首都大学東京 博士(理学)学位論文(課程博士)
論 文 名 土壌栄養塩の空間的不均質性と競争が植物個体群内の個 体の成長に及ぼす影響に関する実験生態学的研究(英文)
著 者 中村 亮二
審査担当者
主 査
委 員
委 員
上記の論文を合格と判定する
平成 年 月 日
首都大学東京大学院理学研究科教授会 研究科長
DISSERTATION FOR A DEGREE OF DOCTOR OF SCIENCE
TOKYO METROPOLITAN UNIVERSITY
TITLE: Experimental studies on ecological effects of nutrient distribution pattern and competition on growth of individual plants in a population
AUTHOR: Ryoji NAKAMURA EXAMINED BY
Examiner in chief
Examiner
Examiner
QUALIFIED BY THE GRADUATE SCHOOL OF SCIENCE TOKYO METROPOLITAN UNIVERSITY
Dean Date
Experimental studies on ecological effects of nutrient distribution pattern and competition on growth of
individual plants in a population
土壌栄養塩の空間的不均質性と競争が植物個体群内の個体 の成長に及ぼす影響に関する実験生態学的研究(英文)
Ryoji Nakamura
Department of Biological Sciences Graduate School of Science Tokyo Metropolitan University
Minami-Osawa 1-1, Hachioji, Tokyo 192-0397, Japan
Tokyo Metropolitan University 2008
Contents
Summary
1–4 Chapter I General Introduction
5–16 Chapter II Root growth and plant biomass in Lolium perenne before the roots reach a nutrient-rich patch in soil
17–41 Chapter III Effects of nutrient distribution pattern and aboveground competition on size of individuals in Ipomoea tricolor populations
42–54 Chapter IV Effects of pattern and amount of nutrients on root placement and competition of morning glory, Ipomoea tricolor
55–72 Chapter V General Discussion
73–80 Acknowledgements
81–82 Literature cited
83–93 Summary in Japanese
94–96
Summary
Distribution of nutrients is spatially and temporally heterogeneous at various scales in soil.
Heterogeneous distribution of soil nutrients would control resource acquisition, growth, and even survival of the plants if the heterogeneous distribution occurs at the scale to which a single plant can respond. Thus, there have been many studies on ecological consequences for plants of the heterogeneous nutrient distribution since the 1990s. Our knowledge about the consequences, however, is restricted mainly at the individual plant level and the plant-plant interaction level, and we still know very little about population- or community-level effects. In this thesis, therefore, I have focused on the population-level effects of the heterogeneous distribution of nutrients.
Development of a plant population should be the assemblage of individual plant growth.
Thus, in this thesis, the growth of individual plants was examined in the population development under heterogeneous environments. In the process of population development, plants in a population can be categorized into four cases based on their experiences; access of plants to nutrient-rich patches and competition between neighbouring plants. The four cases are as follows; (case 1) before reaching the patches without competition, each plant individually explores soil; (case 2) after reaching the patches without competition, each plant would exhibit various responses to the patches and could have benefit from the patches; (case 3) before reaching the patches with competition, each plant individually explores soil and plant growth is somehow reduced by competition; (case 4) after reaching the patches with competition, each plant would exhibit various responses to the patches and plant growth is somehow reduced by competition.
Three experimental studies were conducted, and each study examined one of the four cases; plant growth and soil exploration by roots before reaching a nutrient-rich patch without
competition in chapter 2 (case 1), interactions between nutrient distribution pattern and aboveground competition affecting growth of higher- and lower-ranked plants in a population in chapter 3 (case 4), and effects of nutrient distribution pattern and competition on plant growth in different amounts of nutrients in chapter 4 (case 4).
In chapter 2, soil exploration by roots and plant growth in a heterogeneous environment were investigated to determine whether roots can selectively explore a nutrient-rich patch, and how nutrient heterogeneity affects biomass allocation and total biomass before a patch is reached. Lolium perenne L. plants were grown in a factorial experiment with combinations of fertilization (heterogeneous and homogeneous) and day of harvest (14, 28, 42, or 56 days after transplanting).
The plant in the heterogeneous treatment was smaller in its mean total biomass, and allocated more biomass to roots. The distributions of root length and root biomass in the heterogeneous treatment did not favour the nutrient-rich patch, and did not correspond to the patchy distribution of inorganic nitrogen. Specific root length (length/biomass) was higher and root elongation was more extensive both laterally and vertically in the heterogeneous treatment. These characteristics may enable plants to acquire nutrients efficiently and increase the probability of encountering nutrient-rich patches in a heterogeneous soil. However, heterogeneity of soil nutrients would hold back plant growth before a patch was reached. Therefore, although no significant selective root placement in the nutrient-rich patch was observed, plant growth before reaching nutrient-rich patches was different between heterogeneous and homogeneous environment.
In chapter 3, whether nutrient distribution pattern interacted with aboveground competition to affect plant size in Ipomoea tricolor populations was investigated. Six plants per pot were grown in a factorial experiment with combinations of heterogeneous or homogeneous nutrient distribution pattern and presence or absence of aboveground competition. Plants were harvested and ranked by their aboveground biomasses. In analyses of plant sizes in all ranks simultaneously, mean plant size was significantly affected by nutrient distribution pattern, aboveground competition, and their interaction. In analyses of plant sizes of each rank, aboveground competition affected plant size,
which was found in all ranks. Nutrient distribution pattern affected plant size in the higher ranks, but not in the lowest rank. Selective root placement into nutrient-rich patches under heterogeneous conditions was observed. Our results suggest that the magnitude of the effect of nutrient distribution pattern on plant size changed among ranks owing to changes in aboveground competition and size-dependent growth rate. Size-dependent growth rate could explain the significant effect of nutrient distribution pattern independent of the effect of aboveground competition. Nutrient distribution pattern would then interact with aboveground competition and consequently affect size structure in a population.
In chapter 4, an experiment was carried out to examine effects of the amounts of nutrients, nutrient distribution pattern and competition, on root placement, plant biomass and intensities of competition. The effects of nutrient distribution pattern and the intensity of competition would be prominent particularly in a low nutrient condition. In a low nutrient condition, growth of plants in a heterogeneous condition would, therefore, be larger than that in a homogeneous condition by means of selective root placement, and the intensity of competition would be severer in a heterogeneous condition than that in a homogeneous condition. A plant of Ipomoea tricolor in a pot was grown with or without neighbours in heterogeneous or homogeneous nutrient conditions at three different levels of amounts of nutrients. Root biomass in a nutrient-rich patch was larger than that in a nutrient-poor patch in heterogeneous conditions. Average plant biomass did not significantly differ between heterogeneous and homogeneous conditions that provided the same amount of nutrients. Plant biomass increased as increments of the total amount of fertilization, but decreased when plants had neighbours. An index of competition did not significantly differ between nutrient distribution treatments, but was larger under low-nutrient conditions than under high-nutrient conditions.
Selective root placement was strongly affected by nutrient distribution pattern. Growth of individual plants was, however, only marginally affected by nutrient distribution pattern because the total amount of nutrients and number of competitors determined the available amount of nutrients of an individual plant. Competition is inevitably intensified regardless of nutrient distribution when the
amounts of nutrients are limited. Therefore, the total amount of nutrients has more profound effects on growth of a plant than nutrient distribution pattern, which can be enhanced by competition, especially, in low nutrients.
Growth of individual plants was restrained when the plants had not reached the patches.
However, nutrient acquisition after reaching the patches could improve the growth of individual plants at various stages of population development. Access to the patches could also cause differences in size between neighbour plants under belowground competition. The size differences between neighbours would be increased by aboveground competition and size-dependent growth rates. Aboveground competition brought about obvious effects of nutrient heterogeneity especially on growth of higher-ranked plants. In addition, effects of competition on plant growth were intensified even under heterogeneous environments when the total amount of nutrient supply was limited. This suggests that population development in heterogeneous environments would depend on the total amount of nutrients. In conclusion, nutrient heterogeneity conspicuously interacts with both above- and below-ground competition to affect growth of individual plants in a population in heterogeneous environments, and nutrient heterogeneity would control advantages of the individual plants under competition in the population development.
Key-words: aboveground competition, amount of nutrients, belowground competition, nitrogen distribution, nutrient heterogeneity, population development, rank of plant size, root foraging, selective root placement
Chapter I
General Introduction
Distribution of nutrients is spatially and temporally heterogeneous at various scales in soil. Because plants are sessile, the heterogeneous distribution of soil nutrients would dominate resource acquisition, growth, and even survival of plants. Thus, ecological consequences for plants of heterogeneous distribution of nutrients have been intensively studied since the 1990s. Our knowledge of the consequences, however, is limited mainly at the individual plant level and the plant-plant interaction level, and we still know very little about population- or community-level effects. In this thesis, therefore, I have focused on the population-level effects of the heterogeneous distribution of nutrients.
In this chapter, concepts of heterogeneity were overviewed in the ecological context. The concepts included not only abiotic aspects, such as physical environments, but also biotic ones, such as species distribution. Then, concepts of nutrient heterogeneity were introduced and previous studies on its effects on plant growth were reviewed. Finally, I describe the theme of this thesis that focused on the population-level effects of the nutrient heterogeneity on growth of individual plants in a population.
1. An overview of heterogeneity concepts in ecology
Early concepts of heterogeneity in biogeography and protoecology
Heterogeneity is not a very novel concept in ecology. Studies of von Humboldt and other plant and animal geographers in the 19th century recognized distinct patterns in the distributions of species or community types (McIntosh 1991) and Humboldt described patterns of vegetation and climates. In
the mid-19th century that was the time of protoecology, distribution of organisms floating in water column (i.e., “plankton” since 1880s) had been studied, and a dispute about homogeneity of the spatial distribution of the plankton occurred among the protoecologists (McIntosh 1991). In the terrestrial ecosystems, Pound and Clements (1900) evaluated terrestrial vegetation with a quadrat to quantify its heterogeneity, and reported species abundance and its distribution mode. Clements sometimes advocated his views on the community as a homogeneous superorganism at least at the climax stage (McIntosh 1991), but was one of the founders of spatiotemporal heterogeneity concepts of environments and organisms at various scales.
An assumption of homogeneity in ecological studies
During the 1920s and 1930s, theoretical (especially mathematical) studies of population ecology assumed homogeneity and equilibrium of environments for simplification. As a result, “a view that ecological dynamics are played out in local habitats that are spatially homogeneous and temporally equilibrium rapidly gained force” at the beginning of the 1950s (Wiens 1999). Because theories provided frameworks for asking questions, empirical studies were constrained by the assumption of equilibrium and homogeneity. The view was widely accepted partly because the simplification was practical. In addition, there was a prevailing view in natural history or early ecology that nature was balanced and ordered. Consequently, the view assumed that the nature, or at least substantial aspects of it, was homogeneous (see also Wiens 1999).
A paradigm shift: an introduction of temporal and spatial variation into models
A few early ecologists had questioned homogeneity in a community in the sense of spatial distribution. The earliest examination of distribution of homogeneity in space using probability statistics was conducted in the 1920s (McIntosh 1991). A plant ecologist, H.A. Gleason, and a marine biologist, T Svedberg, showed that most organisms are non-randomly distributed and are most often aggregated or clumped (Clark and Evans 1954). The spatial heterogeneity that is the
spatiotemporal distribution of organisms had been known by the 1950s to affect profoundly on populations, interactions between species, and community properties or functions (McIntosh 1991).
In the mid-1960s and early-1970s, then, theoreticians introduced spatiotemporal variation into their models (e.g., MacArthur and Levins 1964, 1967; Levin and Paine 1974). Their results showed spatial heterogeneity allows a population to persist competing individuals and predators to coexist with their prey (e.g., Levin 1970). The spatial heterogeneity also enhanced local biodiversity (e.g., Levin and Paine 1974). For example, although a species is a weak competitor in a constant environment, the species may be good at colonizing or occupying gaps immediately after disturbances. The weak species may coexist with a strong competitor, if new gaps are frequently created (e.g., Paine 1979).
After the ‘rediscovery’ of heterogeneity in the 1990s, heterogeneity has been questioned more systemically than ever; definition of heterogeneity, the measurement and quantification of heterogeneity; questions of how environmental heterogeneity impacts upon organisms and ecological processes and how the organisms respond to environmental heterogeneity (e.g., Shorrocks and Swingland 1990; Kolasa and Pickett 1991; Caldwell and Pearcy 1994; Hutchings et al. 1999;
Levin et al. 1993; Rhodes et al. 1996; Tilman and Kareiva 1997).
2. Nutrient heterogeneity
Previous studies have shown that heterogeneous distribution of soil nutrients affects plant behaviour and performance in distinct ways, as compared with homogeneous distribution providing equal quantities of the resources (e.g., Shorrocks and Swingland 1990; Kolasa and Pickett 1991;
Caldwell and Pearcy 1994; Hutchings et al. 1999). If co-occuring plants perceive and respond differently to given types of the nutrient heterogeneity, this may affect their interactions, and influence population or community structure.
Fine scale nutrient heterogeneity in space
Assessing fine scale heterogeneity: quantifying spatial distribution pattern of soil resource
Scale is important when we focus on the nutrient heterogeneity (Kotliar and Wiens 1990; Sparrow 1999). Heterogeneity at population- and ecosystem-scale is synonymous with ‘environmental gradients’ (Sparrow 1999). Different responses by single organisms to these scales of the spatial heterogeneity are thus only differences in performances between spatially separated locations (Stewart et al. 1999). The nutrient heterogeneity at the fine scale to which an organism can respond is important because the fine scale heterogeneity would cause differences in growth, survival and reproduction between neighbour plants in the same location (Robinson 1994; Stewart et al. 1999).
Although the quantification of patterns and scales of the heterogeneity has been a developing field since the 1960s (Caldwell and Pearcy 1994), appropriate tools for detecting and quantifying spatial patterns are still unavailable. However, developments of geostatistical approaches such as semivariance analysis enabled us to assess scale and structure of variability (e.g., Webster 1985) or biological processes such as species composition in plant communities (e.g., Jackson and Caldwell 1993b).
The distribution of various resources in soil even at centimeter-scale was clarified by the geostatistical techniques. For example, a combined index of soil fertility incorporating information on soil ammonium, nitrate, phosphate and potassium showed strong autocorrelation at scales of less than 1 metre (Jackson and Caldwell 1993a,b). The spatial heterogeneity in soil resources at the scales also occurs in various plant communities, such as sagebrush steppe (Jackson and Caldwell 1993a,b), deciduous woodland (Farley and Fitter 1999), upland hardwood forest (Gross et al. 1995), desert (Schlesinger et al. 1996), tropical forest (Gonzalez and Zak 1994), warm-temperate conifer forest (Lister et al. 2000).
Definition of a patch
Although nutrient concentration varies at large extent, the localized peak of nutrient concentration in soil is usually called a ‘patch’ or ‘nutrient-rich patch’, and the background a ‘nutrient-poor patch’ or simply ‘background’. Fitter (1994) firstly pointed out the difference between the nutrient-rich patches that vary spatially and the patches that vary temporally. The spatial or temporal patches are characterized by three attributes: distribution (spatial pattern and temporal predictability), extent (size and durability), and number (abundance and frequency). In addition, contrast (the degree of difference between patch and matrix, sensu Kotliar and Wiens 1990) or quality (sensu Wijesinghe and Hutchings 1999) is also an important aspect of patch attributes.
Effects of spatial nutrient heterogeneity on plant performance
The effects of nutrient heterogeneity on single plants
A single plant can respond to nutrient-rich patches with alteration of attributes. The attributes such as root growth, nutrient uptake rates, root:shoot interactions, and whole-plant growth have been studied (see also reviews in Fitter 1994; Fitter et al. 1999; Robinson 1994; Hutchings et al. 1999; Hodge 2004). Plastic responses by roots have been considered as the principal mechanism by which plants cope with heterogeneous supplies of nutrients in soil. When roots encounter a nutrient-rich patch, for example, they often grow and proliferate within it. Previous studies showed that morphological plasticity of roots is a phylogenetically and taxonomically conserved trait within a wide range of angiosperm species (Kembel and Cahill 2005). Roots experiencing nutrient-rich patches are also known to enhance their physiological ion-uptake capacities compared with other roots of the same plant outside the patches.
It is still unclear how the root proliferation within nutrient-rich patches enhances nutrient acquisition and thus plant growth in heterogeneous soil, as compared with the plant growth in
homogeneous soil providing the same amount of nutrients. Some studies supported the growth increment in heterogeneous soil (e.g., Drew and Saker 1975; Birch and Hutchings 1994), but others did not (see also Robinson 1994; Kembel and Cahill 2005). Thus, foraging behavior of single plants should be compared before and after encountering nutrient-rich patches.
The effects of nutrient heterogeneity on a population
Compared with researches on a single plant, studies about effects of nutrient heterogeneity on plant populations are still limited. Recent studies suggest that nutrient heterogeneity can affect interactions between plants, and thus size structure. Then productivity of plant populations or communities is also affected (e.g., Casper et al. 1999; Day et al. 2003a,b). Since roots can proliferate in nutrient-rich patches, nutrient heterogeneity causes spatial aggregation of root systems and severe belowground competition (Caldwell 1987; Casper et al. 1999; Day et al. 2003c). Plants with roots in nutrient-rich patches are advantageous to neighbouring plants with roots in nutrient-poor patches, and to plants with less obvious root responses to the patches (e.g., Hodge et al. 1999; Robinson et al. 1999;
Fransen et al. 1999). As a result, differences in patch availability between plants cause size inequalities in a population (Facelli and Facelli 2002; Day et al. 2003a). Shoot size inequalities in monocultures of the two grasses Festuca rubra and Anthoxanthum odoratum actually tended to be greater under heterogeneous soil than homogeneous soil (Fransen et al. 2001).
Populations grown under heterogeneous conditions with a patchy nutrient supply produced more biomass than populations grown under homogeneous conditions even though the same amount of nutrient is supplied (e.g., Facelli and Facelli 2002; Day et al. 2003a,b; Maestre and Reynolds 2006a). For example, Day et al. (2003a) showed that Cardamine hirsuta populations grown under heterogeneous conditions achieved greater yields than populations in homogeneous conditions after 31 days of growth. The increment of population yield in a heterogeneous soil is mainly explained by the following two scenarios. Firstly, plants in a population under heterogeneous conditions could proliferate their roots in nutrient-rich patches and thus acquire nutrients more
efficiently than plants under homogeneous conditions (Day et al. 2003a). Secondly, because root mass in nutrient-poor patches is less than that in the same place under homogeneous conditions, the belowground competition under heterogeneous conditions may become less severe compared to that under homogeneous conditions in the entire environment that includes all nutrient-rich and -poor patches (Day et al. 2003b).
Population productivity and population structure are also altered by interaction between nutrient heterogeneity and other abiotic factors (Maestre and Reynolds 2006a). Maestre and his colleagues conducted microcosm experiments to evaluate simultaneously the effect of several factors, such as nutrient heterogeneity, the atmospheric concentration of carbon dioxide, and the amount of nutrients: the effects of nutrient heterogeneity were pronounced under elevated CO2 concentration in a population of Plantago lanceolata and under higher total amounts of nutrients in populations of Plantago and Holcus lanatus (Maestre and Reynolds 2006a; Maestre et al. 2007).
The effects of nutrient heterogeneity on a community
Only a few studies investigated effects of nutrient heterogeneity on plant community. Recent experiments have predicted that nutrient heterogeneity would affect many aspects of a community because co-occurring species often differ in their abilities to respond to patchily distributed nutrients (Campbell et al. 1991; Einsmann et al. 1999; Wijesinghe et al. 2001) and thus in their growth (Bliss et al. 2002; Wijesinghe et al. 2005). Actually, nutrient heterogeneity significantly affected community structure, productivity, species composition, and species richness (e.g., Wijesinghe et al.
2005; Maestre et al. 2005, 2006; Maestre and Reynolds 2006b, 2007a,b). However, the effects of nutrient heterogeneity on a plant community are not fully revealed because we still do not know well about growth of individual plants in a community under heterogeneous environments.
3. Scope of this thesis
Growth of individual plants in a population in a heterogeneous environment
A process of population development under a heterogeneous environment
Growth of individual plants in a developing population under heterogeneous environments should be examined because the population development is the assemblage of each plant growth. Our knowledge on population developmental processes under heterogeneous environments is very limited though we have already known that yield and structure of a population differed between heterogeneous and homogeneous environments (Day et al. 2003a,b). Therefore, it is still unclear how differences in population yield and structure would occur.
Four cases of plant growth through population development in a heterogeneous environment
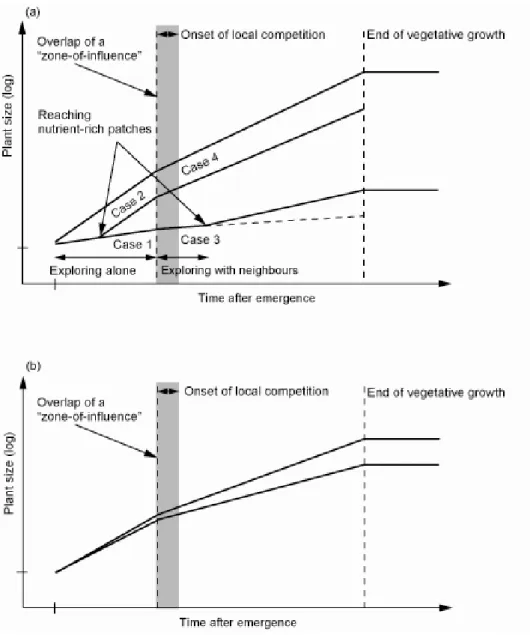
Growth of an individual plant in a developing even-aged population under a heterogeneous environment can be characterized in a diagrammatic form (Fig. 1-1a). Given that individual plants are distributed in a regular pattern in a population, each plant can potentially reach a nutrient-rich patch and the differences in timing to reach the patches could depend on patch distribution and soil exploration by roots (Hutchings et al. 2003). It is critical for each plant whether or not to reach the patches, because the plant with its roots in the patch would grow better (Birch and Hutchings 1994).
Growth of individual plants in a population also depends on whether or not competition occurs (Fig. 1-1). Interaction between neighbours affects plant growth after “zones of influence”
(sensu Casper et al. 2003) have overlapped (see also Nagashima 1999). After then, the growth of plants is somehow restricted, and the interactive effects result in an altered population development
(reviewed by Hutchings 1997).
In this thesis, I proposed that access to nutrient-rich patches and competition are important for plant growth in a population. Therefore, we can categorize the growth of individual plants in a developing population in a heterogeneous environment into four cases (Fig. 1-1a): (case 1) before reaching the patches without competition, each plant individually explores soil; (case 2) after reaching the patches without competition, each plant would exhibit various responses to the patches and could have benefit from the patches; (case 3) before reaching the patches with competition, each plant individually explores soil and plant growth is somehow reduced by competition; (case 4) after reaching the patches with competition, each plant would exhibit various responses to the patches and plant growth is somehow reduced by competition.
Evaluation of previous studies on the four cases
In case 1, we should understand how plants explore soil nutrients. Because most soil nutrients exist in patches, roots within the patches are important for nutrient acquisition and, consequently, plant growth (Hutchings et al. 2003). Our knowledge about root behaviour “before” reaching nutrient-rich patches is very limited though there are many researches about root responses to the patches “after”
reaching them (i.e., in case 2, see also review Hodge 2004).
Competition of the case 3 plants seems to be similar to competition under a homogeneous nutrient-poor environment that has been intensively examined (e.g., Harper 1977), except for soil exploration. The soil exploration by a plant in case 3 is important for its own growth, or even its survival. When a plant in case 3 reaches the patches, the plant is then categorized in case 4. If the plant has reached the patches in advance of neighbours, the plant occupies at least a part of a patch (Raynaud and Leadley 2004) and monopolizes its nutrients (Scwinning and Weiner 1998).
Effects of the interaction between aboveground competition and nutrient heterogeneity on a plant in case 4 are not fully understood especially in quantitative ways. Aboveground competition is pointed out to affect foraging behaviour of plants in a population under heterogeneous
environments (Jackson and Caldwell 1992). The aboveground competition reduces growth of plants through reduction of available light. The light reduction would alter morphological and/or physiological root responses of plants to the patches (Jackson and Caldwell 1992; Bilbrough and Caldwell 1995; Huante et al. 1998). The root responses should be also affected by nutrient heterogeneity. In addition, mode of aboveground competition can be asymmetric even in a heterogeneous environment, because large plants would disproportionally suppress growth of smaller plants in a population (Weiner 1990). Studies to date, however, have mainly focused on effects of belowground competition rather than aboveground competition (e.g., Hodge et al. 1999;
Robinson et al. 1999).
The total amount of nutrients would be important for the growth of plants especially in case 4. Therefore, we should examine how the total amount of nutrients alters effects of nutrient heterogeneity on plants because plant responses to nutrient-rich patches would be different depending on the total amount of nutrients (Lamb et al. 2004). In addition, competition would be more intense in a nutrient-poor environment than that in a nutrient-rich environment (Tilman 1987, 1988; Tilman and Pacala 1993; Cahill 1999; Pugnaire and Luque 2001). However previous studies on the competition effects on plant growth in a heterogeneous environment included only one nutrient level (e.g., Bliss et al. 2002; Day et al. 2003c).
Researches in this thesis
I have examined the effect of nutrient heterogeneity on plant growth in this thesis. In chapter2, differences in root growth and root characteristics were compared between heterogeneous and homogeneous conditions to evaluate soil exploration by roots especially before the roots reach a nutrient-rich patch. In chapter 3, interactions of nutrient heterogeneity and aboveground competition were examined. In chapter 4, interactions of nutrient heterogeneity, above- and belowground competition, and amounts of nutrients were investigated.
An experimental approach
In this thesis, three garden experiments were carried out because nutrient heterogeneity is too complex to be controlled under natural conditions. The garden experiments allow us to evaluate effects of nutrient heterogeneity on plant growth under the equivalent environments except for the nutrient heterogeneity. Then, the garden-experiment approach is a better way when we try to understand some specific issues that are parts of the ecological dynamics in nature (Townsend et al.
2003).
In my researches, the same total quantity of nutrients is provided in homogeneous patterns and as well as heterogeneous patterns, so that I can distinguish effects of patchy nutrient supply and quantitative effects of nutrient amounts (see also Wijesinghe and Hutchings 1997, 1999). However, not all studies have adopted this approach to the nutrient heterogeneity. Some authors added extra nutrients to nutrient patches over homogeneous background supply (e.g., Robinson 1994), which is inappropriate to compare plant growth in a heterogeneous condition with that in a homogeneous condition because the total amounts of nutrient supply are different between them.
Moreover, almost all studies mentioned in chapters 2, 3 and 4, including my own experiments, have evaluated effects of nutrient heterogeneity by “biomass” of plants rather than their
“fitness”. Although survivorship and fecundity tend to correlate closely with plant biomass or a
“size” of a plant (e.g., Solbrig 1981), a more detailed experiment of nutrient-heterogeneity effects on the fitness should be carried out.
Figure 1-1 A schematic diagram of plant growth in an even-aged population under a heterogeneous (a) or a homogeneous (b) environment. In the heterogeneous environment, limited amounts of soil nutrients exist in a patchy manner and a plant can respond to the scale of nutrient patches. In the homogeneous environment, soil nutrients evenly exist in the scale at which a plant can experience. A solid line represents growth of each plant individual in the population. Plants are assumed to distribute in a regular pattern in the population. Local competition between neighbouring plants begins after the “zone of influence” has overlapped. For more details, see the text in chapter 1.
Chapter II
Root growth and plant biomass in Lolium perenne exploring a nutrient-rich patch in soil
Abstract
We investigated soil exploration by roots and plant growth in a heterogeneous environment to determine whether roots can selectively explore a nutrient-rich patch, and how nutrient heterogeneity affects biomass allocation and total biomass before a patch is reached. Lolium perenne L. plants were grown in a factorial experiment with combinations of fertilization (heterogeneous and homogeneous) and day of harvest (14, 28, 42, or 56 days after transplanting). The plant in the heterogeneous treatment was smaller in its mean total biomass, and allocated more biomass to roots.
The distributions of root length and root biomass in the heterogeneous treatment did not favour the nutrient-rich patch, and did not correspond to the patchy distribution of inorganic nitrogen. Specific root length (length/biomass) was higher and root elongation was more extensive both laterally and vertically in the heterogeneous treatment. These characteristics may enable plants to acquire nutrients efficiently and increase the probability of encountering nutrient-rich patches in a heterogeneous soil. However, heterogeneity of soil nutrients would hold back plant growth before a patch was reached. Therefore, although no significant selective root placement in the nutrient-rich patch was observed, plant growth before reaching nutrient-rich patches differed between heterogeneous and homogeneous environments.
Key-words: morphological plasticity, nitrogen distribution, nutrient heterogeneity, plant growth, root proliferation
Introduction
The distribution of soil nutrients is patchy. When roots encounter a nutrient-rich patch, they proliferate within it, and often show various morphological responses and enhanced nutrient uptake capacities relative to roots of the same plant elsewhere (Hodge 2004). These plastic responses are thought to contribute to efficient nutrient acquisition from heterogeneous environments (Hodge 2004). In some cases the growth of a plant is greater when nutrients are spatially heterogeneous rather than homogeneous (e.g., Birch and Hutchings 1994). Moreover, nutrient acquisition from nutrient-rich patches is important in competition for limited soil resources (Hodge et al. 1999;
Robinson et al. 1999).
To acquire nutrients from a rich patch, roots first have to reach the patch, otherwise growth of the plant will be restricted. Whether roots can reach a nutrient-rich patch depends on the size of the root system and its initial distance from the patch (Wijesinghe et al. 2001; Hutchings et al.
2003). Active root responses such as rapid root placement within a nutrient-rich patch (Weiner 1990) and the ‘precise forager’ strategy (Campbell et al. 1991; Einsmann et al. 1999; Rajaniemi and Reynolds 2004) could improve nutrient acquisition under competition. Therefore, it is important to focus on the process of exploration by roots before they reach a nutrient-rich patch.
Compared with the plastic root responses within a nutrient-rich patch, we do not know much about soil exploration before roots have reached such a patch. Several studies, however, suggest that roots can actively explore a patch by means of nutrient gradients around it (e.g., Jackson and Caldwell 1993a). New roots, for example, can develop in response to the perception of soil nutrients (Malamy 2005; see also Zhang and Forde 1998). In an experiment with the weedy annual Abutilon theophrasti, many plants placed many of their roots in the same nutrient-rich patch and drew nutrients from it, and their roots initially tended to grow radially but not evenly (Casper et al.
1999, 2003). These results suggest that the plants grew their roots selectively to nutrient-rich patches.
Additionally, root proliferation within a nutrient-rich patch reduced the growth of roots outside the patch (Drew et al. 1973; Robinson 1994), probably because of asymmetric translocation of assimilates within the plant. Limited carbon investment in roots also alters the morphological responses of roots to a nutrient-rich patch (e.g., Huante et al. 1998; Bilbrough and Caldwell 1995).
Before reaching a nutrient-rich patch in heterogeneous soil, roots will encounter fewer nutrients than in a homogeneous soil containing the same total amounts of nutrients. To improve nutrient acquisition and plant growth, roots in a nutrient-poor environment should increase their ratio of root length to biomass (specific root length, SRL), thus increasing the absorptive root area (Bilbrough and Caldwell 1995). A plant in heterogeneous soil should also allocate more biomass to roots relative to shoots than a plant in homogeneous soil because, in general, plants allocate more biomass to organs needed to acquire resources that are limiting growth (Bloom et al. 1985).
Here, we investigated how nutrient heterogeneity affects plant growth and soil exploration before a nutrient-rich patch is encountered. We predicted that (1) the total plant biomass before the roots reach a nutrient-rich patch in a heterogeneous soil would be less than that in a homogeneous soil, since the plant cannot acquire a large amount of nutrients; (2) a plant in a heterogeneous soil would grow roots more in the direction of the patch than in the opposite direction in response to gradients, while a plant in a homogeneous soil would grow roots evenly in all directions; and (3) before the roots reach a nutrient-rich patch in a heterogeneous soil, a plant would respond to the nutrient-poor environment with a higher SRL and larger biomass allocation to roots.
We measured plant growth in heterogeneous and homogeneous soils. To determine whether plants selectively explore a nutrient-rich patch, we compared root length and biomass inside an area with a nutrient-rich patch to those inside the remainder of the microcosm without the patch.
We also measured the distribution of inorganic nitrogen (N) in each environment. We compared total root length, total root biomass, and SRL per plant between environments, and measured lateral and vertical root elongation.
Materials and methods Plant species
We grew Lolium perenne L. because it shows obvious root morphological responses to locally supplied inorganic N (Robinson and Rorison 1983). This species grows with other grass species in pastures in which N inputs are patchy and unpredictable (Grime et al. 1988).
Experimental apparatus
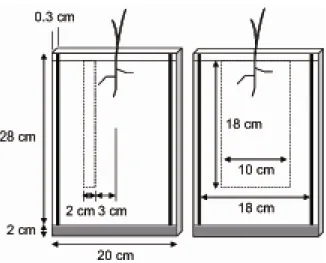
Plants were grown in microcosms built out of two rectangular glass plates (20 cm width × 30 cm height) joined by strips of Plexiglas on the sides and a piece of nylon sponge (20 cm × 2 cm) on the base for drainage. Before the plates were joined, sand sieved through a 2-mm mesh was deposited on one plate (18 cm width × 27 cm height × 0.3 cm thick). Next, solid slow-release inorganic fertilizer (Magamp K, 6-40-6 NPK, Hyponex Japan, Osaka, Japan) was placed on the sand in a heterogeneous or homogeneous pattern (Fig. 2-1). Finally, the other glass plate was secured to the first, sandwiching the rooting space between them. Several microcosms were bundled together and wrapped to exclude light from the roots.
Experimental design
Seeds were soaked for 24 h in water and then germinated on filter paper in Petri dishes. When the root length of the seedlings was 1–1.5 cm after 3–4 days, one seedling was carefully transplanted into the centre of each microcosm (9 cm from each side) on 5 May 2007 (day 0). Any seedlings that died within the first week were replaced with spares sown at the same time.
The fertilizer was supplied patchily or evenly. In the heterogeneous treatment, a nutrient-rich patch (2 cm × 18 cm) was placed on one side of the microcosm 3 cm from the centre (Fig. 2-1). In the homogeneous treatment, fertilizer was supplied in a central 10-cm band to 18 cm
depth (Fig. 2-1). Both treatments supplied 1.2 g fertilizer.
After transplantation, microcosms were laid out in a fully randomized design with two fertilization treatments and 40 replicates. The microcosms were set in a vinyl tunnel in the experimental garden of Tokyo Metropolitan University (Hachioji, Tokyo: 35°37′N, 139°23′E). The temperature during the experiment ranged from 18 to 30°C. Tap water was supplied as required throughout the experiment (two or three times a week, or daily just after transplantation). Ten microcosms per treatment were harvested on each of days 14, 28, 42, and 56.
An additional six microcosms in each treatment were prepared for soil analysis (described below) and laid out in a fully randomized design. Three microcosms per treatment were harvested on each of days 28 and 56.
Measurement of root length
In ten randomly selected microcosms in each treatment, we observed root growth and recorded it on days 7, 14, 21, 28, 35, 42, 49, and 56 on acetate sheets with marker pens of different colours. We also recorded the presence or absence of previously recorded root segments.
Soil analysis
We analysed the spatial distribution pattern of NH4+-N and NO3–-N within each microcosm. We divided the soil into nine 2-cm-width vertical bands (i.e., 2 cm × 27 cm × 0.3 cm) with a knife, and collected the entire sand and root complex in each band. The samples were held temporarily in a freezer before determination of the moisture content and extraction of NH4+-N and NO3–-N. A representative 3-g sample was extracted in 30 mL 2 M KCl solution for 1 h; the concentrations of NH4+-N and NO3–-N in the extract were then determined colorimetrically using a continuous-flow autoanalyser (Futura; Alliance Instruments, Frépillon, France). The concentrations were expressed on a dry-sand weight basis.
Plant analysis
At harvest, shoots were cut at the soil surface. Root segments in similar 2-cm bands as above were collected, washed, and scanned (GT-9800F; Epson, Tokyo, Japan) at 300 dpi resolution. The shoot and roots were dried at 70°C and weighed. We excluded root segments in a band, in a heterogeneous replicate on day 14, a homogeneous replicate on day 28 and that on day 42, because the root segments were too small to be weighed by a microbalance (PG503-S DeltaRange, Mettler Toledo, Columbus, OH). The total length of root segments in each band was measured from the scans with the Root Measure v.1.80 macro (Kimura and Yamasaki 2003) in NIH Image 1.63 software (http://rsb.info.nih.gov/nih-image/). One plant in the homogeneous treatment died between days 49 and 56, probably because of disease, and so was not harvested or further analysed.
Statistical analysis
We analysed the concentration of inorganic N, total biomass, root length, root biomass, SRL, and adjusted shoot biomass using a fixed-model, two-way analysis of variance (ANOVA), with fertilization (heterogeneous or homogeneous) and day of harvest (14, 28, 42, 56) as main factors.
The distance from the centre (1–3, 3–5, 5–7, 7–9 cm) was added as another main factor during the analysis of the spatial distributions of N concentration, root length, and root biomass per band. Data were checked for deviations from normality and for homogeneity of variance before analysis (Zar 1999; Crawley 2005), and log-transformed if necessary. We corrected for the problem of elevated Type I statistical error due to multiple testing by adjusting P values to control for false discovery rate (Benjamin and Hochberg 1995) as described by Verhoeven et al. (2005).
We examined the effects of factors on N concentration per microcosm, total biomass, and adjusted shoot biomass by univariate ANOVA, and on total root length, total root biomass, and SRL per plant by multivariate ANOVA. To examine the spatial distribution pattern of N concentration, we compared N concentration per band between bands on opposite sides of the centre at each distance. First, we calculated the differences between N concentrations within a microcosm. Then
we examined the effects of factors on the differences in NH4+ and NO3– concentrations by multivariate ANOVA. When, for example, N was distributed symmetrically on both sides of a microcosm, the difference in concentration at each distance should be zero. Positive differences indicate that concentration of N was larger on the patch side than on the other side. A few outliers of N caused by random effects were excluded from this comparison analysis in a homogeneous replicate on day 56. We also calculated the differences in root length and biomass between the paired bands. However, there was no root in some paired bands for all 10 replicates at harvest, and roots were obtained from only one sample of 10 replicates in other paired bands. Those paired bands were omitted from the following statistical analyses, and the rest were dealt with equally as one-factor data, because the data are neither balanced nor appropriate for ANOVA (Zar 1999). To test the difference in root length, we used the Kruskal–Wallis rank sum test instead of one-way ANOVA on account of heteroscedasticity (Bartlett’s test, P < 0.001). For the difference in root biomass, we used one-way ANOVA (Bartlett’s test, P = 0.07).
We examined the relationship between lateral and vertical root elongation in root-growth data recorded on days 7, 14, 21, 28, 35, 42, 49, and 56 (n = 10 in each treatment). Lateral root elongation was defined as the lateral distance from the centre to the furthest tip of a root segment.
Vertical root elongation was defined as maximum rooting depth. First, we used major-axis regression analysis for the relationship between lateral and vertical root elongation. We compared the 95% confidence intervals (CIs) of the slopes of the regression analysis between heterogeneous and homogeneous treatments. Then we used one-factor ANCOVA for lateral and vertical root elongation, respectively, and analysed the effects of fertilization and day of harvest on each root elongation. Day of harvest was used as the ordered independent variable. In one-factor ANCOVA, we first confirmed the linearity of slopes of the linear regressions of lateral or vertical root elongation against day of harvest, and then compared the slopes. When the slopes were not significantly different, we tested adjusted means. When the slopes were significantly different, we used the Johnson–Neyman technique to identify the values of the independent variable (day) over
which values of the dependent variable (lateral or vertical root elongation) were significantly different between the fertilization treatments (Huitema 1980). For this test, the level of significance was set at P = 0.05.
We also used two-way ANCOVA to examine the effects of fertilization and day of harvest on biomass allocation between shoots and roots, with shoot biomass as the dependent variable, treatment as the independent variable, and root biomass as a covariate. A test of homogeneity of slopes revealed no differences between treatments, satisfying an assumption of ANCOVA. We calculated means of shoot biomass adjusted for the means of the covariate (root biomass) to represent differences in biomass allocation between shoots and roots between treatments (Sokal and Rohlf 1995). All analyses were performed with the statistical software R version 2.2.1 (http://www.r-project.org/).
Results
Treatment effects on N distribution in soil
The spatial distribution of the concentrations of NH4+-N and NO3–-N varied with treatment (Figs.
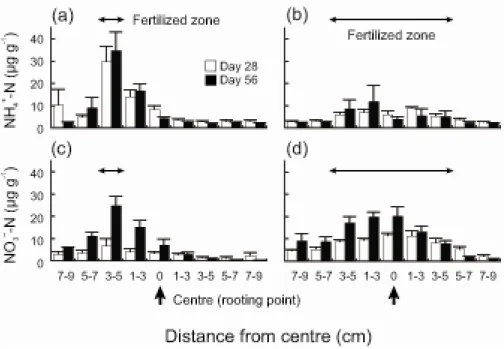
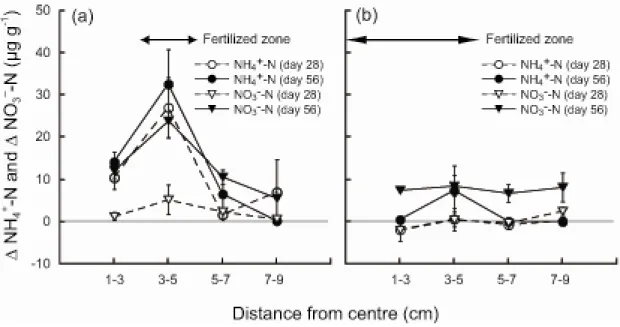
2-2, 2-3). N concentration was compared between bands at the same distances from the centre of a microcosm. The N concentration in the heterogeneous treatment tended to be distributed asymmetrically, but was symmetrically distributed in the homogeneous treatment (Fig. 2-2).
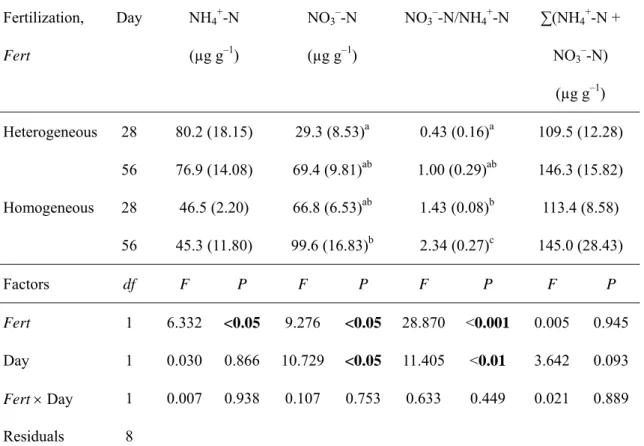
Differences in N concentrations between paired bands were significantly affected by fertilization, day of harvest, distance from the centre, and the interaction between fertilization and distance (MANOVA: P < 0.0012 for each factor). Differences in NH4+-N concentration between paired bands were affected by fertilization, distance, and their interaction (univariate ANOVA: P < 0.005 for each).
Means ± SE of the difference at each distance in the homogeneous treatment were close to zero (Fig.
2-3). This suggests that NH4+-N in the homogeneous treatment was distributed equally between both halves of the microcosm. In contrast, in the heterogeneous treatment, means of the difference at a
distance of 3–5 cm were highly positive and the highest among distances (Fig. 2-3). Differences were smaller at 1–3 and 5–7 cm. This fine-scale NH4+ distribution in heterogeneous soil corresponded to the placement of the nutrient patch at 3–5 cm from the centre.
Differences in NO3–-N concentration were affected by fertilization, distance, their interaction, and day (univariate ANOVA: P < 0.007 for each). The distribution of NO3–-N in the homogeneous treatment at each distance was generally equal on both sides of the centre (Fig. 2-3).
In contrast, in the heterogeneous treatment, the mean of the difference at 3–5 cm was highly positive on day 56, and slightly positive on day 28 (Fig. 2-3).
Fertilization affected the concentration of NH4+-N in each microcosm: means in the heterogeneous treatment were significantly larger than those in the homogeneous treatment (Table 2-1). Fertilization and days both affected the concentration of NO3–-N: means were significantly larger in the homogeneous treatment, and increased significantly during the experimental period (Table 2-1). Means of the ratio of NO3–-N to NH4+-N in the homogeneous treatment were thus significantly larger than those in the heterogeneous treatment, and both increased significantly during the experiment (Table 2-1). There was no difference between treatments in the sum of the concentrations of NH4+-N and NO3–-N (Table 2-1).
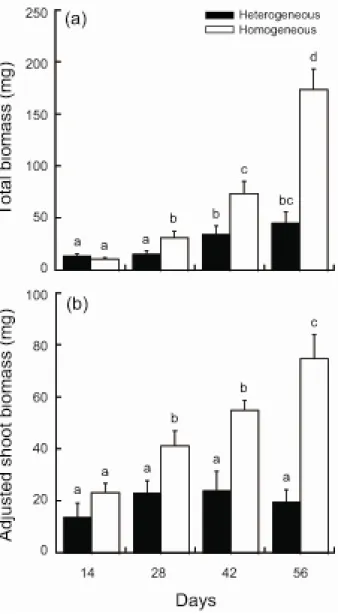
Plant growth
Means of total biomass were significantly affected by fertilization, day, and their interaction (ANOVA, Table 2-2). The means increased during the experimental period, and those in the homogeneous treatment were significantly greater than those in the heterogeneous treatment on days 28, 42 and 56 (Fig. 2-4).
Root growth
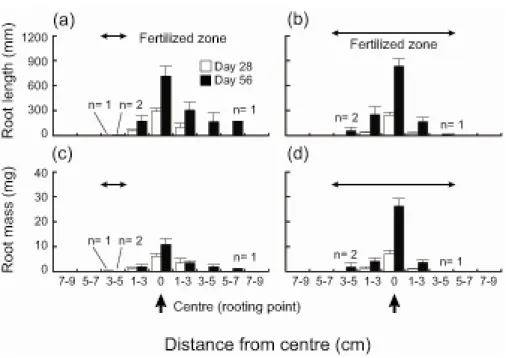
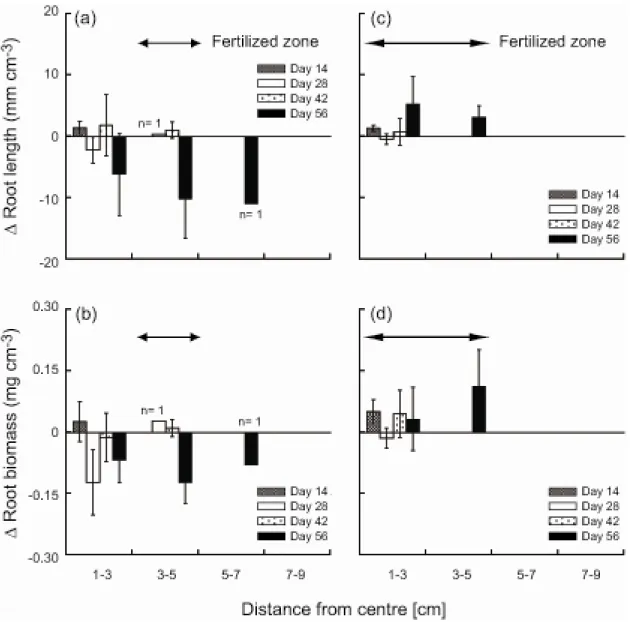
Root biomass and length in the heterogeneous and homogeneous treatments tended to distribute symmetrically on both sides of a rooting point (Fig. 2-5). In the heterogeneous treatment, root
segments in a band assigned as a nutrient-rich patch were obtained on days 28 (n = 1 out of 10) and 56 (n = 2 out of 10) (Fig 2-5). There was no obvious difference in root segments or aboveground parts between the plants with their roots in the patch and those with no roots in the patch (personal observation by R.N.). There was no significant difference between means of the difference in root length in paired bands among distances (Kruskal–Wallis rank sum test: χ2 = 9.348, df = 10, P = 0.499; Fig. 2-6a,c). Means of the difference in root biomass also did not differ (one-way ANOVA:
F10,69 = 1.156, P = 0.335; Fig. 2-6b,d). None of the means differed from zero at P = 0.05 (Fig.
2-6a–d).
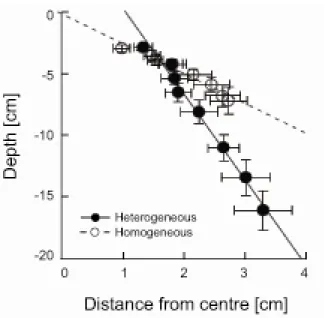
There was a significant difference between treatments in the relationship between lateral and vertical root elongation, because 95% CIs for the slopes of the major-axis regression analysis did not overlap (heterogeneous treatment: -7.95 to -6.20; homogeneous treatment: -3.15 to -2.10; Fig.
2-7).
Both lateral and vertical root elongation increased during the experimental period, and more so in the heterogeneous treatment (Fig. 2-7). Means of lateral root elongation were significantly larger in the heterogeneous treatment during the experimental period; the slopes of the linear regressions of lateral root elongation against day of harvest were almost the same between the treatments (ANCOVA: F1,12 = 0.448, P = 0.516), but the adjusted means were significantly different (ANCOVA: F1,13 = 22.108, P < 0.001). The mean of the lateral root elongation in the heterogeneous treatment on day 56 ranged over the distance from the centre to the nutrient-rich patch (i.e., 3 cm;
Fig. 2-1). In vertical elongation, roots grew deeper in the heterogeneous treatment, and differences between treatments were observed during the later experimental period (Fig. 2-7). The slopes of the linear regressions of vertical root elongation against day of harvest were different between treatments (ANCOVA: F1,12 = 70.585, P < 0.001). This indicates a significant effects of fertilization and the interaction of fertilization and day on vertical root elongation. Fertilization treatments showed significant differences in vertical root elongation between days 17.7 and 56 (Johnson–Neyman technique, P = 0.05).
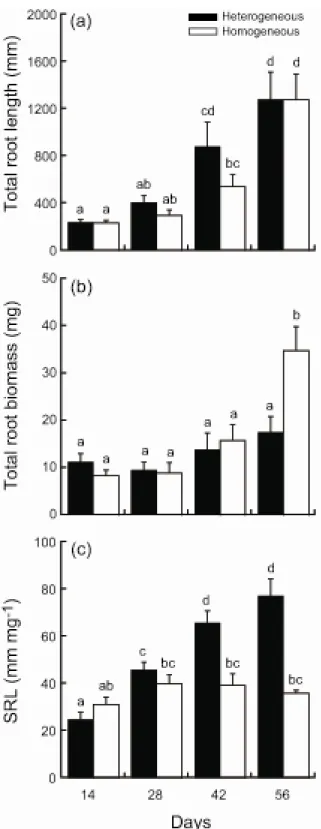
Total root length, total root biomass, and SRL per plant were significantly affected by fertilization, day, and their interaction (MANOVA, Table 2-3a). Means of total root length on day 42 were greater (but not significantly so) in the heterogeneous treatment (univariate ANOVA, Table 2-3b; Fig. 2-8a). Mean root biomass on day 56 was significantly greater in the heterogeneous treatment (Fig. 2-8b). This difference caused the marginally significant interaction (Table 2-3b).
SRL per plant was significantly affected by fertilization, day, and their interaction (univariate ANOVA, Table 2-3b). Means of SRL increased significantly during the experimental period in the heterogeneous treatment, but not in the homogeneous treatment (Fig. 2-8c). This caused a significant interaction in univariate ANOVA (Table 2-3b). Means of SRL on days 42 and 56 were significantly greater in the heterogeneous treatment (Fig. 2-8c).
Biomass allocation
Adjusted shoot biomass was significantly affected by fertilization, day, and their interaction (Table 2-2). Means increased significantly in the homogeneous treatment during the experimental period, but not in the heterogeneous treatment (Fig. 2-4b). This difference caused the significant interaction (Table 2-2). Means on days 28, 42, and 56 were significantly greater in the homogeneous treatment, suggesting a greater biomass allocation to roots in the heterogeneous treatment (Fig. 2-4b).
Discussion Inorganic N
Soil analyses showed that the distributions of NH4+ and NO3– concentrations within each microcosm reflected the patterns of fertilization at the beginning of the experiment (Figs. 2-2, 2-3). In the heterogeneous treatment, the concentration of N outside the patch was relatively low, and most N was distributed in and near the patch (Fig. 2-2). Although the distribution of NO3– is generally homogeneous because of its high diffusion rate (Marschner 1995), our results showed that NO3–
would remain locally at centimetre-scale. In addition, patterns of fertilization could cause differences in the chemical composition of inorganic N between heterogeneous and homogeneous treatments.
Indeed, a lower NO3––N/NH4+-N ratio was observed in the heterogeneous treatment.
Plant growth
Our results are consistent with our first prediction that total plant biomass before roots reach the nutrient-rich patch in heterogeneous treatment would be less than that in homogeneous treatment.
Although the same amounts of nutrients were provided, the mean total biomass in the heterogeneous treatment was less than that in the homogeneous treatment on days 28, 42, and 56 (Fig. 2-4a).
Because roots grew in a highly variable manner, extending both towards the patch and away from it (Fig. 2-6), most plants in the heterogeneous treatment did not reach the nutrient-rich patch. A few plants, however, reached the patch, and their root segments obtained from the patch were very small (Fig. 2-5), suggesting that nutrient acquisition by these root segments might be negligible. Despite the gradient in nutrient content, root growth of the plants was almost symmetrical. As a result, plant growth was limited, and plants did not benefit from the heterogeneous environment.
Previous studies have suggested a causal relationship between increases in root proliferation within nutrient-rich patches and increments in plant biomass in heterogeneous environments (see Hutchings et al. 1999 and references therein). Hutchings et al. (2003) also predicted that heterogeneity would limit plant performance when nutrient-rich patches could not be reached. In our study, only a few plants in the heterogeneous soil reached the nutrient-rich patch, and the mean plant biomass was smaller than that in the homogeneous soil. Thus, our results imply the importance of patches for plant growth in a heterogeneous environment, but further research on changes in plant growth associated with root morphological and physiological responses before and after reaching the patch is needed.
Root exploration for a nutrient-rich patch
Root biomass and length in the homogeneous treatment were distributed symmetrically within each microcosm, corresponding to the distribution of N concentration (Figs. 2-2b, 2-4c,d). These results were consistent with our second prediction. On the other hand, these distributions in the heterogeneous treatment did not match the patchy distribution of N (Figs. 2-2a, 2-4a,b).
The concentration of NO3– capable of inducing root responses could depend on growing conditions. The mean concentration of NO3–-N in the nutrient-rich patch in the heterogeneous treatment on day 56 was 24.9 µg g–1. This value was higher than the mean concentration in a nutrient-rich patch in which roots of a single plant of L. perenne proliferated (ca. 17.9 µg g–1; Hodge et al. 1998), and would thus be sufficient for a root response to the patch. In the presence of a neighbour, the concentration of NO3–-N that induced significant root proliferation of L. perenne within a nutrient-rich patch was much lower (ca. 2.0 µg g–1 on day 56 in Hodge et al. 1999) than in the absence of a neighbour. This fact suggests that roots of L. perenne with neighbouring competitors are more sensitive to low concentrations of NO3–-N than are roots without neighbouring competitors.
In general, L. perenne is a nutrient-demanding species (Grime et al. 1988). Previous studies have often shown selective root proliferation of this species to a nutrient-rich patch, even if the patch contained much higher concentrations of N than in our study (e.g., 1,000 µg g–1, Fitter 1976). Therefore, it was unlikely that the roots avoided the nutrient-rich patch in the heterogeneous treatment.
The extensive root growth in the heterogeneous treatment caused lateral root elongation beyond the nutrient-rich patch. If the plants were grown for longer, the extensive root growth should increase the probability of encountering the nutrient-rich patches (Crick and Grime 1987; Hutchings et al. 2003) and thus the plants would be able to acquire large amounts of nutrients from the heterogeneous environment. Deeper root elongation may also benefit plants by reaching deeper moisture and the nutrients within it. This possibility suggests the need to examine the interactions