which is a saturated-calomel electrode (SCE) were utilized for conducting an electrochemical method. The electrolyte solution was sustained at room temperature. The carbon steel sample was polished, degreased, and rinsed.
III.
R
ESULTSA
NDD
ISCUSSIONThe mechanism of guar-gum inhibition on mild steel is based on adsorption at the electrode/solution interface. As mentioned above, guar gum has heterocyclic pyrane moiety. The
adsorption of guar gum is possible due to the existence of hetero-oxygen atom of the in its structure. Such adsorption could happen by coordinate-type-linkage via the transport of sole pairs of oxygen atom electrons to the mild steel surface. This will lead to obtaining an unchanging chelate five-membered ring with ferrous cation Fe+2. Because of the proximity factor present in such chelation amid O1 and O2 with Fe+2, it appears to be inextricably linked as shown in Figure 1.
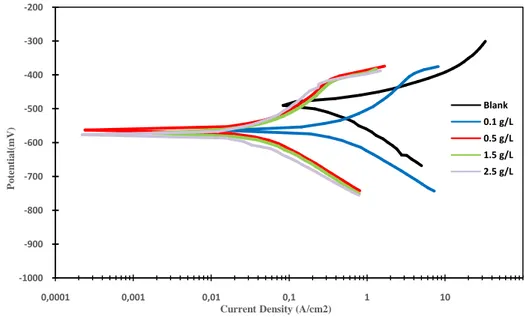
The influence of different concentrations of guar gum inhibitors (0.1, 0.5, 1.5, and 2.5 g/L) on the anodic and cathodic polarization of mild steel was researched in 1M acetic acid in 2204 tank water. Typical polarization curves without and within several concentrations of guar gum inhibitor at 298K are shown in Figure 2.
Figure 2.Curves of polarization of carbon steel in 1Molar acetic acid in at different concentrations of guar gum inhibitor in 2204 tank water at 298K.
Figure 2 illustrates that the increase in the inhibition concentration decreases the current densities. Also, examination of curves indicates that by increasing the inhibitor concentrations the metal potential shifts to be more positive. In addition, the lack of electrons is the reason for shifting the metal potential. As a result, the anodic reaction is accelerated and the cathodic reaction is slowed, as shown in Figure 2.
Corrosion rate relays on current-density and the metal potential. Furthermore, low current density and more positive potential of the metal means a lower corrosion rate. Hence, the optimum concentration for the guar gum inhibitor is 2.5 g/L because of the lack of electrons; thus,
the shift of the metal potential to more positive values leads to slower corrosion rate.
Table 3 presents various electrochemical parameters without and within numerous concentrations of the guar-gum inhibitor, such as corrosion-potential (Ecorr), corrosion-current density (icorr), and the computed inhibitor efficiency (%). The computed efficiency of the inhibitor could be estimated by using the following equation:
Ei =
icorr− icorrinh icorrinh
, (1) where:
Ei is inhibitor efficiency (percentage);
-1000 -900 -800 -700 -600 -500 -400 -300 -200 0,0001 0,001 0,01 0,1 1 10 P o te n tial (m V)
Current Density (A/cm2)
Blank 0.1 g/L 0.5 g/L 1.5 g/L 2.5 g/L
icorr and icorrinh are the values of the corrosion current-density in the absence and presence of the guar-gum inhibitor.
They are measured by using the extrapolation of the cathodic Tafel slopes to the corrosion-potential. Table 3 presents the values of the corrosion rate (mm/yr), inhibitor efficiency (E%), and the coverage of the surface (θ) obtained by inhibitor efficiency per 100 for carbon steel corrosion in 1M acetic acid and in the existence of inhibitors tested at altered concentrations.
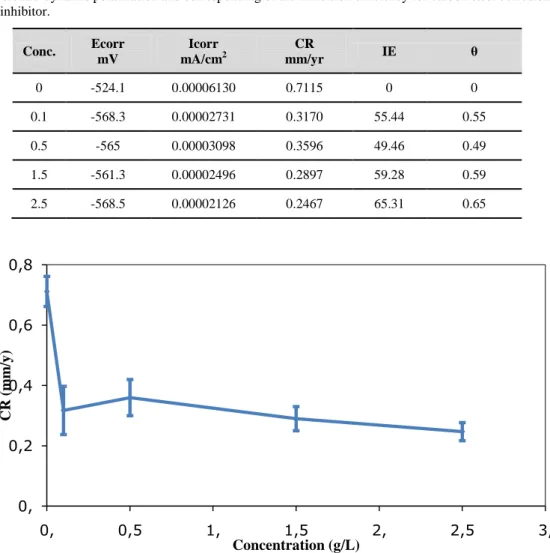
Figure 3 demonstrates the corrosion rates of mild steel without and with altered concentrations of guar gum (green inhibitor) in 1M of acetic acid. The optimum corrosion rate is ascertained in 2.5 g/L of guar gum.
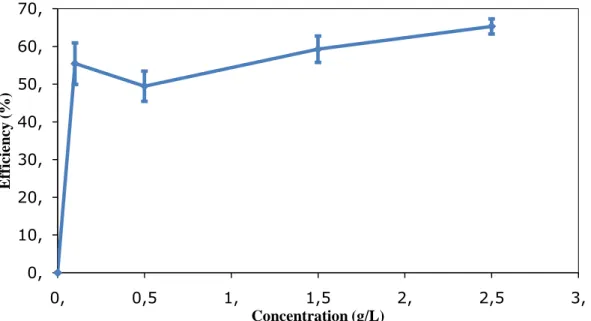
Figure 4 shows the efficiency of the guar-gum as a corrosion inhibition in the presence of 1M of acetic acid in 2240 tank water, and the highest efficiency is observed in 2.5 g/L of guar-gum.
Table 3
Parameters of potentio-dynamic polarization and corresponding of the inhibition efficiency for carbon steel corrosion without and with guar-gum inhibitor.
Conc. Ecorr mV Icorr mA/cm2 CR mm/yr IE θ 0 -524.1 0.00006130 0.7115 0 0 0.1 -568.3 0.00002731 0.3170 55.44 0.55 0.5 -565 0.00003098 0.3596 49.46 0.49 1.5 -561.3 0.00002496 0.2897 59.28 0.59 2.5 -568.5 0.00002126 0.2467 65.31 0.65
Figure 3.Corrosion rate of carbon steel in 1Molar acetic acid without and with altered concentrations of guar gum as corrosion inhibitor. 0, 0,2 0,4 0,6 0,8 0, 0,5 1, 1,5 2, 2,5 3, CR (m m /y ) Concentration (g/L)
Figure 4. Efficiency of carbon steel in 1Molar acetic acid without and with altered concentration of guar gum as corrosion inhibitor.
VI.
C
ONCLUSIONSExperiments were carried out in order to investigate the influence of the green inhibition, namely guar gum, on the mild-steel corrosion in the weak acid medium in 2240 tank water. The results of this study can be summarized as follows:
1. The presence of the hetero-oxygen atom in the guar gum structure is a factor that allows adsorption on the mild steel surface.
2. As a result, the adsorption process gives an unchanging five-membered chelate ring with ferrous cations Fe2+ and it appears to be inextricably linked.
3. The results show that the decrease in the current-densities is associated with enhancing inhibition concentration, and also leads to shifting the metal potential more positively due to the lack of electrons.
4. It is observed that the cathodic reaction is slowed and the anodic reaction is accelerated. 5. The optimum concentration for the guar gum
inhibitor and high efficiency is observed in 2.5 g/L of natural polymer. Thus, guar-gum could be used as a corrosion inhibitor.
A
CKNOWLEDGMENTThe authors would like to thank the dean of the Basra Engineering Technical College for his support in the Corrosion Engineering Lab.