l I
1 )
1
[
Memoirs of the College of EdUCatiOn]
Akita University (Natural Science) 45, 1-22 (1993)
Studies on the Mechanism of Cell Division (II)
Furrowing Determination and Cross Wall Formation
Masahiko
YAMAMOTO*(Received july, 28 1993)
ABSTRACT - With the progress of cell division, the superficial cytoplasm of the cell becomes firm as a girdle of the gel just prior to cell division. The girdle is formed by the beginning of anaphase in the area, corresponding with the equatorial plane of mitotic spindle. This means that the potentiality of the contraction of the furrow has been established on the cell surface by the above described period of time, in close connection to the position of the mitotic spindle.
The second step of cytokinesis is achieved by the formation of a partitioning plane in an equator of the dividing cell. The writer previously reported a new cell plane formation as a leaflet structure, by the thermal exposure of grasshopper spermatocytes (1993). This is veritable to the cross wall formation, in cleaving sea urchin egg appearing as a plane, by the coalescence of PAS stained vacuoles or granules, arranged linearly along the equatorial plane of the mitotic spindle. The partitioning plane was also ascertained in Caecerra eggs when they were centrifugalized at the end of meiotic division (pseudocleavage).
As a result, cytokinesis is accomplished by collaboration of the contraction of furrowing surface, and the formation of cross wall based upon the coalescence of pre-existing substances with an action of thy mitotic apparatus.
INTRODUCTION
Although karyokinesis and cytokinesis are of course linked, the plane of cell division (cleavage) is accomplished finally in close connection with the function of mitotic apparatus. The mitotic apparatus plays an important role in anaphase movement of separating chromosomes. Presently, it has been believed that the spindle associated vesicles release Ca
2+to initiate their movement, mush as the salcoplasmic reticulum releases Ca
2+to initiate skeletal muscle contraction. (Murray, 1985; Wolniak, 1988). Moreover, the mitotic apparatus accomplishes in determining where and when cell division takes place. The division furrow is generally established at an equator of the mitotic spindle perpendicular to the long axis of the spindle. The writer previously reported an effect of the displacement of the mitotic spindle at various stages of mitosis, and ascertained when it was displaced beyond metaphase, that the furrow was, in most cases, formed irrespective
*
Biological Laboratory, Dept. of Education, Akita University, 1-1, Tegata-Gakuencho,
Akita City, JAPAN
L1r*~~of the final position of the mitotic spindle (Yamamoto, 1964). Considering the data obtained, the writer came to the conclusion that cytokinesis was governed by the mitotic apparatus in the first half of cell division.
In grasshopper spermatocytes and the eggs of some marine invertebrates, the initiation of cytokinesis is, in the normal course of development, determined on a superficial cytoplasm (cortex) of the prospective furrowing region, in close connection to the position of the mitotic spindle (Yamamoto, 1964). By centrifugation experiments on the eggs of sea urchin,
H. pulche-rrimus, the writer assumed a period of the determination for cleavage, that it would be established on the superficial cytoplasm as a girdle or a belt, made of the gel just prior to early anaphase (Yamamoto, 1992). At present the critical stage, a period of determination, is evidently permitted, and it is now designated as 'point of no return' in the researchers of
~hisfield.
Further illustrations on the validity of this conclusion have been done by many researchers from several aspects ofstudies. Furthermore, it has been evident that the superficial cytoplasm of the prospective 'furrowing region becomes firm by an assembly of microtubles and some actin bundles (Blose, 1979). According to 'The Cell'
(A.Brav et al. 1989), a beltlike bundle of actin filaments and myosins, known as contractile ring, appear during the cell division ; forces generated by this ring, contract an equatorial plane of the cell leading to eventual separation of two daughter cells. In sea urchin egg about to divide, myosin molecules are at first distributed evenly beneath plasma membrane, and then, move to an equatorial region as a contractile ring form (Schroeder, 1973 ; Langanger and others, 1986).
Up to the present, four major theories about the mechanism of cell division (below) have been established. The researcher's name who first advocated, is written at the end of the theory in parenthesis.
(1)Contractile ring theory (Chambers, 1938) ; (2) Spindle elongation theory (Dan, 1943) ; (3) New membrane formation theory (Motomura, 1950) and (4) Expanding membrane theory (Swann and Mitchison, 1958). The writer, making allowance for the above mentioned theories, carried out various experiments to ensure the mechanism, using several kinds of procedures; micrurugical, physical and chemical treatments on cells and eggs in division. The species names used, will be shown on all such occasions.
Before retirement from the university, the writer would like to recollect his researches extend- ing about forty years, he wishes to review his series of works concerning the mechanism of cell division. After long complications, the writer has reached to the conclusion that cytokinesis is accomplished by the collaboration of the contraction of cell surface and the formation of plane of partition (cross wall), based on the fusion of vacuoles or granules which are lined along the prospective plane of cell in division. In the present paper, the writer would like to review the series of experiments for an analysis of cell division, in commemoration of his career as a researcher of developmental biology.
Before going further, the writer wishes to express his cordial thanks to Mr. and Mrs. Ryukichi
Harada, the former professor of T6hoku University, and to Mr. and Mrs. Tadataka Fujieda, the
former principal of Sannohe High School, and to Mr. and Mrs. Makoto Shoji, the director of the
hospital, Haranomachi Sendai, for their kind advice and encouragement during the course of the
writer's research and career.
RESULTS AND DISCUSSION 1. Destruction of Mitotic Apparatus by Podophyllin
To determine the critical stage for the completion of furrowing under an influence of mitotic apparatus, the writer at the beginning of his research, studied the actions of some mitotic inhibitors, using several kinds of animal cells in the course of cell division. At the onset of this paper, he wishes to pick up one of his experiments on the effect of podophyllin as a representative, and to refer to its action on the fertilized eggs of a bivalve, Caecella chinensis.
Podophyllin is a chief ingredient of Podophyllum peratum (Mendrake), and it has been used in medical tratment on Condylomata as a mitotic inhibitor. In the treatment within a meristem of Allium cepa, prometaphase was accumulated in the absence of mitotic spindle to complet divi- sions, and the chromosomes shortend slightly but remained attached at centrimere (Sullivan and Wechsler, 1947). King et al. (1946) reported that the mode of an action of this chemical was almost the same as colchicin, a famous mitotic inhibitor of a fabulous price at his start of research.
When the fertilized eggs were exposed to sea water containing 0.5 per cent podophyllin, just after the last half of the stages of maturation division, then, reared continuously in this condition, there appeared a clear spot in the center of the treated eggs (Fig. 2 A). This is probably the constituents of the mitotic apparatus disorganized by the effect of podophyllin. The formation of astral rays was first arrested however, they were formed later, showing feeble rays of radiation outwords. The first cleavage was prolonged for about 50 minutes or more, and a rate of cleavage reached 47 per cent of the total treated eggs. Among the divided eggs, nearly half of them was cleft into blastomeres in an equal size (2 cell stage), although the control eggs showed a distinct unequal cleavage (Fig. 2, C). The development of the treated eggs when they were put back to sea water, was irregular in most cases, and the blastomeres later became dispersed as in the development of fertilized sea urchin eggs, reared in Ca free sea water.
When the eggs were exposed to higher concentration of podophyllin (2
X10-
6per sent), at the stage just prior to furrowing, the astral rays distinctly observed before the treatment, were extinguished within 5 to 10 minutes. The portions where asters had been found, became gradually homogeneous, then, two clear spots were observed. Meanwhile, the eggs begun to cleave dividing the egg into blastomeres of an unequal size, whereas remaining eggs in the prolonged cleavage, commonly cleft later forming blastomeres in an equal size (2 cell stage).
In the preceding paper (Yamamoto, 1992), the writer ascertained the critical stage of the
gelation on cell surface when preparation for cell division was determined. This stage is con-
sidered to settle during the beginning of anaphase. Presently, this is permitted by cytologists, and
is refered to as 'point of no return'. When the mitotic apparatus was destroyed beyond the critical
stage, the cell proceeded normal course of cytokinesis in several kinds of cells in division. Figs. 2,
Da-Dc show the process of destruction of mitotic apparatus, when fertilized eggs of sea urchin,
Hemicentrotus pulcherrimus were immersed to podophyllin beyoud anaphase. After the immersion
for 40 minutes and more, furrowing began in some cases, without the presence of the definite
mitotic apparatus.
2. Micrurugical Analysis (Microdissection)
In the foregoing chapter, the writer obtained autonomous contraction of superficial cytoplam (furrowing) without presence of mitotic apparatus. Hence, what is an actual condition of this phenomenon involved in the 'point of no return'? Possibly, it must occur some kind of changes taking place on the cortex of the cell at the stage after the beginning of anaphase. Ordinarily, the rule for cell division is that a furrow bisects the developing cell through an equatorial plane of mitotic spindle. Therefore, it may be thought that the mitotic spindle exerts some affections around the equatorial periphery of the cell cortex, necessary for furrowing by this time. In the writer's previous report (Yamamoto, 1964), using grasshopper spermatocytes, the furrow was determined in close connection with the position of bundles of mitochondria, investing along the surface of the mitotic spindle. Hence, it seems that an inducing substance governing surface changes, will not be derived from the mitotic spindle alone. For this reason, to ascertain structural changes occurring on cell surface, the writer (1957) carried out microdissection experiment using dividing first spermatocyte of grasshopper, Chrysochraon japonicus. In the spermatocyte, at the stage beyond metaphase and entering anaphase, when the cell was punctured at a point around a polar region, disintegration of protoplasmic membrane immediately propagated in all direc- tions, but it stopped at the equatorial part (future furrowing region) of the cell (Fig. 2, G). The opposite half of the cell remained intact for several period of time, then, its cell wall gradually became swollen and, by sudden disintegration, it disappeared completelly. These phenomena were more conspicuous in the cell at advancing mitotic stages with slightly deepened furrow.
Before and during metaphase, when the spermatocytes were punctured at any point of cell surface, the superficial membrane collapsed abruptly, propagating disintegration toward all over the surface. Hence, the mitotic apparatus exposed outside the cell, remeined still in a medium (Fig.
2, F). This indicates that, beyond early anaphase, the structural difference of the superficial cytoplasm is established on the cortex of the prospective furrowing region, as a girdle of the gel at the position corresponding with the plane across an equator of the mitotic spindle.
Chambers (1939) punctured the polar region of dumb-bell shaped eggs of sea urchin, Arbacia punctulata, in an isosmotic solution of KCl, and found that, in spite of ensuring tear, the cleavage furrow continued its advance, and that the equatorial region remained for a long time than the other part of the egg.
Based on the observation of displacement of pigment granules, residing in the cortical surface of the eggs of sea urchin, cetrifugalized under condition in hydrostatic pressure circumstance, Marsland (1951) reported that, at the initiation of cleavage, the superficial protoplasm grew firm, especially in the region of incipient furrow. According to Mazia (1961), the hypothesis involves three levels of inferences that; furrowing involves a gel that sol-gel transformation is an indicator of a contractile mechanism, and that the contractile mechanism is expressed in a constricting ring around the equatorial periphery. For this reason, it may be said at that time, that the writer's experiment was estimated to have ensured the morphological changes taking place during cell in division.
Recently, the actual structure of furrowing region (contractile ring) was observed by an elecron
microscopy. This structure was composed of microfilaments of 30 to 70 A. oriented around the
prospective furrowing region as a ring (Schraeder, 1973). In the course of development of the
studies of this field, this has been supported by many researchers in a variety of materials. At present, the role of the microfilaments is considerd to exert forces inducing invasion of the furrow.
For instance, when the microfilaments formation is blocked using several kinds of drugs, the contractile ring is never formed, therefore the cleavage is completely inhibited (Browder et ai, 1980). The microfilaments residing in contractile ring, were elucidated to be composed of actins found in the skeletal muscles. Several years later, Mabuchi et al. (1973 and 1977) continually extracted myosin from the cortex of sea urchin egg. Accordingly, the force establishing the furrow is furnished with the shrinkage, similar to the muscle contraction.
3. Displacement of Mitotic Apparatus
In the previous papers (Yamamoto, 1964, 1965 and 1992), the writer indicated the structure of mitotic apparatus on some kinds of cells, by an application of centrifugal force at various stages of mitosis. Getting over several difficulities, he has performed experiments, and explained some aspects concerning the structure and the function of mitotic apparatus. In those days when the writer set to works, even the high speed centrifuger was not equipped in his laboratory, because of the scantiness comming from deficiency of experimental tools by the defeat of the World War IT. Consequently, the mitotic apparatus was hardly to be displaced through an ordinary electric driven centrifuger. He, at that situation, thought: Is there any simple and skillful method to move and to segregate major organells present in the cell? He found out skillful method of centrifuga- tion after immersion of cell into the medium of low osmotic pressure. With pretreatment, if the mitotic apparatus is a cohelent body having properties of the gel, it should be easily displaced by an ordinary driven centrifuger. Therefore, in the experiment, he beforehand immersed each stage of eggs of sea urchin into 58 per cent of hypotonic sea water. In the case of unfertilized eggs centrifugalized, they were remarkably stratified into three layer; (1) oil droplet layer at the centripetal side, (2) clear hyaloplasmic layer including nucleus, and (3) yolk granule layer on the centrifugal side. Because of a transparency of hyaloplasmic layer, not only nucleus but also cortical granules were clearly observed just beneath the egg surface. By careful observation of a point of sperm attachment on the hyaloplamic layer side, a behavior of a spermatozoon in a process of invation and its penetration into the egg interior were precisely observed (Yamamoto, 1990). Similarly, by the continuous observetion, the changes of the mitotic apparatus during mitosis, and the behavior of furrowing process were clearly recognized as shown in Fig. 2, H-J and K-O. With an advance of cleavage, the superficial cytoplasm around the prospective furrowing region, seemed to increase its thickness (slightly reddish in colour). The change of the thickness was also ascertained by sectioned preparations, that were stained by Lillie's PAS method during furrowing (Yamamoto, 1990). Besides, he found many PAS positive granules in the egg interior gathering up toward the prospective cleavage plane, during the course of development just preceding and during furrowing (Yamamoto, 1989).
The problems of the mechanism of cell division that we expect to know, is the final determina-
tion of furrowing potency in the superficial cytoplasm prceding cell division. How does it
determine? Is it based upon an action of mitotic apparatus? If this is true, till when does the
preparation for division accomplish on the cell surface? For this reason, to know the critical stage
when determination for cell division does occur, and to ascertain an action of the mitotic
apparatus on the formation of furrow, the writer carried out above described centrifugation experiments to displace the mitotic apparatus, using mainly the eggs of sea urchin in every developmental stages of mitosis.
In an application of centrifugal force before metaphase, the first cleavage plane was, in almost all the cases, formed concerning the final position of the displaced mitotic apparatus. While, when centrifugation was done beyond the second half of cell division (passing through the stage of an initiation of chromosome separation), the egg cleft automatically irrespective of the final position of the displaced mitotic apparatus.
From the data obtained, he came to the conclusion that the mitotic apparatus played an important role for cell division by the first half of mitosis, then, by reaching near the critical stage, the determination of furrowing was established on the superficial cytoplasm, limiting on an area of the prospective furrow. Therefore, the cell acquires the furrowing potency, and it is able to divide without the presence of the mitotic apparatus.
After about ten years, he introduced experiments with high speed eletrically driven centrifuger, and again proposed to test the same experiments described above (Yamamoto, 1975). The fertilized eggs (same species) were centrifugalized at various mitotic stages, after the immersion of hyponic sea water for 5 and 10 minutes. The centrifuged eggs were somewhat stretched in contrast to the foregoing experiments, toward the direction of cenrifugal force, stratifying into four definite layers. In the present centrifugation, one semitransparent cytoplasm layer was added further, onto the centrifugal yolk layer. The hyaloplasmic layer was conspicuously evident containing nucleus just beneath the oil drop layer.
In the case of the unfertilized eggs after centrifugation, when they were inseminated and observed continuously from this time, the behavior of the mitotic apparatus was clearly obser- vable.
Itwas formed at the centripetal side in parallel with the long axis of the stretched egg.
Therefore, the first cleavage plane was formed perpendicularly to the axis, dividing the egg into an unequal blastomeres (Fig. 3, H-J). The second cleavage took place only in the blastomere of the centrifugal side. With further development, successive cleavage occurred, however, the blast- omere on the centripetal side kept still whithout cleavage notwithstanding the presence of the nucleus.
Motomura (1946) carried out experiment, using air turbin driven ultracentrifuger in combina- tion with an air compressor (force, over 1 x 10
5G. M.). He designated the unsegmented blastomer in the centripetal side as the giant cell. He infered cleavage inhibition probably based upon displacement of cortical cyroplasm for cleavage. Apart from the cleavage inhibition, the writer's simple technique described in this paper, seems to correspond with the experiments, done using more high grade centrifuger. By the pretreament of hypotonic sea water, he could get the same effects as in the usage of high speed centrifuger. This was fortunate to him propagating subsequent pursuits of experiments as stated follows.
When the eggs centrifugalized before and during metaphase, the furrow was formed in close
connection with the final position of the mitotic apparatus. While, in the case when the eggs were
centrifugalized during anaphase, the furrow was formed in the original position where the mitotic
apparatus had form ely been found. Namely, the furrow was formed in the position without an
existence. of the mitotic apparatus.
From the above facts, it can be said that the mechanism of furrowing is localized on the equator of cell surface, then its contraction drives furrowing inward. After proposing an assumption, the writer carried out following experiments, using other ways and means to testify the mechanism of furrowing.
4. Toughning of Fertilization Membrane
Congo red is one of the special stain for mitotic apparatus, and it should stain spindle or mitotic figure into deep orange to light red in the living state ('Biological Stain', written byH.
J.
Conn, 1961). Based upon the explanation, the writer chose Congo red for a vital staining dye of the mitotic apparatus, using mainly the eggs of a sea urchin,Hemicentrotus pulcherrimus
(Yamamoto, 1964). After setting to the treatment, Cong red could not stain mitotic apparatus of developing eggs in the living state. However, contrary to the writer's expectation, it was able to stain fertilization membrane, at the period just after an elevation of fertilization membrane. In the staining, not only fertilization membrane itself but also perivitelline space were totally stained into orange or reddish in colour. The nature of the fertilization membrane was somehow denatured, increasing its toughness and losing elasitisity. Hence, when fertilized eggs just prior to membrane elevation were immersed to sea water containing Congo red, an elevation of fertiliza- tion membrane was completely, or in some cases, partially blocked, except around sperm penetra- tion point (Fig. 4, A). Accordingly, most of the treated egg were covered with stained tight membrane over the whole surface. Under this condition, the external changes of the body of the egg contour with developmental progress, were perfectly arrested. In the following, the effets of tight membrane on the process of cleavage were described.When fertilized eggs were exposed to 0.03 per cent of Congo red sea water, 40-45 seconds after fertilization, an elevation of fertilization membrane was interrupted in the midway, forming partially elevated membrane around a sperm entry point. This is probably due to denaturalization of the fertilization membrane during elevation. In the eggs mentioned before, the mitotic appa- ratus was formed regardless of the position of partially elevated membrane at the beginning.
Then, the axis of the spindle gradually moved round in parallel with the extent of perivitelline space, enveloped by denatured membrane (Fig. 4, A).
The first cleavage plane was formed through the central portion of the elevated membrane in 79 per cent of the eggs treated (Figs. 4, A and C). In some eggs having considerable small pervitelline space or spaces, the cleavage was almost suppressed in spite of the progress of karyokinesis. They became finally into multinucleated eggs (Figs. 4, F and G).
Generally, during the course of cleavage, it has been noticed that the egg contour becomes somewhat stretched along an axis of the mitotic spindle, prior to an initiation of furrowing. From topographic mesurement of a behavior of kaoline granules attaching onto the egg surface, Dan (1951) found an obvious elongation of spindle with the progress of cytokinesis. By an en- velopment of tight membrane, the elongation of the egg contour toward a direction of a long axis of the spindle was, of course, somewhat arrested. In this condition, whether cleavage does occur or not? As shown in Figs. 4, F and G, the invation of the furrow was, in most cases, suppressed.
However, no matter how small a rupture of the denatured membrane took place, an invation of the plane of partition appeared rapidly, dividing the egg into two daughter blastomeres. Surpr-
isingly at this occasion, the invation of furrowing was completely omitted.
An inhibition of the spindle elongation, by the resistance of the intimate encircling membrane, led to the suppression of the invasion of furrow, irrespective of the existence of full grown mitotic apparatus. However, the rapid formation of a partitioning plane, taking place at the time of membrane break down, leads to an assumption that the materials participating in the formation of cleavage plane (cross wall) are supposed to subsit along the position of the prospective plane of cleavage.
In the case of grasshopper spermatocyte (later stages of mitosis), when they were observed after the direct immersion to warmed Ringer's solution (50°C) for two minutes, there appeared a feeble platelet on the prospective division plane of the cell, across an equatorial plane of mitotic spindle (Yamamoto, 1964). This seems to be due to the furrowing inhibition, caused by an inhibition of furrowing cortex by high temperature. From the results, it should be considered, that this platelet is certainly the cell plate seen in the plant cell in division. The writer's platelet seems to be the vestigial homologue of cell plate or phragmoplast in the plant cell in division (Gunning, 1985).
Therefore, it can be said that the platelet, described before, corresponds to the cleavage plane or the boundary (diastema) as observed by Motomura (1950).
Considering the data, it may be said that cytokinesis should be carried out by two main processes; one is the contraction of egg surface (furrowing), and the other is new membrane formation (cross wall) that partitions the cell into two daughter blastomeres. As stated above, because of the increase of an internal pressure caused by the suppression of growth and elongation of egg during the course of cleavage, the cytokinesis of the treated eggs were consecutively interrupted. However, by a release of the internal pressure when the denatured membrane was torn even slightly, cytokinesis moved again establishing a partitioning membrane of the platelet in the cell equator, without the invasion of furrowing.
In the fertilized egg having some extent of perivitelline space or spaces, the egg could continue segmentations, though somehow slow, into blastula stage. In this case, the hatching was com- pletely arrested because of the denaturalization of the fertilization membrane. The embryo continued to develope toward gastrula, growing somewhat larger untill it was full to the brim of the denatured membrane. At this stage, the embryo remained still without further development, meanwhile, several mesenchyme cells were formed on the vegetal wall of the embryo. The mesenchymal cells newly formed, moved slowly upward, forming a ring some what away from the animal pole.
The initiation of invagination (above eggs) was inhibited in most cases. After a while, the wall of the vegetal side gradually began to invaginate, making a dome of cell masses as shown in Fig.
5, I and J. Subsequently, a crowd of the secondary mesenchymal cells were formed from the tip
of the dome, projecting long pseudopodia toward the animal pole, (Fig. 5, L). Each pseudopodium
continued stretching until it reached about twice as long as the normal one. The stretching of the
pseudopodia is probably based upon the strong induction from the animal pole. Once they were
attached, the tip of the dome seemed to be pulled upward by the contraction of the pseudopodia,
pulling an archenteron into blastocoel (small in size) (Fig. 5, K). Hardim (1988) ablated the
secondary mesenchyme cells using laser irradiation, that the archenteron could only elongate
about two-thirds of the full length.
It
must be realized however, that when the bundles of the pseudopodia were cut off by a sudden expansion of the roof of the embryo (by membrane rupture), the embryo continued an invagina- tion process, and finally established longer archenteron inside the blastocoel (Yamamoto, 1965).
The informations concerning the mechanism of gastrulation were reported by Dan and Okazaki (1956), and Gustafson and Kinnanedr (1956) and other researchers. According to Dan and Okazaki, the invagination process contains two main phases. The first step is slow but auto- nomous, depending essentially on the factors residing in the vegital pole itself. The second step is due to the contraction of pseudopodia, emmitted from the tip of the invaginating archenteron to the blastulal wall of the aninal pole.
Judging from the writer's experiment, it may show that the main role of the pseudopodia is merely a guidepost of invagination, to which the direction of invagination is led rather than their pulling force for gastrulation.
Concerning the denaturalization of the fertilization membrane, an almost the same observations was obtained in the fertilized eggs of an oyster, Crassostrea gigas. Because of the small amount of perivitelline space, the fertilization membrane was formed attached intimately to egg surface. By the exposure to the dye, the fertilization membrane was stained reddish as in the case of sea urchin egg. In these eggs, with an advance of cleavage, the polar lobe formation was arrested to some extent, however, a sudden endoplasmic flow out took place by the rupture of the denatured membrane. In this case, the egg completed cleavage forming blastomeres of an unequal size (Fig.
4, M and N). This shows clearly the denaturalization of fertilization membrane occurring also in this species.
5. Cytokinesis without Mitotic Apparatus
In the prceding chapter, the writer repoted an inhibition of egg contour during the process of cleavage. Here, what kind of changes does occur, when the fertilized egg was compressed to inhibit external change of the egg during the course of cell division? For this purpose, the eggs of sea urchin, Hemicentrous pulcherrimus, were exposed to sea water containing crude methylen blue (Yamamoto, 1965). As the result of the experiment, jelly coat was stained into blue in the fist step, then it began to contract till it was precipitated onto egg surface, attaching intimately as an elastic coat of bluish membrane (Fig. 1, C). This change was certified by the following procedures.
By pressing the treated egg strongry enough between two cover slips, the precipitated jelly coat
was deprived as a coat or membrane, as shown in the figure (Fig. 1, D). In other evidence, we could
occassionary find one kind of a protozoa (Hypotrichida) swimming around egg surface, in a space
just under the jelly coat (Fig. 1, E--G). When small drops of methylen blue solution (0.001 per cent
in sea water) were added, the protozoa was pushed inward to the egg interior under the
precipitated jelly coat (Fig. 1, H). This shows the strong contraction of the precipitated jelly coat
by an action of this reagent. Further, as the result of the contraction, a small cytoplasmic
projection budded out from the egg, making a tiny protuberance, then, as an advance of
development, it gradually grew in a form of a midget dome (Fig. 5, C). The behavior of the dome
will be described and discussed later. The volume of the dome depends upon to some degree the
concentration of the reagent. These phenomena evidently show an active contraction of the jelly
coat. In addition, when the unfertilized egg was pretreated with acid sea water to deprive the jelly
coat, it never formed an egg protuberance after the exposure to this reagent.
As memtioned before, when the fertilized egg was exposed to methylen blue just after insemina- tion, there appeared a small cytoplasmic projection from the egg surface, at the beginning of exposure as shown in Fig. 5, D and E. This is probably due to an outflow of an endoplasm through a penetration defect of a spermatozoon. The volume of the projection grew gradually, that reached finally in the volume, almost the same size to an original egg. The growth of the projection seems to occur by an internal pressure, accompanying with a force of the formation of an perivitelline space or spaces, under the restriction of stiffening fertilization membrance.
During the growth of an egg protuberance, a nucleus in an early stage of mitosis (even the glimpse of mitotic figure was not recognized) flew out toward the egg protuberance. Meanwhile, the protuberance was divided into two blastomeres, however, its pattern of cleavage was done with cross wall formation. This is probably due to an absence of cortex, so that furrowing could not occur. With subsequent segmentations (egg protuberance), each blastomere was disperced separately as in gevelopment in Ca free condition. This may be due to the deficiency of the hyaline membrane.
Contrary to the above occasion, mitotic apparatus was initially formed in the egg side (Fig. 5, J
-K),
from which most of an endoplasm gradually flew out into the egg protuberance. Because of
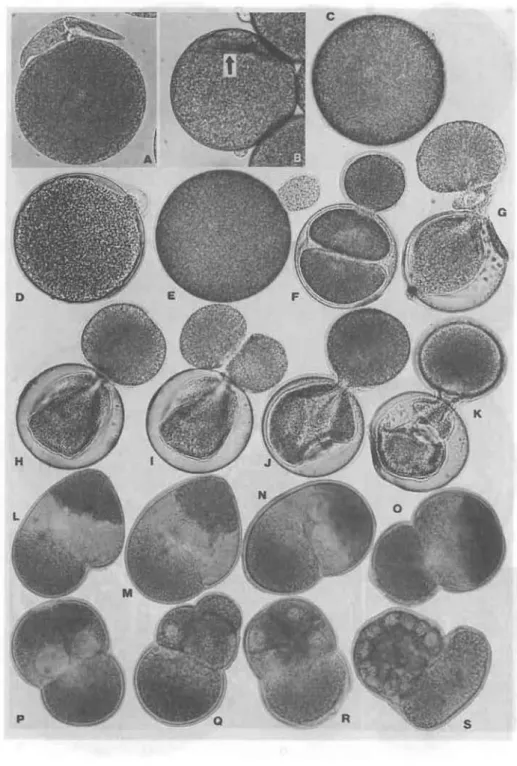
FIGURE I
Photomicrographs showing precipitated jelly coat by an exposure to crude methylen blue
(egg of sea urchin;
H.pulcherrimus). (A) Unfertilized egg showing jelly coat, observed in sea
water containing a number of carbon particles. (B) Disappearance of jelly coat, after the
treatment of acid sea water. (C) Contraction of jelly coat after the exposue. Precipitated jelly
coat is seen (dark definit line adhering closely to egg surface).
(D)Precipitated jelly coat,
deprived (by pressing the treated egg between two cover slips). (E) - (F) A cell of Protozoa
swimming around egg surface (closely attached on the egg). (H) The cell (pointed by on arrow)
merged into the egg interior by the contraction of the jelly coat.
an release of most endoplasm toward the protuberance, the egg itself later became like a castoff skin of insect made of cortex alone (Fig. 5,
J).At the time of cleavage, even in the drastic change (cortex alone without endoplasm), the furrow appeared in an intimate relationship to the position of mitotic apparatus, where it was present before the extrusion. In this case, the furrow, only with a thin layered cortex remaining in the fertilization membrane, deepend as shown in the figures (Fig.
5J and
K).Here, it may surely be postulated that mitotic apparatus participates in the first half of mitosis, and after the establishment of the determination of furrowing, the furrow deepens, as a breaking out of dam, without most endoplasm. Here, the writer would like to emphazize : Cytokinesis consists of two main processes; one is the active contraction of cell surface, and the other is the partitioning of cell by the establishment of a membrane newly formed.
The interpretation availabel is a review of Hiramoto (1981), who sucked out mitotic apparatus from sea urchin egg at metaphase. He pointed out that the mitotic apparatus is not necessary for a whole stage of cell division, and the furrowing results depending upon the active contraction of cell cortex. Moreover, some of the best evidence for the role of superficial cytoplasm in the formation of furrow came from studies of many researchers. Hoffmann Beling (1959) made sure that cytokokinesis was carried out in the same mechanism to muscle contraction. He observed that telophase model (glycerol-extracted fibroblats in which cytokinesis began) completed their furrowing, when APT was added under the conditions, indentical with those that elicited contraction from corresponding 'muscle models' (Mazia, 1961).
As shown in the present report, the former, contraction of cortex alone, is thought to be closely comformity with Hoffmann Berling. And, the latter, cytokinesis without the presence of cortex, leads to a consept of cross wall formation as in the plant cell.
6. Cleavage
induction by Meiotic SpindleThe initiation of meiosis in eggs of a bivalve, Caecella chinensis is the breakdown of a germinal vesicle that occurs about fifteen minutes after fertilization. After a while, the first polar body is eliminated at the animal pole. Then, second meiotic division follows at the same area, eliminating a second polar body about 15 minutes after the first meiotic division. The elimination of two polar bodies is achieved by subsequent divisions through the meiotic spindles. The meiotic spindles are very small in size comparing to the mitotic spindle (participating cell division), and they are formed at the point close to the animal pole.
To ascertain the functional differences of meiotic and mitotic spindles, which take part in maturation division and cell division respectively, the writer carried out an experiment of centrifugation, to shift meiotic spindle from the original peripheral position (animal pole) toward egg interior, using Caecella eggs in various stages of meiosis (Yamamoto, 1971 and 1973).
When the fertilized eggs were centrifugalized just after germinal vessicle breakdown (Meiosis
I ), they were stratified into distinct three layers (Fig. 5, L). The female pronucleus just formed,
was moved into the second hyaloplasmic layer, occupying in almost the central part of the
hyaloplasm. After a while, about 30 per cent of the treated eggs were divided into two blastomeres
of an equal size. In these cases, the spindle participating call division, was formed in the egg
interior as a small one, then it grew up larger comparing to the original small meiotic spindle. The
enlarged meiotic spindle (reaching almost the same size of mitotic spindle) seems to function as
the mitotic one, however, the pattern of cleavage differed greatly (Fig. 5, M-O), comparing to the normal cleavage (unequal cleavage). Furthermore, an initiation of cleavage occurred twenty minutes earlier than the normal cleavage. What does this cleavage acceleration mean? Does it suggest the modification of the meiotic spindle to the mitotic one? This fact implicates many important problems about the mechanism of meiosis and mitosis.
Appart from the discussion, the writer designated this kind of cleavage as 'pseudo-cleavage', because of an imcompletion of synkaryon of a male and a female pronuclei. To make clear the fact, the writer engaged in operations to make sectioned and stained preparations. According to the observations, he ascertained the period (meiotic stage of the control egg), when pseudo-cleavage took place.
Itwas the metaphase or the early anaphase. Therefore, considering the above fact, he came to the consideration that mitotic appapatus for meiosis, when it was shifted into egg interior and kept at this situation, could acquire a potentiality for inducing cleavage.
Just after the completion of pseudo-cleavage, one half of blastomeres containing a small transparent sphere (centripetal side), formed second polar body. Judging from the observations, the transparent sphere will be surely a male pronucleus. After synkaryon, blastmere described above, having conjoined nucleus, continued develepment until it reached morula stage (Fig. 5, P -8). This proves, one half of the blastomere corresponds to an oocyte itself, and the other half, still such a bulky sphere (never divide), is no doubt, a first polar body itself (giant polar body).
To fill up the space, the writer wishes to offer writer's experiense to comming students : 'On est heureux quand on travaille bien'.
In the eggs centrifugalized before germinal vesicle breakdown (ten minutes after fertilization), the materials constituting nucleus were moved to hyaloplasmic layer, then back to the original position forming meiotic spindle (later eliminating polar bodies). The retardation of development was almost the same as a duration of centrifugation. When centrifugation was began just after the end of the breakdown, and continued for 20 minutes, upon rapid formation of the mitotic apparatus (in large volume), pseudo-cleavage took place 11 minutes later. This is interesting that the time of the initiation of cleavage of the treated egg corresponds with a time of an elimination of second polar body. In this experiment, since the displacement was done before the formation of the mitotic apparatus. Therefore, the mitotic apparatus rapidly formed, could not be thought to be an original mitotic apparatus itself. Accordingly, the mitotic apparatus described above might be established by using materials of original meotic spindle, of course, partly by using new materials for mitosis in the adjustment of new circumstance.
In the previous paper, The writer (1967) inhibited the formation of mitotic apparatus using phenyl urethan as a mitotic inhibitor. In the recovery, it became clear by the writer, that the formation of the mitotic apparatus depended upon endoplasmic conditions proceeding in the egg, without relation to the inhibition of the mitotic apparatus formation. Accordingly, in the present experiment, the formation of the mitotic apparatus was achived in close connection with en- doplasmic condition proceeding autonomously without affection of centlifugation. In responce to endoplasmic condition, mitotic apparatus seems to be formed haleidoscopically, either rapid or slow.
Collectively speaking, it becomes clear that the mitotic apparatus is thought to be newly
formed, using displaced components and new one, into the larger form for cleavage.
CONCLUSION
Considering the data obtained, cytokinesis is established by two major processes; one is furrowing, contraction of superficial cytoplasm encircling prospective furrow region, and the other, cross wall formation between two dividing daughter blastomeres.
Up to the present, many investigations concerning the structural difference in the superficial cytoplasm of prospective furrowing region have been made. As shown in the present paper, it is now considered that the superficial cytoplasm grows firm displaying the structure of the gel, comming close to the period of furrowing. These changes are surely determined under the action of mitotic apparatus, consisting of a enormous number of microtubles or filaments. Albert et al (1989) postulated that first visible sign of cleavage in animal cells was a puchering and a furrowing of the cortex during anaphase. Concerning this, by pressing an fertilized oyster eggs between two cover slips just prior to polar lobe formation, the writer obtained an evidence of puchering action of cortex. As shown in Fig. 4, M-O, there appeared several lines of fold radiating outward from deepening furrow. This means, that there appears some internal force drawing the cortex of furrowing region, and exists an apparent tension lines as a result of a possible contraction of inner pulling force. This is evidently a fact, comprising the puchering actions.
According to Bruce et al (1989), most animal cells possess a dense network of actin filaments and associated proteins, just beneath the plasma membrane. This network constitutes cell cortex which gives mechanical strength to the cell surface, and enables the cell to change up shapes dynamically. Concerning this, it has been shown that microfilaments are gathered to the region of the prospective furrow as a girdle with an advance of cell division (Bray, 1986). By contraction of these microfilaments, the first step of cell division seems progressively to put motion. Bonder et al. (1988) showed clearly the presence of an action of microfilaments in the furrowing cortex, using fluorescent labelling method. Beams and Kessel (1976) reported cortical ring of micro- filaments around the cleavage furrow of Zebra fish. As shown in the above, it is evident that superficial cytoplasmic layer, where furrowing takes place, becomes firm as the nature of the gel, by the end of anaphase. The writer ascertained the gelation of furrowing region in the severEd kinds of animal cells, using mainly micrurugical technique and some other methods. Accordingly, the writer's experiments are thought to contribute to analyze the structure of the furrowing region and to infer the mechanism of cell division, before the period of an advancement of recent studies.
The second step of cell division is the formation of partitioning membrane, between two
daughter cells before the completion of cell division. Evidence for new membrane formation came
from the careful studies of the staining, by using the echinodermal and amphibian eggs. The first
researcher, who found out new cell membrane formation was Motomura (1951), the writer's
professor when he was the research associate in T6hoku University. Motomura advocated new
cell membrane (diastema) between two cleaving blastomeres prior to first cleavage. He observed
the behavior of basophilic or silver stained granules, that they were gradually gathered toward
prospective plane of partition. And, he ascertained, that these granules would probably be the
materials for membrane formation. Moreover, by using his new fixative containing cadomium iodide and the Lugol's solution, and by staining them with acidulated Alucian Blue, he (1967) propagated his opinion about the formation of diastema. According to him, it was formed by assembling the granules of mucosubstance at the equatorial plane prior to the advancement of the furrowing. These granules form the row of vacuoles at the equator by the swelling. The row of vacuoles thus produced, provides the pathway of furrowing and forms the new cell membrane between the daughter blastomeres, by the fusion of those vacuoles.
An evidence of new membrane formation came from the studies on cleavage of frog zygote (de Latt and Bluemink, 1974). According to them, the pigment granules originally found on the egg cortex were not recognized in deepening furrow. This means the presence of partitioning membrane. Byer and Armstrong (1986) also found the new cell membrane, using auto- radiographic patterns of membrane protein in fertilized Xenopus egg during cleavage. In their studies, it became evident that leading edge of cleavage furrow was heavily labeled, and the space between base of furrowing and leading edge consisted of a large area, without radioisotope incorporation of the original egg surface materials. According to the text book written by Gilbert et al. (1991), the membrane at the leading edge of furrowing is derived from pre-existing radiolabeled outer surface of the cortex, but most of the partitioning membrane derive from the other regions that are inaccessible to surface labelling. This was geometrically imagined an increase at least in partitioning plane between two daughter cells. The increase of an extent of new membrane may be partly supplied by the stretching of cell surface, or assembly of new membrane forming materials onto the membrane newly formed.
Itwas noted by Browder (1980), that this new membrane was readily observed since it was unpigmented area just beneath the cortex, whereas the cortex contained pigment granules. This was also clearly photographed in the cleavage plane of a newt egg during cleavage (Selman and Perry, 1970).
The writer's leaflet structure found in orthopteran spermatocyte division (1992), and pseudo- cleavage described in this paper, are surely considered as the real evidence of the presence of new cell wall during cell division.
When the process of cytokinesis is viewed from the inside looking outward, or from the outside looking inward, the answer will be found different. Namely, from looking inward, the contraction of cell surface is a main motive force of cytokinesis, however, from looking outward, it will be performed, depending upon the coalescense of granules or vacuoles present in a cell equator.
Hence, collectively speaking, cytokinesis is accomplished by the collaboration of the contraction of cell surface (with deepening furrow), and the establishment of new cell membrane (with assembling granules or vaculoes being destined to membrane formation). Both processes are performed in close connection with the function of mitotic apparatus.
EXPLAN ATION OF FIGURES
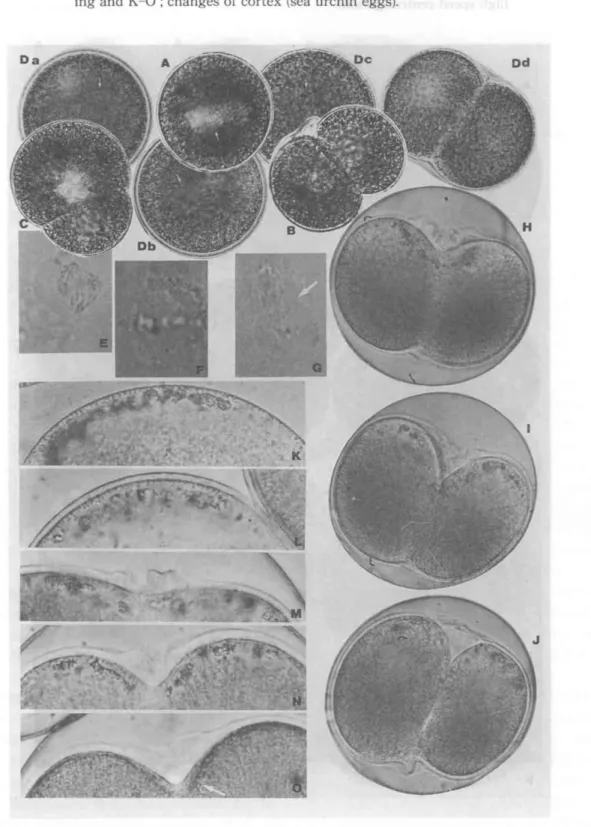
Fig.2 Photomicrographs showing an effect of podophyllin on fertilized eggs: (A) - (C), Caecella
chinensis ; D
a -D
d,sea urchin,
H.pulcherrimus. (A), Clear spot appearing in the central part of the
egg, five minutes after the treatment. Exposure was begun during the completion of meiotic
divisions. (B), Equal cleavage seen later. (C), Unequal cleavage, taking place in the treated egg,
when the exposure was begun later than (A). (D
a ) -(D
d),Disintegration process of mitotic apparatus (accomplished) in sea urchin egg. (D
d ),Cleavage, when fertilized egg was exposed to reagent after anaphase. (E) - (G) Micrurugical treatment on grasshopper spermatocyte. (E) - (F), Disintegration of cell surface punctured at metaphase and anaphase. (G), Disintegration, sto- pped at the cell equator (pointed by an arrow). In this case, a polar tip of the cell was punctured in early anaphase. (H) - (0), Centrifugation on fertilized sea urchin, eggs after treatment of hypotonic sea water. (H) -
(J),Process of cleavage of the eggs centrifugalized before metaphase.
(K) - (0), Photomicrographs showing the changes of superficial cytoplasm. The eggs were centrifugalized before fertilization, then inseminated. (K), Centripetal portion, photographed
10minites after insemination. (L), Formation of hyaline layer. (M) - (0), Process of furrowing. Note, the changes of thickness of cortex, taking place in the furrowing region.
Fig.3 Centrifugation experiments on fertilized eggs of a sea urchin,
H.pulcherrimus. The eggs in the various stages were centrifugalized after treatment of hypotonic sea water. (A), Appearance of cleavage furrow in the original position, where mitotic apparatus was once present. Centri- fugation was done beyond early anaphase. The mitotic apparatus completed, was displaced into hyaloplasmic layer (uppermost layer of centrifugation). (B), After a while, the furrow was induced by displaced mitotic apparatus (secondary furrow).
(C) -(D), Photomicrographs show- ing the original and the secondary induced (vertical direction) furrow. Centrifugation was done as same as (A) (sectioned and stained preparation). (E), Appearance of fine granules, arranged in a plane, across the equator of mitotic apparatus (darkly stained portion). These granules are probably the precursors of new cell membrane.
(F) -(G), Two cell stage, partitioned by the original furrow. (G), Two daughter nuclei are recognized in one blastomere (right side, as shown in an arrow). (H) - (L), Successive cleavage of centrifugalized unfertilized eggs
(8,000r.p.m. for several minutes). (H) -
(J),First cleavage. Mitotic apparatus was formed close to the centripetal side, in parallel with long axis of stretched egg. Later the egg was divided into one large and one slightly small transparent blastomere. (K), Second cleavage. The transparent blastomere (upper side) stood still, never cleft. (L), Third cleavage.
Fig. 4 (A) - (H), Photomicrographs showing cleavage inhibition, by denatured fertilization membrane. The fertilized sea urchin eggs just after insemination, were exposed to sea water containing Congo red for a short period of time, then transfered to ordinary sea water
(H.pulcherrimus). (A), Amphiaster stage. Note, partial elevation of fertilized membrane, therefore,
stretching of egg toward the direction of spindle axis is inhibited. (B) - (C), Inhibition of
furrowing, owing to wrapping by a tight membrane. (D), Cleavage of an egg having small extent
of privitelline space. (E), Complete cleavage inhibition. Perivitelline space is poorly formed. (F)
- (G), Karyokinesis without cytokinesis. Because of the extent of perivitelline space poorly
formed, cleavage is completely blocked by the inhibition of stretching of the egg. (H), Partition-
ing septum formation, suddenly and rapidly established by the rupture of tight membrane. (I)
- (K), Inhibition of gastrulation under restricion of tight membrane. Successive stages showing
behavior of mesenchymes, projecting their pseudopodia toward an animaml pole. (M) - (0),
Photomicrographs showing an effect of pressure on the oyster egg during cleavage. The egg
was pressed between two cover slips during an initiation of polar lobe formation. (M) - (N), Successive deepenings of furrow toward a vegetal pole. Note, inhibition of polar lobe formation.
(0), Superficial lines of fold toward the prospective furrowing region (comprising an actual contraction of the cortex).
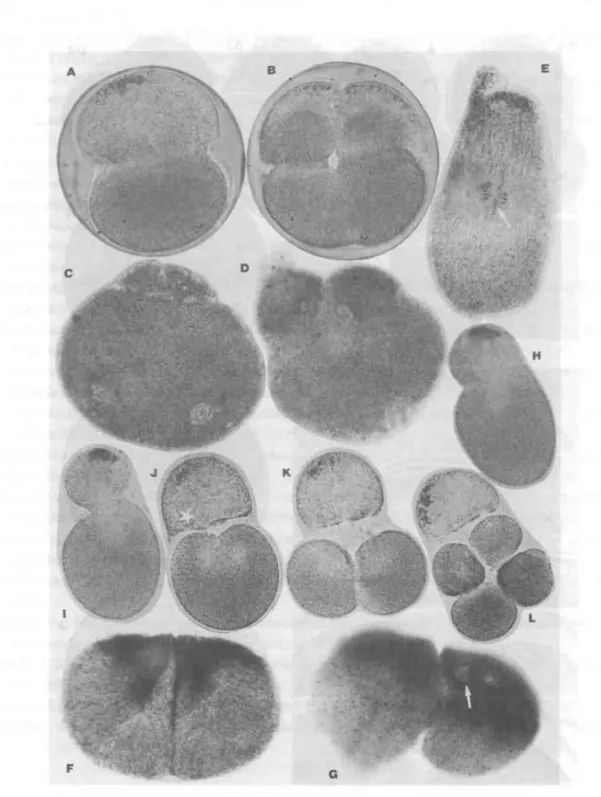
Fig. 5 Photomicrographs showing egg protuberance fOJ;mation on egg surface, and its develop- ment. Unfertilized eggs of sea urchin,
H pulcherrimus,
just after insemination, were exposed to sea water containing crude methylen blue for a short period of time, then transfered to ordinary sea water. (A), A protozoan individual, moving around egg surface in ordinary sea water. (B), In this condition, when some drops of this reagent were added, it was pushed inward under precipitation jelly coat (an arrow). (C) - (E), Process of the formation of egg protuberances by the contraction of jelly coat. (F), Cleavage, seen in the egg side. (G), Cleavage, ocurring between egg and protuberance. Most of the egg content was gashed out into the protuberance, involving constituents of the mitotic apparatus between the two spheres. (H) - (I), Successive changes, showing cleavage in the egg protuberance. The egg protuberance was divided through a flat plane of newly formed membrane (an arrow).(J) - (K), Formation of furrow in the original egg side (constituting cortex alone), remaining under the fertilization membrane. Most endoplasm flew out into the protuberance. (L) - (0), Pseudo~cleavage,occurring in fertilizedCaecella
eggs, centrifugalized just after germinal vesicle breakdown. They were transfered to ordinary sea water and reared. (L) - (N), Giant polar body formation by the pseudo-cleavage. (0), Elimination of second polar body (inversed). (P) - (S), Advance of development of centrifugalized eggs described above. Note, the giant polar body left under developing embryos.REFERENCES
Alverts, B. and others 1989 Molecular Biology of the Cell. Garland Publishing, Inc. N. Y
Beams,H.W. et al. 1976 Cytokinesis. A comparative study of cytoplasmic division in animal cells.
Am. Sci., 64 ;274-290
Blose, S. H. 1979 Proc. Natl. Acad. Sci. USA.76 ;3372-3376
Bray, D.
J.. J.
Heath andJ.
Moss 1986 The membrane associated 'cortex' of animal cells.J.
Cell. Sci., Supp!, 4; 71-88Browder,L. W. 1980 Developmental Biology. Saunders College, Philadelphia.
Byers, T.
J.
and P. B. Armstrong 1986 Membrane protein redistribution duringXenopus
first cleavage.J.
Cell. BioI. 102; 2176-2184Chambers, R. 1938 Behavior of cell surface during cleavage V.
J.
Cell and Compo Physiol. 12;149 Conn,H.J.
1961 Biological Stain. The Williams and Wikkens Company.Cornman,I.and M. E. Cornman 1951 The action of podophyllin and its function on marine eggs.
Annals of N. Y. Acad, Sci.51; 1443-1481
Dan, K 1943 Behavior of cell surface during cleavage V. Perforation experiment. ]. Fac. Sci., Tokyo Univ. Ser V. 6 ; 297
Dan, K 1952 Cyto-embryological studies of sea urchins IT. Blastula stage. BioI. Bull 102 ; 74-89 Dan,K.andK.Okazaki 1956 Cyto-embryological studies of sea urchin III. Role of the secondary
mesenchyme cells in the formation of primitive gut in sea urchin larvae. BioI. Bull,
110; 29-42De Latt, S. W. and]. G. Bluemink
1974New membrane formation during cytokinesis in normal
and cytochalasin B treated eggs of Xenopus laevis. ]. Cell. BioI.,
60 ; 529-540Gunning, B. E. S.
1985Prophase bands, phragmoplasts and spatial control of cytokinesis. J. Cell Sci. Suppl.
2; 157-179Gustafson, T. and H. Kinnander
1956Microaquaria for time-laps cinematographic studies of morphogenesis in swimming larvae and observations on sea urchin gastrulation. Exptl. Cell Research, 11 ;
36~51Hardin, ]. D. and
L.Y. Cheng,
1986The mechanism and mechanics of archenteron elongation during sea urchin gastrulation. Dev. BioI.
115; 490-501Hardin, ].
1988The role of the secondary mesenchyme cells during sea urchin gastrulation by laser ablation. Development,
103; 317-324Hiramoto,
Y.1956Cell division without mitotic apparatus in sea urchin egg. Exptl. Cell Research,
11 ; 630-636Hiramoto, Y.
1981How chromosome moves? (in Japanese). Sci. Asahi, Aug.;
26-36Langanger,
B.et al.
1986The molecular organization of myosin in stress fibers of cultured cells.
]. Cell BioI.,
102 ; 200-209King,
L.S. and M. Sullivan
1946The similarity of the effect of podophyllin and colchicine and their use in the treatment of condylomata accuminata. Science,
104 ; 244-245Mabuchi, 1.
1973A
myosin~likeprotein in the cortical layer of the sea urchin egg. J. Cell. BioI.
59;542-547
Mabuchi, 1. and M. Okuno
1977The effect of myosin antibody on the division of starfish blastomeres. ]. Cell BioI.
74 ; 251-263Mazia, D.
1961The Cell. Biochemistry, Physiology, Morphology edited by ]. Brachet and A. E.
Mirsky. Academic press, N.
Y.Marsland, D.
A.1951The mechanism of cell division. Hydrostatic pressure effects upon dividing egg cells. ]. Cell. Compo Physiol.,
17 ; 15-22Motomura, 1.
1946Modification of the position of the embryonic axis in the ultra-centrifuged eggs of sea urchin. Seibutsu,
1 ; 193-200Motomura, I.
1950Studies of cleavage V. The role of vacuoles in the cleavage plane formation in sea urchin eggs. Sci. Rept. Tohoku Univ. (BioI),
18; 255-261Motomura, 1.
1967Formation of diastema in the cleaving egg of the sea urchin. Sci. Rept of Tohoku Univ. (BioI.)
33 ; 135-142.Marray,
A.and J. W. Szostak
1985Chromosome segregation in mitosis and meiosis. Annu. Rev.
Cell BioI.;
1,289-315Rappaport, R.
1986Establishment of the mechanism of cytokinesis in interphase and mitotic cells.
]. Cell BioI.
43 ; 35-47Schroeder, T.
E. 1973Actin in dividing cells: Contractile ring filaments bind heavy meromyosin.
Proc. Natl. Acad. Sci. USA.
70; 1688-1692Selman, G. and M. Perry
1970How cell cleave? New-Scientist,
46 ; 12-14Singal, P.
K.and
E.J. Saunders
1974An ultrastructural study of the first cleavage of Xenopus
embryo. J. Ultrastruct. Res.,
47; 433-451Sullivan,
T.D. and H. J. Wechsler 1947 The cytological effect of podophyllin. Science, 105; 433 Swann,
M. M.and J. M. Mitchson 1958 BioI. Revs. Cambridge Phil. Soc., 33; 103
Tolle, H., G. Weber and M. Obson 1987 Keratin filament disruption in interphase and mitotic cells.
J. Cell BioI., 43 ; 35-47
Yamamoto, M. 1957 The effect of injury on spermatocyte of grasshopper with a micro needle. Sci.
Rept. Tohoku Univ. (BioI.) 23 ; 59-62
Yamamoto, M. 1964 The effect of high temperature on cell division of grasshopper spermatocyte.
Ibid., 30 ; 179-186
Yamamoto, M. 1964 Note on effect of centrifugal force on sea urchin egg, with special reference to position of mitotic apparatus and formation of furrow. Ibid., 31; 187-195
Yamamoto, M. 1965 The effect of Congo Red on development of sea urchin egg with special reference to cell division and gastrulation. Ibid. 31 ; 45-53
Yamamoto, M. 1965 An experimental studies on cleavage of sea urchin egg by means of egg protuberance. Ibid. 31 ; 55-62
Yamamoto, M. 1965 Some observations of effect of podophyllin on the eggs of a bivalve, Caecella chinensis. Ibid., 24 ; 63-66
Yamamoto, M. 1971 Modification of position of maturation spindle by centrifuging the fertilized eggs of a bivalve, Caecella chinensis. Bull. Mar. BioI. 14 ; 109-115
Yamamoto, M. 1972 Studies on the mechanism of cleavage in the eggs of oyster, Crassostrea gigas.
Mem. ColI. Edu. Akita Univ., 22, 15-18
Yamamoto, M. 1971 Induction of pseudocleavage by centrifuging the eggs of a bivalve, Caecella chinensis. Bull. Mar. BioI. Tohoku Univ. 14; 143-148
Yamamoto, M. 1973 Induction of pseudo-cleavage by centrifuging the eggs of a bivalve, Cecella chinensis. Bull. Mar. BioI. 14; 143-148
Yamamoto, M. 1974 Studies on the mechanism of meiosis on the polar body formation in Caecella eggs. Ibid. 15 ; 29-35
Yamamoto, M. 1975 Studies on the formation of the furrow by centrifuging the eggs of sea urchin,
H.pulcherrimus. Mem. ColI. Edu., Akita University, 25 ; 13-22 (Japanese)
Yamamoto, M. 1989 The staining of cortical cytoplasmic layer and the behavior of PAS stained granules in sea urchin egg. Mem. ColI. Edu. Akita Univ. 40; 9-14
Yamamoto, M. 1990 The process of fertilization in the eggs of sea urchin,
H.pulcherrimus.Ibid., 41 ; 63-67
Yamamoto, M. 1993 Studies on the mechanism of cell division (I). On the structure of mitotic apparatus and its role in cell division. Ibid., 44 ; 77-95
White, J.
G.and
G. G.Barisy 1983 On the mechanism of cytokinesis in animal cells. J. Theor. BioI., 101; 289-316
Wilson, E. B. 1987 The cell in development and Heredity. 3rd. ed. Macmillan Co. LTD., N.
Y.Wolniak, S.
M.1988 The regulation of mitotic spindle function. Biochem. Cell BioI. 66 ; 490-515
FIGURE