Intracellular calcium elevation during plateau potentials
mediated by extrasynaptic NMDA receptor activation
in rat hippocampal CA1 pyramidal neurons.
1
Contents
Introduction
1: Synaptic transmission. ...2 1-1: Chemical synapse. ...2 1-2: Spill over. ...3 2: Glutamate receptors. ...3 2-1: N-methyl-D-aspartate receptors. ...32-2: Voltage dependency of NMDAR. ...5
2-3: Distribution of NMDAR in the central nervous system. ...5
3: Intracellular Calcium ions in Neurons. ...6
4: Hippocampal formation. ...7
5: The objectives of the present study. ...9
Materials and Methods
1: Slice preparations. ... 10 2: Whole-cell recordings. ... 10 3: Stimulation. ... 10 4: Calcium imaging. ... 11 5: Data analysis. ... 11 6: Drugs. ... 12Results
1: Synaptically induced plateau potentials are accompanied by elevation of [Ca2+] i. ... 132: [Ca2+] i elevation during synaptically-induced plateau potentials can be blocked by NMDAR antagonist. ... 14
3: Cd2+ blocks [Ca2+] i elevation during iontophoretically induced plateau potentials but not the change in membrane potential. ... 17
4: Cd2+ blocks [Ca2+] i elevation during plateau potentials generated in the absence of extracellular Mg2+ ions. ... 20
5: [Ca2+] i elevation during iontophoretically induced plateau potentials in basal dendrites. ... 23
Discussion
1: Summary. ... 252: Sources of Ca2+ elevation during NMDAR-mediated plateau potentials. ... 26
3: Types of voltage-gated Ca2+ channels responsible for the Ca2+ elevation associated with NMDAR-mediated plateau potentials. ... 27
4: Possible roles of NMDAR and NMDAR-mediated plateau potentials. ... 28
Acknowledgements
... 30Abbreviations
... 312
Introduction
1: Synaptic transmission.
Neuronal transmission in the central nervous system (CNS) is caused by synaptic transmission, mainly in chemical synapses. At chemical synapses, neurons release molecules to communicate with adjacent neurons.
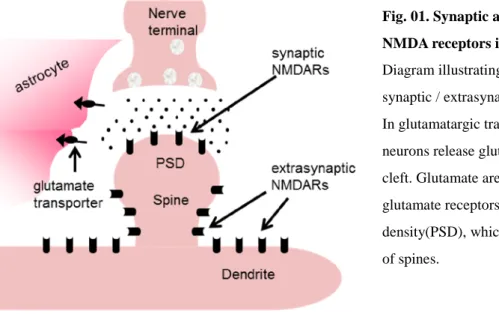
Fig. 01. Synaptic and extrasynaptic NMDA receptors in dendrites.
Diagram illustrating glutamate synapse and synaptic / extrasynaptic NMDA receptors. In glutamatargic transmission, presynaptic neurons release glutamate to the synaptic cleft. Glutamate are thought to activate glutamate receptors in the post synaptic density(PSD), which are located on the head of spines.
1-1: Chemical synapse.
Transmission in chemical synapses forms three main processes, presynaptic transmitter release, postsynaptic reception, and processes in synaptic cleft (Maycox, et al., 1990; Reimer, et al., 2001). Intracellular Ca2+ increase is critical for presynaptic process. Action potential propagation give rise to memebrane potential and activate voltage gated calcium channel (VGCC)s. This induces Ca2+ influx through VGCCs and increase intracellular Ca2+ concentration in the presynaptic terminal. In the presynaptic terminal, there are synaptic vesicles which neurotransmitters are stored inside. Intracellular Ca2+ increase triggers the synaptic vesicle membrane fuse to the presynaptic membrane, and release neurotransmitters out to the synaptic cleft. Released neurotransmitters defuse through the synaptic cleft and reach the postsynaptic cell. Neurotransmitters bind to the transmitter receptors in the postsynaptic membrane, and bring various effects to the postsynaptic cell. Generally, effect which gives rise to the membrane potential is called “excitatory”. On the other hand, effect which hyper-polarize the membrane potential is called “inhibitory”.
3
1-2: Spill over.
In studies of synaptic transmission, it is usually discussed that functional transmitter receptors express on the spine head, facing to the synaptic cleft. Although, receptors are also found in the spine neck, dendritic trunk, and the cell body of neurons. Thus they are capable to be targets for the released neurotransmitters. A phenomenon, referred to as spillover or the leakage of synaptically released neurotransmitters from the synaptic cleft, is suggested to be one of the source activating the extrasynaptic receptors (Huang, 1998; Isaacson, 2000: Kullmann, 2000). The physiological mechanism of spill over is still unclear. One theory is that high frequent synaptic input makes a transmitter “pond” around the synapse which is originated from failure of glial processes to clear the transmitters (Oikonomou, et al., 2012). In hippocampal CA1 area and cerebellar perkinje cells, extracellular application of glutamate transporter antagonist TBOA promotes glutamate spill over (Reichelt & Knopfel, 2002; Huang, et al., 2004). Repetitive synaptic stimulation in the presence of TBOA generates a sustained depolarization in CA1 pyramidal neurons (Suzuki, et al., 2008), which referred to as a plateau potential. Suzuki et al. reported that plateau potentials can be also generated by iontophoretic application of glutamate or NMDA to the apical dendrites of pyramidal neurons.
2: Glutamate receptors.
Excitatory synaptic transmission in the CNS is mainly due to glutamatergic neurotransmission. The main actor of the glutamatergic trasmission is the glutamate receptor families. Glutamate receptor families are classified into two big families, the metabotropic glutamate receptor (mGluR) family and ionotropic glutamate receptor (iGluR) family. Ionotropic glutamate receptors are further classified based on pharmacology and structural homology, including α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR), kainate receptors (KAR), and N-methyl-D-aspartate receptors (NMDAR). They assemble as tetrameric complexes of subunits, and function as ion channels. AMPAR and KAR are ligand gated ion channels, but NMDAR also have voltage dependency to function as an ion channel. While AMPAR and KAR are considered to play major roles in conventional fast synaptic transmission at glutamatergic synapses, the functional roles of the NMDAR are not fully understood.
2-1: N-methyl-D-aspartate receptors.
4
Fig. 02. Trans-membrane architecture of an NMDA receptor.
Glutamate and NMDA binds to GluN2 subunit while glycine binds to GluN1 subunit. The ion channel is permeable to Na+ , K+, and Ca2+. Extracellular Mg2+ gives voltage sensitive block by binding deep within the pore.
The mechanism of the NMDAR function is complicated. In phyiological condition, the pore of the NMDAR ion channel is blocked by extracellular magnesium ions (Mg2+) in the resting membrane potential. Activation of NMDAR requires both glutamate and glycine bindings, and additional mebrane depolarization to reduce extracellular Mg2+ block in the pore of the ion channel. However, Mg2+ block is incompleate in the resting membrane potential, so glutamate binding to NMDARs can evoke inward current flow whithout membrane depolarization (Jahr & Stevens, 1990; Sabatini, et al., 2002).
NMDARs are cation channels, but it is not sure whether they have ion selectivity. This ion channel is permeable to Na+, K+, and Ca2+ in physiological condition. Remarked characteristics of the NMDAR in neuroscience is the permeability of Ca2+. However, some types of AMPAR and KAR are also permeable to Ca2+. NMDARs including GluN2A or GluN2B subunit have high Ca2+ permeability, but GluN2C or GluN2D containing NMDAR's Ca2+ permeability is similar to those of Ca2+ permeable AMPARs and KARs (Traynelis, et al., 2010).
The variety of GluN2 subunits gives diversity to NMDAR function. Subunit specificity in pharmacological characteristics is one of the important nature of the NMDAR. In sensitivities of NMDAR to glutamate, EC50 value of the GluN2A containing NMDAR is highest, which is ten times higher than that of GluN2D containing NMDARs. In contrast, sensitivity of DL-APV, a major antagonist known as none
5
2-2: Voltage dependency of NMDAR.
The most remarkable feature of NMDA-R is in its voltage dependency. The NMDA receptor's voltage dependence follows directly from channel block by extracellular Mg2+. At resting membrane potentials, most subtypes of NMDA receptors undergo rapid channel block by extracellular Mg2+, which decrease NMDAR current conductance. The Mg2+ block escape from the channel pore with membrane depolarization which enables the NMDAR to function as ion channels. Voltage dependent blockage of NMDAR by Mg2+ ion is thought to be controlled by GluN2 subunit (Kuner & Schoepfer, 1996, ), and it has been reported that receptors containing GluN2C or GluN2D subunits exhibit lower sensitivity to extracellular Mg2+ ion compared to those containing GluN2A or GluN2B subunits (Wrington et al., 2008; Qian et al., 2005; Monyer, et al. 1994; Kuner & Schoepfer, 1996; Momiyama, et al. 1996). According to Wrington et al. (2008), the channels of GluN2D or GluN2C containing receptors are incompletely blocked at -100mV and pass maximal current at approximately -35mV.
2-3: Distribution of NMDAR in the central nervous system.
Fig. 03. NMDAR subunit diversity and expression.
The developmental profile of NMDAR subunit expression in the mouse brain at postnatal day 0 (left), 2 weeks following birth (middle), and at the adult stage (right). (Paoletti, et al., 2013)
6
3: Intracellular Calcium ions in Neurons.
Ca2+ generates various intracellular signals in almost every cell types. In the CNS, intracellular Ca2+ concentration have important roles in neuronal transmission and following neuronal activities, including synaptic signaling, induction of short and long term plasticity, and gene transcription (Zucker, 1999; Soderling, 2000; Sabatini, 2001). The best characterized intracellular Ca2+ concentration changes follow from opening of voltage gated calcium channels (VGCCs) and ionotropic receptors (Berridge, 1998; Augustine, et al., 2003; Bloodgood & Sabatini, 2007).
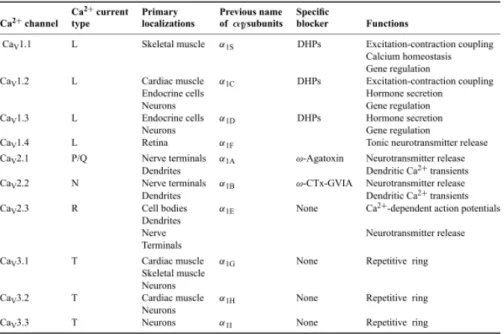
Table 1. Subunit composition and function of Ca2+ channel types. (Catterall, 2000)
Voltage gated calcium channels are important for Ca2+ increase during neuronal activities. They activate by membrane potential depolarization and pass Ca2+ from extracellular to intracellular. VGCC are classified into five types (T, L, P/Q, N, R) by their voltage dependency and antagonist specificities. T type VGCC have low threshold in the membrane potential of -70mV. L type activates above -30mV, and P/Q, N, R type VGCCs activates at -20mV. In dendritic spines and branches, VGCC activate with membrane depolarization due to strong synaptic inputs (Miyakawa, et al., 1992; Christie, et al., 1995). In variety of neurons, including hippocampal pyramidal neurons, it is known that action potentials propagate to the apical dendrites (Waters, et al., 2005). Back propagating action potentials activate VGCCs at least in the proximal dendrites and provide intracellular Ca increase (Callawey and Ross, 1995; Schiller, et al., 1995; Yuste and Denk 1995).
7
endoplasmic reticulum (Dudman, 2007; Hong and Ross 2007; Watanabe, et al., 2006; Nakamura, et al., 2000). Recent studies have revealed spontaneous Ca release events in dendrites and spines of hippocampal CA1 pyramidal neurons, which are ryanodine and inositol 1, 4, 5-trisphosphate (IP3) sensitive (Manita, et
al., 2010; Ross, 2012.).
4: Hippocampal formation.
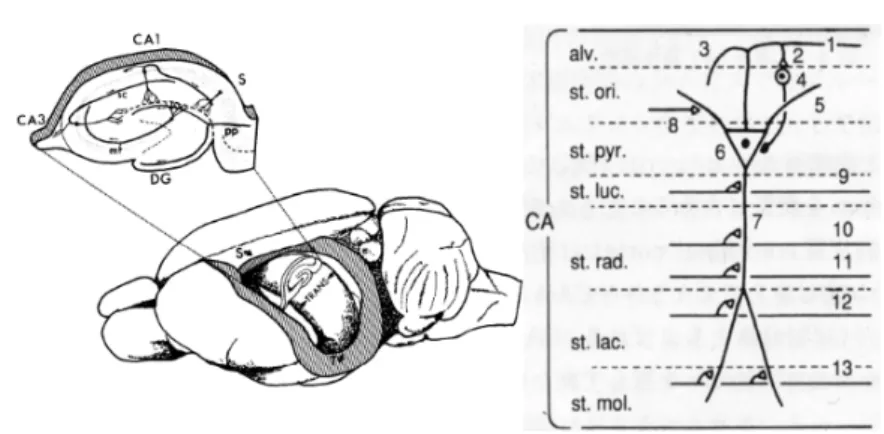
Fig. 04. Hippocampal formation in rat brain.
Left, The position and layers of the hippocampal formation in the rat brain. mf, mossy fiber; sc, shaffer collateral; pp, perforant pathway. Right, layers and projections of hippocampal pyramidal neurons. 1, pyramidal neuron axon; 2, axonal branching to basket cells; 3, input from CA3 areas (Shaffer collateral ); 4, basket cell; 5, basal dendrite of pyramidal neuron; 6, pyramidal neuron cell body; 7, apical dendrite of pyramidal neuron; 8, 9, 10, inputs from septum and commissural fiber; 11, 12, shaffer collateral; 13, input from entorhynal cortex (perforant pathway); alv., alveus; st. ori., stratum oriens; st. pyr., stratum pyramidale; st. luc., stratum lucidum; st. rad., stratum radiatum; st. lac., stratum lacunosum; st. mol., stratum moleculare. (Amaral & Witter, 1989)
The hippocampal formation is one of the best known neuroanatomical structures due to intense study with a variety of descriptive and experimental anatomical methods. Many scientists have been focused on this, since it is thought to be involved in learning and memory (Dingledine, et al., 1999; Nakazawa, et al., 2002; Lisman, et al., 2005). It is also interested clinically for involvement in neurodegenerative disorders and psychiatric disorders, such as epilepsy, Altzheimer's desease, and schizophrenia (Anderson, et al., 2007).
8
9
5: The objectives of the present study.
Previous study reported that repetitive synaptic stimulation during blockade of glutamate transporters or iontophoreticaly application of NMDA generates plateau potential in CA1 pyramidal neuron (Suzuki, et al., 2008). Induction of plateau potential was sensitive to 5,7-dCK (GluN1 subunit antagonist) and had a weak sensitivity to DL-APV (GluN2 subunit antagonist), and persistent to pre-blockade of synaptically located
NMDARs. These results indicate that plateau potential is induced by activation of extrasynaptic NMDARs. Many studies have shown that NMDAR is critically involved in the synaptic plasticity responsible for learning and memory (Zito & Scheuss, 2009; Dingledine et al., 1999). Additionally, NMDAR is believed to be involved in schizophrenia (Sawa & Snyder, 2002; Phillips & Silverstein, 2003; Kantrowitz & Javitt, 2010) and activity-dependent brain damage (Hardingham & Bading, 2010, Parsons & Raymond, 2014). The NMDAR channels permeate Ca2+ ions in addition to Na+ and K+ ions, and the predominant hypothesis for the mechanisms of NMDAR involvement in these phenomena is that the activation of NMDAR elevates intracellular calcium concentrations ([Ca2+]i) that trigger various intracellular signaling systems. An
alternative hypothesis is that the prolonged depolarization due to the activation of NMDAR is responsible for these phenomena.
Thus, it is important to isolate the NMDAR activation, membrane depolarization, and [Ca2+]i for further
discussion. In this study, we examined if the NMDAR-mediated plateau potential is accompanied by an elevation of [Ca2+]i. We performed Ca
2+
imaging and whole-cell recordings from single hippocampal CA1 pyramidal neurons and measured the change in [Ca2+]i during NMDAR-mediated plateau potentials. We
have found that plateau potentials are accompanied by an elevation of [Ca2+]i primarily caused by an influx
10
Materials and Methods
1: Slice preparations.
28 Male Wistar rats (15- to 30-days old) (Tokyo Laboratory Animals Science, Tokyo, Japan) were anesthetized with diethyl ether, decapitated, and the brain was rapidly removed and immersed in ice-cold saline containing (in mM); 124 choline chloride, 2.5 KCl, 0.5 CaCl2, 4 MgCl2, 1.25 NaH2PO4, 26
NaHCO3, and 10 glucose, saturated with 95 % O2 / 5 % CO2. Transverse hippocampal slices (300 µm
thick) were cut in ice-cold saline using a slicer with a vibrating razor blade (Supermicroslicer Zero-1; Dosaka EM, Kyoto, Japan). The slices were incubated for 30 min at 32 °C in artificial cerebrospinal fluid (ACSF) containing (in mM); 124 NaCl, 2.5 KCl, 2.5 CaCl2, 1.5 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 10
glucose, saturated with 95% O2/5% CO2 and then kept for at least 30 min at room temperature before
experiments. All experiments were carried out in compliance with institutional guidelines for animal experiments and every effort was made to minimize the number of animals used. All experimental protocols followed the guidelines of the Ministry of Education, Culture, Sports, Science, and Technology of Japan and those of the U.S. NIH, and were approved by the Tokyo University of Pharmacy and Life Sciences Institutional Animal Care and Use Committee.
2: Whole-cell recordings.
Whole-cell patch clamp recordings were made from CA1 pyramidal neurons. Neurons were visualized with a 40x water-immersion objective lens (Achroplan, 0.75 NA; ZEISS, Oberkochen, Germany, LUMPlanFl/IR, 0.80 NA; Olympus, Tokyo, Japan) using an upright microscope (Axioskop FS; ZEISS, Oberkochen, Germany) equipped with infrared/differential interference contrast (IR⁄DIC) systems and a CCD camera (Hamamatsu Photonics, Hamamatsu, Japan). The whole-cell electrodes fabricated from borosilicate capillaries (#801686; Warner Instruments, Hamden, CT, USA) and pulled on a micropipette puller (Sutter Instrument, Novato, CA, USA) had a resistance of 6 - 9 MΩ. Whole-cell current-clamp recordings were made with an internal solution containing the following (in mM): 120 K-gluconate, 20 HEPES, 2 MgATP, 0.2 NaGTP, and 0.1 fluo-4 adjusted to pH 7.4 with KOH. All experiments were performed at 33 ± 1 °C. Responses were recorded using an AxoClamp 2B amplifier (Axon Instruments, Foster City, CA, USA), filtered at 10 kHz, and digitized at 3 - 10 kHz. Slices were continuously superfused with ACSF at a rate of 2–3 mL ⁄ min.
3: Stimulation.
11
was applied at all other times to avoid drug leakage) through an NMDA (50 mM)-filled glass pipette with a resistance of 110 – 160 MΩ by using a microiontophoresis current generator (SEZ-1100; Nihon Kohden, Tokyo, Japan). NMDA (50 mM) was prepared in distilled H2O and adjusted to pH 7.4 with NaOH. In both
cases, a stimulator (SEN-7203; Nihon Kohden, Tokyo, Japan) was used to trigger current pulses.
4: Calcium imaging.
We made whole-cell patch clamp recordings with microelectrodes filled with the fluorescent Ca2+ indicator fluo-4. The fluo-4 pentapotassium salt (Molecular Probes - Life Technologies, Carlsbad, CA, USA) was dissolved in distilled water to prepare a stock solution. We added the stock solution to the intracellular solution such that the final concentration of fluo-4 became 100 µM. A 150-W tungsten-halogen lamp (FCS 150W; PHILIPS, Amsterdam, Netherlands) was used as a light source. An incident light filter of 480 - 490 nm, a dichroic mirror with a center wavelength of 510 nm, and a barrier filter of 520 nm was used. Fluorescence images (512 × 512 pixels, 14-bit intensity) were captured at a 30 - 50 Hz frame rate with an EM-CCD camera (C9100-12; Hamamatsu Photonics, Hamamatsu, Japan) and were recorded using AquaCosmos software (Hamamatsu photonics, Hamamatsu, Japan).
5: Data analysis.
12
6: Drugs.
Ethylene glycol bis(β-aminoethyether)-N,N,N,N-tetraacetic acid (EGTA) was purchased from Dojindo Laboratories (Kumamoto, Japan). Cadmium chloride hemi(pentahydrate) (Cd),
6-Cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX), and Picrotoxin (PTX) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Tetrodotoxin (TTX),
(S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385), 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP), 5,7-dichloro-4-hydroxyquinoline-2-carboxylic acid (5,7-dCK),
DL-2-amino-5-phosphonopentanoic acid (DL-APV), DL-threo-β-Benzyloxyaspartic acid (TBOA), and N-methyl-D-aspartic acid (NMDA) were purchased from Tocris Bioscience (Bristol, UK).
Drugs Effects Cd CNQX PTX TTX LY367385 MPEP 5,7-dCK DL-APV TBOA NMDA
Non-specific voltage gated calcium channel blocker. AMPA receptor antagonist.
GABAA receptor antagonist.
Voltage gated sodium channel blocker.
Specific antagonist for mGluR1 type metabotropic glutamate receptor. Specific antagonist for mGluR5 type metabotropic glutamate receptor. Specific antagonist for GluN1 subunit of NMDA receptor.
Specific antagonist for GluN2 subunit of NMDA receptor. Glutamate transporter blocker.
Specific agonist for GluN2 subunit of NMDA receptor.
13
Results
1: Synaptically induced plateau potentials are accompanied by elevation of [Ca2+]i.
In our previous study (Suzuki et al., 2008), we induced NMDAR-mediated plateau potentials in hippocampal pyramidal cells in two ways: by delivering repetitive synaptic stimulations and by iontophoretically applying sodium glutamate or NMDA locally onto the apical dendrites. We proposed there that the potential was mediated by activation of extrasynaptic NMDAR. To examine if the synaptically-induced plateau potential is accompanied by a change in [Ca2+]i, in this study we carried out
simultaneous recordings of membrane potentials and [Ca2+]i from CA1 pyramidal neurons in acute rat
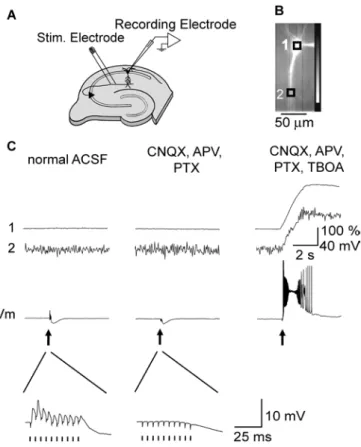
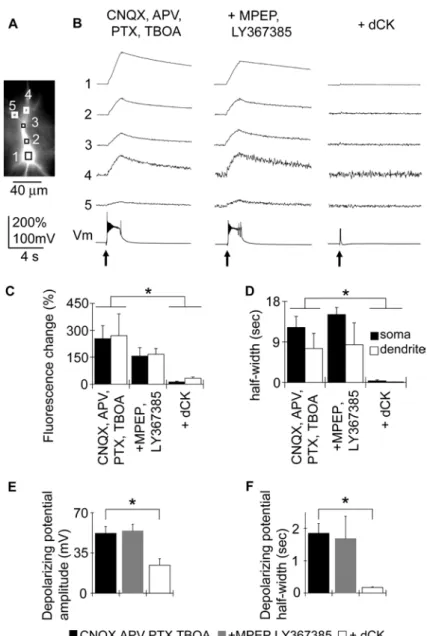
hippocampal slice preparations. We made whole-cell patch clamp recordings with microelectrodes filled with the fluorescent Ca2+ indicator fluo-4. To avoid saturating the dye with Ca2+ during synaptically induced plateau potentials, a high amount of EGTA (10 mM) was added to the internal solution of the recording pipette. The schematic drawing of our experimental arrangements is shown in Fig. 05A. Synaptic responses were elicited by delivering repetitive current pulses through a bipolar tungsten electrode or a glass pipette placed in the str. radiatum 100 – 200 µm from the str. pyramidale and laterally 100 - 450 µm away from the test cell. In normal ACSF, repetitive synaptic stimulation (100 Hz, 10 pulses) evoked excitatory postsynaptic potentials (EPSPs) followed by inhibitory postsynaptic potentials (IPSPs) (Fig. 05C). Expanded traces of the membrane potentials showed that EPSPs were evoked by each of the stimulations. In this condition, no noticeable change in [Ca2+]i was detected either at the soma or dendrites.
A mixture of 10 µΜ CNQX, 50 µΜ DL-APV, and 100 µΜ PTX blocked EPSPs and the early phase of
IPSPs (Fig. 05C) leaving slow components of IPSPs. The reason we added 50 µΜ DL-APV in this
experiment was to block activation of NMDAR located at the synapses and examine plateau potentials induced by activation of extrasynaptically located NMDAR.
In our previous study, we showed that spill over of glutamate needs to be facilitated by blocking glutamate uptake in order to trigger plateau potentials synaptically. Traces on the right in Fig. 05C show the responses for repetitive synaptic stimulation after applying 50 µΜ TBOA, a non-selective blocker for glutamate transporters, in the presence of a mixture of 10 µΜ CNQX, 50 µΜ DL-APV, and 100 µΜ PTX. A plateau
14
Fig. 05. Synaptically induced Ca2+elevation and plateau potentials.
(A) Schematic drawing of experimental arrangements. A synaptic response was elicited by applying short current pulses through a bipolar tungsten electrode or a glass pipette placed in the stratum (str.) radiatum 50–100 µm from str. pyramidale. (B) Fluorescence image of a CA1 pyramidal neuron filled with 100 µM fluo-4. The intracellular solution included 10 mM EGTA. Scale bar, 50 µm. The recorded neurons were typically 150 µm away from the stimulating electrode. (C) Change in fluorescence and membrane potential of the neuron elicited by synaptic stimulations (100 Hz, 100 ms, arrows) in normal ACSF (left); in the presence of 10 µM CNQX, 50 µM DL-APV (APV), and 100 µM Picrotoxin (PTX) (middle); and after
15
2: [Ca2+]i elevation during synaptically-induced plateau potentials can be blocked by NMDAR
antagonist.
To determine the source of [Ca2+]i elevation during synaptically-induced plateau potentials, we examined
the effects of antagonists for mGluR because it has been shown that the activation of mGluR can trigger Ca2+release from intracellular Ca2+ stores and induce Ca2+ waves in hippocampal pyramidal neurons (Nakamura et al., 1999, 2000; Ross, 2012). Since Nakamura et al. (2000) reported that the class I mGluRs, mGluR1 and mGluR5, are responsible for generating Ca2+ waves, we applied a mixture of MPEP (10 µΜ), a specific antagonist for mGluR5, and LY367385 (50 µΜ), a specific antagonist for mGluR1, to examine if Ca2+ release is involved in Ca2+ elevation accompanying plateau potentials. The mixture of MPEP and LY367385 did not show significant effects on the amplitude of the rise in [Ca2+]i at the soma and dendrites
(Fig. 06), indicating that the [Ca2+]i elevation that accompanies synaptically induced plateau potentials is
not due to the activation of mGluRs. In this condition, 50 µΜ DL-APV was already included in ACSF to
block synaptically located NMDAR. We have shown in our previous study that either 30 µΜ 5,7-dCK, a specific and potent antagonist for the glycine binding site of NMDAR, or 200 µΜ D-APV abolished the
persistent membrane depolarization leaving only a small depolarization and hyperpolarization (Suzuki et al., 2008). The rightmost panel of Fig. 06B shows the responses for repetitive synaptic stimulation after applying 30 µΜ 5,7-dCK in the presence of a mixture of 10 µΜ CNQX, 50 µΜ DL-APV, 100 µΜ PTX, 10
µΜ MPEP, 50 µΜ LY367385, and 50 µΜ TBOA. The plateau potential was abolished, and the rise in [Ca2+]i was almost completely blocked, showing that the synaptically induced plateau potential and the
16
Fig. 06. Ca2+ elevation and depolarizing potential during synaptically induced plateau potentials can be blocked by NMDAR antagonist.
(A) Fluorescence image of a neuron filled with the Ca2+ indicator fluo-4. Scale bar, 40 µm. (B) Left: Synaptically induced Ca2+ responses recorded in the presence of 10 µM CNQX, 50 µM DL-APV (APV), 100
17
3: Cd2+ blocks [Ca2+]i elevation during iontophoretically induced plateau potentials but not the change in
membrane potential.
Although the experiments shown in Fig. 06 indicate that the Ca2+ elevation accompanying synaptically induced plateau potentials depends on the activation of NMDAR, it does not necessarily mean that this Ca2+ elevation was due to the entry of Ca2+ ions through NMDAR. Because the NMDAR antagonist 5,7-dCK blocks the plateau potential itself, it is possible that the [Ca2+]i elevation is due to a mechanism
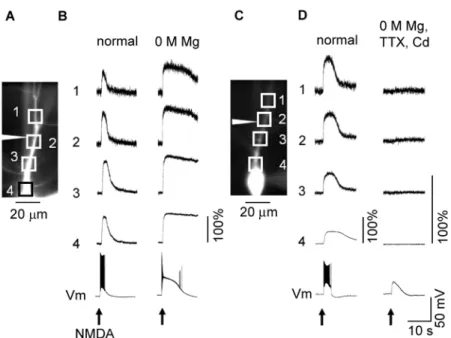
secondary to the activation of NMDAR, namely the activation of voltage-gated Ca2+ channels. It is also possible that the plateau potential depends partially on inward current through voltage-gated Ca2+ channels. We could not examine this possibility simply by testing the effects of voltage-gated Ca2+ channel blockers on synaptically induced plateau potentials because the blockers would inhibit synaptic transmission. To circumvent this, we tested the effects of voltage-gated Ca2+ channel blockers on iontophoretically induced plateau potentials. We placed a glass pipette filled with 50 mM NMDA close to the apical dendritic shaft of dye-stained pyramidal cells bathed in normal ACSF and applied a current pulse (200 nA, 400 ms duration) to eject NMDA on to the dendrites. Despite the presence of 1.5 mM Mg2+ ions in the ACSF, the NMDA application induced plateau potentials as we reported previously, and the potentials were accompanied by a rise in [Ca2+]i both along the dendritic shaft and at the soma (Fig. 07B). The amplitude of the fluorescence
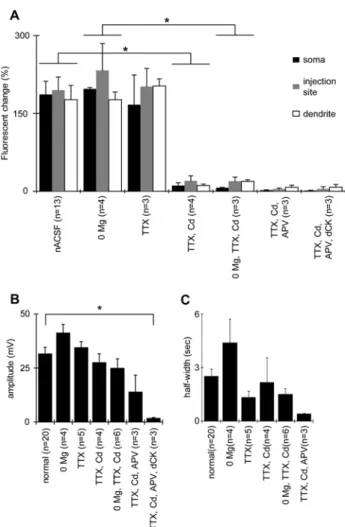
change was 194 ± 25% (n = 13) at the injection site, 176 ± 27% (n = 13) at the distal region of the stimulated dendrite, and 186 ± 25% (n = 13) at the cell body (Fig. 09A).
Repetitive firing of fast action potentials was always observed during the NMDA-induced plateau potentials. Since previous reports have shown that back-propagating action potentials are accompanied by a rise in [Ca2+]i at the dendrites and soma (Jaffe et al., 1992; Christie et al., 1995; Manita et al., 2011), a part
of this rise in [Ca2+]i could be related to back-propagating spikes. However, in the presence of TTX (1 µM)
that blocks fast action potentials, the amplitude of the fluorescence change accompanying NMDA-induced plateau potentials was 201 ± 34% (n = 3) at the injection site, 202 ± 13% (n = 3) at the distal region of the stimulated dendrite, and 166 ± 57% (n = 3) at the cell body (Fig. 09). These values were not significantly different from the values measured in normal ACSF, implying that the rise in [Ca2+]i was not due to the
generation of action potentials.
A mixture of 1 µM TTX and 200 µM Cd2+
blocked most of the rise in [Ca2+]i associated with plateau
potentials, leaving a much smaller rise in [Ca2+]i near the stimulation site and the soma, as shown in Fig.
07B. In this condition, the amplitude of the fluorescence change accompanying iontophoretically induced plateau potentials was 20 ± 1% (n = 4) at the injection site, 11 ± 5% (n = 4) at the distal region of the stimulated dendrite, and 11 ± 3% (n = 4) at the cell body (Fig. 09A). Compared to the values in normal ACSF, the reduction in amplitude in the presence of TTX and Cd was 90%, 94%, and 94%, respectively. This implies that the entry of Ca2+ through voltage-gated Ca2+ channels is the primary source for the elevation of [Ca2+]i during the plateau potentials generated in this condition.
18
peak values of filtered traces. The amplitude and half-width of the plateau potentials measured at the cell body in the mixture of 1 µM TTX and 200 µM Cd2+
was 27.7 ± 3.9 mV and 2.18 ± 1.38 ms (n = 4), respectively. These values were not significantly different from the amplitude and half-width values of 31.7 ± 2.9 mV and 2.53 ± 0.40 ms (n = 20), respectively, found in normal ACSF (Fig. 09B, C).
The plateau potential was resistant to further application of 50 µM DL-APV but was abolished in the
19
Fig. 07. Cd2+blocks Ca2+ elevation during iontophoretically induced plateau potentials.
(A) Fluorescence image of a neuron filled with the Ca2+ indicator fluo-4. Triangular shape indicates the position of a glass pipette used to apply NMDA iontophoretically. Squares show ROIs. (B) Responses of a neuron during iontophoretic application of NMDA (arrow, 200 nA, 400 ms). Traces 1–5 show fluorescent changes recorded from the five ROIs indicated in (A). Bottom traces show changes in membrane potential recorded from the cell body. Far left: In normal ACSF. Note that an elevation of Ca2+ accompanies the plateau potential induced by iontophoretic application of NMDA. Mid left: In the presence of 1 µM TTX and 200 µM Cd2+. Mid right: In the presence of 1 µM TTX, 200 µM Cd2+, and 50 µM
DL-APV. Far right: In
the presence of 1 µM TTX, 200 µM Cd2+, 50 µM DL-APV, and 30 µM 5,7-dCK. (C) Low-pass filtered
20
4: Cd2+ blocks [Ca2+]i elevation during plateau potentials generated in the absence of extracellular Mg 2+
ions.
In the experiments shown in Fig. 07, NMDAR-mediated plateau potentials were induced in the presence of 1.5 mM Mg2+ in the bathing solution. Although the blockade of Ca2+ elevation by Cd2+ indicates that the Ca2+ elevation is not due to Ca2+ entry through NMDAR but through voltage-gated Ca2+ channels, the possibility remains that Ca2+ entry through NMDAR did make a small contribution, perhaps because the NMDAR might not have been sufficiently activated due to the blockade of NMDAR with Mg2+ ions. To test this possibility, we examined the effect of Cd2+ in a condition where Mg2+ ions were not present in the bathing medium. In the Mg2+-free condition, iontophoretic application of NMDA to the proximal apical dendrite induced a plateau potential with a much longer duration 4.41 ± 1.32 s (n = 4), but the amplitude of the sustained potential was 41.4 ± 3.9 mV (n = 4). This was not significantly different as compared to the potential induced in the presence of 1.5 mM Mg2+. The amplitude of the change in dye fluorescence was 264 ± 71% at the injection site, 164 ± 15% at the site distal to the injection site, and 197 ± 3% at the soma (n = 4). These values were not significantly greater than the values observed in the presence of Mg2+, however the elevated level lasted much longer (Figs. 4 and 5).
With the addition of 1 µM TTX and 200 µM Cd2+
in the Mg2+-free condition, the amplitude of the change in fluorescence was significantly reduced, as was the case with 1.5 mM Mg2+ in the bathing medium. The amplitude of the changes in fluorescence was 19.5 ± 7.9% at the injection site, 18.9 ± 3.1% at the site distal to the injection site, and 6.83 ± 0.79% at the soma (n = 3) (Fig. 09A, Fig. 08B). The amplitude of the sustained membrane potential during the plateau potential in this condition was 25.1 ± 4.2 mV (n = 6), which was not significantly different from the value in the bathing medium with 1.5 mM Mg2+ (Fig. 09B). Thus, either with or without Mg2+ ions in the bathing medium, iontophoresis of NMDA generates plateau potentials accompanied by an elevation of [Ca2+]i, primarily due to the activation of voltage-gated
21
Fig. 08. Cd2+blocks Ca2+ elevation during plateau potentials in the absence of Mg2+ ions.
Ca2+ imaging during iontophoretically induced plateau potentials in the absence of extracellular Mg2+. (A) Fluorescence image of a neuron filled with the Ca2+ indicator fluo-4. Triangle indicates the position of a pipette filled with 50 mM NMDA used for iontophoresis. (B) Responses of the neuron during iontophoretic application of NMDA (arrows, 200 nA, 400 ms). Left: Responses in normal ACSF. Right: Responses in Mg2+-free ACSF. Traces 1–4 show fluorescent changes recorded from the four ROIs shown in (A). Bottom traces show membrane potentials recorded from the cell body. (C) Fluorescence image of a different neuron than the one shown in (A). Triangle indicates the position of a pipette filled with 50 mM NMDA. (D) Responses of the neuron during iontophoretic application of NMDA (arrows, 200 nA, 400 ms). Left: Responses in normal ACSF. Right: Responses in the presence of 1 µM TTX and 200 µM Cd2+
22
Fig. 09. Effects of channel blockers on Ca2+ elevation during NMDA-induced plateau potentials.
Pooled data of responses during NMDA-induced plateau potentials are shown as histograms. (A) Maximal amplitude of the fluorescence change observed from the soma, injection site, and the dendrite distal from the injection site. nACSF: normal ACSF; 0 Mg: Mg2+-free ACSF; TTX: 1 µM TTX added to nACSF; TTX, Cd: 1 µM TTX and 200 µM Cd2+ added to nACSF; 0 Mg, TTX, Cd: 1 µM TTX and 200 µM Cd2+
added to Mg2+-free ACSF; TTX, Cd, APV: 1 µM TTX, 200 µM Cd2+, and 50 µM DL-APV added to nACSF; TTX,
Cd,APV, dCK: 1 µM TTX, 200 µM Cd2+, 50 µM DL-APV, and 30 µM 5,7-dCK added to nACSF. (B, C)
23
5. [Ca2+]i elevation during iontophoretically induced plateau potentials in basal dendrites. Recent studies have shown that the activation of NMDAR in the basal dendrites of cortical pyramidal neurons gives rise to plateau potentials that are accompanied by an elevation of [Ca2+]i (Major et al., 2008;
Oikonomou et al., 2012). In the present study, we tested if the iontophoretic application of NMDA onto the basal dendrites of CA1 pyramidal neurons triggers NMDAR-mediated plateau potentials as well as if the potentials were accompanied by an elevation of [Ca2+]i. Fig. 10 shows the activity of CA1 pyramidal
neurons in response to iontophoretic application of NMDA to proximal basal dendrites in normal ACSF containing 1.5 mM Mg2+ ions, and indicates that a plateau potential can be triggered, which is accompanied by an elevation of [Ca2+]i. The elevation of [Ca
2+
]i was observed not only at the dendrites to which the
NMDA was applied but also at the dendrites to which NMDA was not applied. The amplitude of the change in fluorescence was 98 ± 25% at the injection site and 251 ± 42% at the soma (n = 10) (Fig. 10B, C). In the presence of 1 µM TTX and 200 µM Cd2+
, the fluorescence change was almost totally abolished, with the amplitude of the change being 6.6 ± 5.6% at the injection site and 4.1 ± 3.6% at the soma (n = 3) (Fig. 10B, C). However, the amplitude and duration of the NMDA-induced plateau potential were not different from those in normal ACSF, (32 ± 2 mV and 1.72 ± 0.32 s, respectively) or from those in normal ACSF with 1 µM TTX and 200 µM Cd2+
24
Fig. 10. Cd2+ blockes Ca2+ elevation during NMDA-induced plateau potentials in basal dendrites.
(A) Fluorescence image of a neuron filled with the Ca2+ indicator fluo-4. Triangle indicates the position of a pipette filled with 50 mM NMDA used for iontophoresis. (B) Responses of the neuron during iontophoretic application of NMDA (arrows, 200 nA, 400 ms). Traces 1–5 show fluorescent changes recorded from the five ROIs indicated in (A). Bottom traces show membrane potentials recorded from the cell body. Left: Responses in normal ACSF Right: Responses in the presence of 1 µM TTX and 200 µM Cd2+
. (C) Pooled data for comparing the changes in fluorescence in normal ACSF and in the presence of 1 µM TTX and 200 µM Cd2+. The mixture of 1 µM TTX and 200 µM Cd2+
significantly suppressed fluorescence. Error bars represent the mean ± SEM. *p < 0.05, **p < 0.01. (D) Pooled data for comparing the amplitudes and half-width of membrane potentials recorded from the soma in normal ACSF and in the presence 1 µM TTX and 200 µM Cd2+
25
Discussion
1: Summary.
In this study, we have shown that extrasynaptic NMDAR-mediated plateau potentials in hippocampal CA1 pyramidal neurons are accompanied by an elevation of intracellular Ca2+. Although the plateau potentials were generated in the presence of a mixture of blockers for voltage-gated Na+ channels and Ca2+ channels, the elevation of intracellular Ca2+ was markedly suppressed in this condition. This implies that the primary source for the Ca2+ elevation during NMDAR-mediated plateau potentials is entry of Ca2+ through voltage-gated Ca2+ channels. The main role of NMDAR is to provide membrane depolarization for secondary processes, such as activation of VGCCs.
Fig. 11. Role of NMDARs in excitatory synaptic activity.
Diagram showing the summary of this study. Yellow arrows indicate Ca2+ flows, and blue indicate Na+ flows. (A) Many studies persist that major role of the NMDAR is to provide [Ca2+]i increase by
entering extracellular Ca2+ through NMDAR-ion channels. (B) In this study, we claim that the main source of [Ca2+]i increase during NMDAR activity is due to activation of VGCCs. The locations of
activated NMDARs, synaptic or extrasynaptic, may work to detect the strength of inputs from the pre-synaptic neuron.
26
2: Sources of Ca2+ elevation during NMDAR-mediated plateau potentials.
There have been many studies examining NMDAR-mediated Ca2+ elevation in neurons. Many of these studies claimed that Ca2+ elevation was due to the entry of Ca2+ through NMDAR based on observations that NMDAR antagonists blocked the Ca2+ elevation (Muller & Connor, 1991; Yuste & Denk, 1995; Koester & Sakmann, 1998; Mainen et al., 1999; Ngo-Anh et al., 2004; Nimchinsky et al., 2004; Noguchi et
al., 2005; Sobczyk et al., 2005; Sobczyk & Svoboda, 2007) (Fig. 11A). These studies did not prove that the
Ca2+ elevation was indeed due to the entry of Ca2+ through NMDAR. However, they did not rule out the possibility that membrane depolarization and Ca2+ elevation due to NMDAR activation may have triggered secondary Ca2+ elevation.
Release of Ca2+ from internal stores is one such secondary process. Emptage et al. (1999) demonstrated that synaptically evoked transient Ca2+ in the dendritic spines of hippocampal pyramidal cells is NMDAR-mediated and that the main source of Ca2+ is the release of Ca2+ from internal stores. It has been shown that Ca2+ entry can trigger Ca2+ waves, which are large Ca2+ elevations due to the release of Ca2+ from intracellular Ca2+ stores, on the condition that mGluRs are activated and the level of inositol 1, 4, 5-trisphosphate (IP3) is sufficiently high (Nakamura et al., 2000; Manita & Ross, 2009; Fitzpatrick et al.,
2009). In the present study, the antagonists for mGluRs significantly suppressed Ca2+ elevation at the soma, but not at the dendrites, showing that Ca2+ release is partially responsible for the Ca2+ elevation associated with plateau potentials.
27
study did not rule out the involvement of other types of Ca2+ channels because the blockers for other Ca2+ channels would block synaptic transmission; therefore, could not be used to test this possibility. Thus, our conclusion is consistent with previous studies examining the source of the NMDAR-mediated Ca2+ elevation.
3: Types of voltage-gated Ca2+ channels responsible for the Ca2+ elevation associated with NMDAR-mediated plateau potentials.
As mentioned above, a few studies have reported that voltage-gated Ca2+ channels mildly contribute to the Ca2+ elevation associated with the activation of NMDAR. However, in our study, the Ca2+ elevation was markedly suppressed by bath application of Cd2+ ions, implying that the Ca2+ elevation associated with the NMDAR-mediated plateau potentials is mainly due to entry through voltage-gated Ca2+ channels. Our conclusion is based on the blockage of voltage-gated Ca2+ channels by Cd2+ ions. Bloodgood et al. (2007) did not use Cd2+ or Ni2+ because of concerns that these ions may suppress NMDAR and quench the fluorescence signal. In our study, Cd2+ did not block the NMDAR-mediated plateau potential, as shown in Fig. 07. Christie et al. (1995) and Nakamura et al. (2000) measured the rise of Ca2+ associated with Na+ action potentials and Ca2+ waves, respectively, in the presence of 200 µM Cd2+. These facts assured us that 200 µM Cd2+
would not interfere with the activation of NMDAR or the Ca2+ imaging.
The family of voltage-gated Ca2+ channels consists of 10 subtypes: L-(Cav1.1, Cav1.2, Cav1.3, Cav1.4),
P/Q-(Cav2.1), N-(Cav2.2), R-(Cav2.3), and T-(Cav3.1, Cav3.2, Cav3.3) type (Catterall et al., 2005). It is of
interest which types are most likely to be responsible for the Ca2+ elevation associated with the NMDAR-mediated plateau potential. L-, N-, P/Q-, and T-type are unlikely to be involved because the mixture of specific blockers for these channels had only mild effects on NMDAR-mediated Ca2+ elevation in the above-mentioned studies, leaving R-type as the only type left unexamined. According to Bloodgood
et al. (2007), a mixture of various types of voltage-gated Ca2+ channels, including 300 nM SNX-482, to block R-type Ca2+ channels suppressed glutamate-induced transient Ca2+ by only 28%. However, at this concentration, SNX-482 might not have fully suppressed R-type Ca2+ channels (Newcomb et al., 1998) and other sources might have contributed the Ca2+ elevation. Thus, it is likely that the R-type Ca2+ channel is responsible for the Ca2+ elevation that accompanies NMDAR-mediated plateau potentials, and this notion is consistent with previous studies.
The R-type Ca2+ channel has been shown to be involved in many aspects of neuronal activities. Christie et
28
shift the gating towards the depolarized range. However, a specific blocker for the R-type Ca2+ channels expressed in hippocampal neurons is not yet available. Characterizing the properties of R-type Ca2+ channels would be critically important for understanding the function of NMDAR.
4: Possible roles of NMDAR-mediated plateau potentials.
Since that first report by Schiller et al. (2000), there have been many studies reporting on NMDA-spikes or NMDAR-mediated plateau potentials in basal dendrites and apical tuft of neocortical pyramidal neurons (Schiller, et al., 2000; Milojkovic, et al., 2005; Rhodes, 2006; Gordon, et al., 2006; Milojkovic, et al., 2007; Major, et al., 2008; Larkum, et al., 2009; Polsky, et al., 2009; Chalifoux & Carter, 2011; Okinomou,
et al., 2012). In hippocampal CA1 pyramidal neurons, smaller number of studies have reported
NMDA-spikes, NMDAR-mediated plateau potentials and NMDAR-mediated depolarizing after potentials (Wei, et al., 2001; Enoki, et al., 2004; Suzuki, et al., 2008; Manita, et al., 2011). The "super slow after burst" reported by Lozovaya (2004) may have similar processes underlying the phenomenon. We surmise that these NMDAR-mediated activities in hippocampal neurons show similar properties reported for neocortical pyramidal neurons, although it awaits further studies to conclude if it really is the case.
As stated above, we think it likely that activation of GluN2D containing NMDAR is responsible for the NMDAR-mediated plateau potentials in our study. If the potential can be generated in physiological conditions , however, the activities should be a consequence of various mechanisms including activation of synaptically located NMDAR and voltage-gated Ca2+ channels. We would like to suggest to examine involvement of GluN2D containing NMDAR in analyzing the NMDAR mediated sustained depolarization in neocortical pyramidal neurons as well. For example, Milojkovic, et al. (2007) reported APV sensitive post-plateau potentials in addition to NMDA dependent plateau potential in the basal dendrites of prefrontal cortex layer 5 pyramidal neurons. Activation of separate types of NMDAR with different kinetic properties and location might be involved in generating the potentials.
In hippocampal pyramidal neurons, NMDAR-mediated spikes / plateau potentials were observed only in artificial conditions, such as under blockade of glutamate transporters or GABA receptors (Enoki, et al., 2004), and whether the potential can be generated in physiological conditions is a matter of speculation. One possible condition in which generation of NMDAR-mediated spikes / plateau would be facilitated, is a situation in which clear theta oscillation is seen (Buzsaki 2002). A possible depolarization and hyperpolarization in the basal dendrites and apical tufts due to potential gradient (Bragin, et al., 1995), possible waxing and waning GABAergic inputs, and possible AChR-mediated modulation of various cellular properties during theta oscillation might provide favorable conditions for NMDAR-mediated potentials.
29
accompany synaptic inputs in hippocampal CA1 pyramidal neurons and has shown that the elevation is primarily due to activation of voltage gated Ca2+ channels (Miyakawa, et al., 1992). In neocortical layer 5 pyramidal neuons, Markram, et al. (1994) have reported similar results. Thus, the primary role of NMDAR, irrespective of the location and types, seem to be providing sustained depolarization (Suzuki, et al., 2008), not providing large Ca2+ elevation. As has been reviewed by many researchers working on dendritic integration (Stuart, et al., 2007), electrical activities of dendrites are target of diverse regulatory mechanisms, and the NMDAR-mediated electrical activity also is a target of various processes (Zito, et al., 2009; Antic, et al., 2010; Paoletti, et al., 2013).
Mechanisms for large elevation of intracellular Ca2+, including Ca2+ entry through NMDAR, Ca2+ entry through voltage-gated Ca2+ channels and Ca2+ release from intracellular stores, also are subject to various regulatory mechanisms (Ross, 2012; Verkhratsky, 2004). Although activation of NMDARs would give rise to large elevation of intracellular Ca2+, it is not a direct consequence of NMDAR activation rather a consequence of the activation of secondary processes that provide large source of Ca2+.
30
Acknowledgements
First of all, I would like to thank my research adviser, Professor Hiroyoshi Miyakawa, for his continous encouragement and valuable advices for a long period of time. I would also like to thank Associate Professor Takako Morimoto, Lecturer Masashi Inoue, and Assistant Professor Yoichi Seki for their helpful advices and comments. Next, I would like to express my great appreciation to Professor A.Kamikouchi at Nagoya University, Professor Kenichi Homma, Professor Sato Hommma, and Assistant Professor R.Enoki at Hokkaido University for their helpful advices and comments.
31
Abbreviations
[Ca2+]i, intracellular calcium concentrations; 5,7-dCK, 5,7-dichlorokynurenic acid; ACSF, artificial
cerebrospinal fluid; AMPAR, α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ANOVA, analysis of variance; CA1, cornu ammonis field 1 of the hippocampal formation; CNQX,
6-Cyano-7-nitroquinoxaline-2,3-dione; D-APV, D-2-amino-5-phosphonopentanoic acid; DL-APV,
DL-2-amino-5-phosphonopentanoic acid; EGTA, Ethylene glycol
bis(β-aminoethyether)-N,N,N,N-tetraacetic acid; EPSP, excitatory postsynaptic potential; iGluR, ionotropic glutamate receptor; IP3, inositol 1, 4, 5-trisphosphate; IPSP, inhibitory postsynaptic potential; KA-R,
kainate receptor; LY367385, (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid; mGluR, metabotropic glutamate receptor; MPEP, 2-methyl-6-(phenylethynyl)pyridine; NMDA,
32
References
Agnati, L. F., Fuxe, K., Nicholson, C. & Sykova, E. (2000) Volume transmission revisited. Progress in
Brain Research 125, Elsevier Science, Amsterdam.
Agnati L. F., Guidolin D., Guescini M., Genedani S. & Fuxe K. (2010) Understanding wiring and volume transmission. Brain Res. Rev., 64, 137-159.
Amaral, D., G. & Witter, M., P. (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience, 31(3), 571-591.
Andersen, P., Morris, R., Amaral, D., Bliss, T. & O'Keefe, J. (2007) The Hippocampus Book. Oxford
University Press, New York.
Antic, S.D. Zhou, W-L. Moore, A.R. & Short, S.M. (2010) The decade of the dendritic NMDA spike. J.
Neurosci. Res., 88, 2991-3001.
Augustine, G., J., Santamaria, F. & Tanaka, K. (2003) Local calcium signalling in neurons. Neuron, 40, 331–346.
Berridge, M., J. (1998) Neuronal calcium signaling. Neuron, 21, 13–26.
Bloodgood, B. L. & Sabatini, B. L. (2007) Nonlinear regulation of unitary synaptic signals by CaV2.3
voltage-sensitive calcium channels located in dendritic spines. Neuron 53, 249-260.
Bloodgood, B. L., Giessel, A. J., Sabatini, B. L. (2009) Biphasic Synaptic Ca Influx Arising from Compartmentalized Electrical Signals in Dendritic Spines. PLos Biology, 7, issue 9, e1000190.
Bragin, A., Jandó, G., Nádasdy, Z., Hetke, J., Wise, K., Buzsáki, G. (1995) Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J. Neurosci., 15, 47-60.
Burnashev, N., Zhou, Z., Neher, E. & Sakmann, B. (1995) Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J. Physiol., 485, 403-418.
Buzsaki, G. (2002) Theta oscillations in the hippocampus. Neuron, 33, 325-340.
33
Catterall, W., A. (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol.,
16, 521-555.
Catterall, W. A., Perez-Reyes, E., Snutch, T. P. & Striessnig, J. (2005) International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol.
Rev., 57, 411-425.
Chalifoux, J. R., & Carter, A. G. (2011) Glutamate spillover promotes the generation of NMDA spike.
J.Neurosci., 31, 16435-16446.
Chamberland, S. & Topolnik, L. (2012) Inhibitory control of hippocampal inhibitory neurons. Front.
Neurosci., 6, 165.
Christie, B. R., Eliot, L. S., Ito, K., Miyakawa, H. & Johnston, D. (1995) Different Ca2+ channels in soma and dendrites of hippocampal pyramidal neurons mediates spike-induced Ca2+ influx. J. Neurophysiol., 73, 2553-2557.
Cull-Candy, S., G. & Leszkiewicz, D., N. (2004) Role of distinct NMDA receptor subtypes at central synapses. Sci. STKE, 2004, re16.
Dingledine, D., Borges, K., Bowie, D., & Traynelis, S. F. (1999) The glutamate receptor ion channels.
Dudman, J., T., Tsay, D. & Siegelbaum, S., A. (2007) A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long‑term plasticity. Neuron, 56, 866–879.
Emptage, N., Bliss, T. V. P. & Fine, A. (1999) Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spikes. Neuron, 22, 115-124.
Enoki, R., Kiuchi, T., Koizumi, A., Sasaki, G., Kudo, Y. & Miyakawa, H. (2004) NMDA receptor-mediated depolarizing after-potentials in the basal dendrites of CA1 pyramidal neurons. Neurosci. Res., 48, 325-333.
Fitzpatrick, J. S., Hagenston, A. M., Hertle, D. N., Gipson, K. E., Bertetto-D'Angelo, L. & Yeckel, M. F. (2009). Inositol-1,4,5-trisphosphate receptor-mediated Ca2+ waves in pyramidal neuron dendrites propagate through hot spots and cold spots. J. Physiol., 587, 1439-1459.
34
Gordon, U., Polsky, A. & Schiller,J. (2006) Plasticity Compartments in Basal Dendrites of Neocortical Pyramidal Neurons. J.Neurosci., 26, 12717-12726.
Hansen, K., B., Bräuner-Osborne, H. & Egebjerg, J. (2008) Pharmacological characterization of ligands at recombinant NMDA receptor subtypes by electrophysiological recordings and intracellular calcium measurements. Comb. Chem. High Throughput Screen., 11(4), 304-315.
Hardingham, G. E. & Bading, H. (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci., 11, 682-696.
Herman, M., A. & Jahr, C., E. (2007) Extracellular glutamate concentration in hippocampal slice. J.
Neurosci., 27, 9736-9741.
Hong, M. & Ross, W., N. (2007) Priming of intracellular calcium stores in rat CA1 pyramidal neurons. J.
Physiol., 584, 75–87.
Huang, E., P. (1998). Synaptic transmission: spillover at central synapses. Curr. Biol., 8(17), R613-R615.
Huang, Y., H., Sinha, S., R., Tanaka, K., Rothstein, J., D. & Bergles, D., E. (2004) Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J.
Neurosci., 24, 4551-4559.
Isaacson, J., S. (2000). Spillover in the spotlight. Curr. Biol., 10(13), R475-477.
Jaffe, D. B., Johnston, D., Ross, N. L., Lisman, J. E., Miyakawa, H. & Ross, W. N. (1992) The Spread of Na+ Spikes Determines the Pattern of Dendritic Ca2+ Entry into Hippocampal Neurons. Nature, 357, 244-246.
Jahr, C., E. & Stevens, C., F. (1990). A quantitative description of NMDA receptor channel kinetic behavior.
J. Neurosci., 10, 1830–1837.
Kantrowitz, J. T. & Javitt D. C. (2010) N-methyl-D-aspartate (NMDA) receptor dysfunction or
dysregulation: The final common pathway on the road to schizophrenia. Brain Res. Bull., 83, 108-121.
35
Kovalchuk, Y., Eilers, J., Lisman, J., Konnerth, A. (2000) NMDA receptor-mediated subthreshold Ca2+ signals in spine of hippocampal neurons. J. Neurosci., 20, 1791-1799.
Kullmann, D. M. (1999). Synaptic and extrasynaptic roles of glutamate in the mammalian hippocampus.
Acta Physiol Scand., 166(2), 79-83.
Kullmann, D., M. (2000) Spillover and synaptic cross talk mediated by glutamate and GABA in the mammalian brain. In: Agnati, L., F., Fuxe, K., Nicholson, C. & Sykova, E. (Eds) Volume transmission revisited. Prog. Brain Res., 125, Elsevier Science, Amsterdam, pp339-361.
Kuner, T. & Schoepfer, R. (1996) Multiple structural elements determine subunit specificity of Mg2+
block in NMDA receptor channels. J. Neurosci., 16, 3549–3558.
Larkum, M.E., Nevian, T., Sandler, M., Polsky, A. & Schiller, J. (2009) Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: A new unifying principle., Science, 325, 756-760.
Lau, C. G., Takeuchi, K., Rodenas-Ruano, A., Takayasu, Y., Murphy, J., Bennett, M. V. and Zukin, R. S. (2009) Regulation of NMDA receptor Ca2+ signaling and synaptic plasticity. Biochem. Soc. Trans., 37, 1369-1374.
Lisman, J., E., Talamini, L., M. & Raffone. (2005) A.Recall of memory sequences by interaction of the dentate and CA3: a revised model of the phase precession. Neural Netw., 18(9), 1191-1201.
Lozovaya, N.A., Grebenyuk, S.E., Tsintsadze, T., Feng, B., Monaghan, D.T. & Krishtal, O.A. (2004) Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape 'superslow' afterburst EPSC in rat hippocampus. J. Physiol., 558, 451-463.
Mainen, Z. F., Malinow, R. & Svoboda K. (1999) Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature, 399, 151-155.
Major, G., Polsky, A., Denk, W., Schiller, J. & Tank, D. W. (2008) Spatiotemporally graded NMDA spike/plateau potentials in basal dendrites of neocortical pyramidal neurons. J. Neurophysiol., 99, 2584-601.
Major, G., Polsky, A., Denk, W., Schiller, J. & Tank, D.W. (2008) Spatiotemporally graded NMDA spike/plateau potentials in basal dendrites of neocortical pyramidal neurons. J. Neurophysiol., 99, 2584-2601.
36
frequency of spontaneous elementary Ca2+ release events in the dendrites of pyramidal neurons. J.
Neurosci., 29, 7833-7845.
Manita, S. & Ross, W., N. (2010) IP3 mobilization and diffusion determine the timing window of Ca 2+
release by synaptic stimulation and a spike in rat CA1 pyramidal cells. Hippocampus, 20, 524–539.
Manita, S., Miyazaki, K. & Ross, W. N. (2011) Synaptically activated Ca2+ waves and NMDA spikes locally suppress voltage-dependent Ca2+ signaling in rat pyramidal cell dendrites. J. Physiol., 589, 4903-4920.
Markram, H. & Sakmann, B. (1994) Calcium transients in apical dendrites evoked by single sub-threshold excitatory post-synaptic potentials via low voltage-activated calcium channels. Proc. Natl. Acad. Sci. USA,
91, 5207-5211
Mathie, A., Sutton, G. L., Clarke, C. E. & Veale, E. L. (2006) Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol. Ther., 111, 567-583.
Maycox, P., R., Hell, J., W. & Jahn, R. (1990) Amino acid neurotransmission: spotlight on synaptic vesicles.
Trends. Neurosci., 13, 83-87.
Mayer, M., L. (2005) Glutamate receptor ion channels. Curr. Opin. Neurobiol., 15, 282-288.
Metz, A. E., Jarsky, T., Martina, M. & Spruston, N. (2005) R-type calcium channels contribute to afterdepolarization and bursting in hippocampal CA1 pyramidal neurons. J. Neurosci., 25, 5763-5773.
Milojkovic, B.A. Radojicic, M.S. & Antic, S.D. (2005) A strict correlation between dendritic and somatic plateau depolarizations in the rat prefrontal cortex pyramidal neurons. J. Neurosci., 25, 3940-3951.
Milojkovic, B.A., Zhou, W-L. & Antic, S.D. (2007) Voltage and calcium transients in basal dendrites of the rat prefrontal cortex. J.Physiol., 585, 447-468.
Miyakawa, H., Ross, W. N., Jaffe, D. B., Callaway, J. C., Ross, N. L., Lisman, J. E. & Johnston, D. (1992) Synaptically Activated Increase in Calcium Concentration in the Dendrites of Hippocampal CA1 Pyramidal Cells is Primarily Due to Voltage-Gated Ca2+ Channels. Neuron, 9, 1163-1173.
Momiyama, A. (2000) Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. J. Physiol., 523, 621–628.
37
Nakamura, T., Barbara, J. G., Nakamura, K. & Ross, W. N., (1999) Synergistic release of Ca2+ from
IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron, 24, 727-737.
Nakamura, T., Nakamura, K., Lasser-Ross, N., Barbara, J. G., Sandler, V. M. and Ross, W. N. (2000) Inositol 1,4,5-trisphosphate (IP3)-mediated Ca
2+
release evoked by metabotropic agonists and
backpropagating action potentials in hippocampal CA1 pyramidal neurons. J. Neurosci., 20, 8365-8376.
Nakazawa, K., Quirk, M., C., Chitwood, R., A., Watanabe, M., Yeckel, M., F., Sun, L., D., Kato, A., Carr, C., A., Johnston, D., Wilson, M., A. & Tonegawa, S. (2002) Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science, 297(5579), 211-218.
Newcomb, R., Szoke, B., Palma, A., Wang, G., Chen, Xh., Hopkins, W., Cong, R., Miller, J., Urge, L., Tarczy-Hornoch, K., Loo, J. A., Dooley, D. J., Nadasdi, L., Tsien, R. W., Lemos, J. & Miljanich, G. (1998) Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry, 37, 15353-15362.
Ngo-Anh, T. J., Bloodgood, B. L., Lin, M., Sabatini, B. L, Maylie, J. & Adelman, J. P. (2004) SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat. Neurosci., 8, 642-649.
Noguchi, J., Matsuzaki, M., Ellis-Davies, G. C. & Kasai, H. (2005) Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron, 46, 609–622.
Nyitrai, G., Kékesi, K. A. & Juhász, G. (2006) Extracellular level of GABA and Glu: in vivo microdialysis-HPLC measurements. Curr. Top Med. Chem., 6(10), 935-940.
Oikonomou, K. D., Short, S. M., Rich, M. T. & Antic, S. D. (2012) Extrasynaptic glutamate receptor activation as cellular bases for dynamic range compression in pyramidal neurons. Front. Physiol., 3, article 334, 1-22.
Paoletti, P. (2011) Molecular basis of NMDA receptor functional diversity. Eur. J. Neurosci., 33, 1351–1365.
Paoletti, P., Bellone, C. & Zhou, Q. (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature Rev. Neurosci., 14, 383-400.
38 System Disorders. Neuron, 82(2), 279-293.
Petralia, R. S., Wang, Y. X., Hua, F., YI, Z., Zhou, A., Ge, L., Stephenson, F. A., & Wenthold, R. J. (2010) Organization of NMDA receptors at extrasynaptic locations. Neuroscience, 167, 68–87.
Petralia, R., S. (2012) Distribution of extrasynaptic NMDA receptors on neurons. Scientific World Journal,
2012, 267120.
Pharmacological Review, 51, 7-61.
Phillips, W. A. & Silverstein, S. M. (2003) Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behavioral and Brain Sciences, 26, 65-82.
Polsky, A., Mel, B. & Schiller, J. (2009) Encoding and decoding bursts by NMDA spikes in basal dendrites of layer 5 pyramidal neurons. J.Neurosci., 29, 11891-11903.
Reichelt, W. & Knopfel, T. (2002) Glutamate uptake controls expression of a slow postsynaptic current mediated by mGluRs in cerebellar Purkinje cells. J. Neurophysiol., 87, 1974-1980.
Reimer, R., J., Fremeau, R., T., Jr, Bellocchio, E., E. & Edwards RH. (2001) The essence of excitation. Curr.
Opin. Cell Biol., 13, 417-421.
Rhodes, P. (2006) The properties and implications of NMDA spikes in neocortical pyramidal cells.
J.Neurosci., 26, 6704-6715.
Ross, W. N. (2012) Understanding calcium waves and sparks in central neurons. Nat. Neurosci., 13, 157-168.
Sabatini, B., L., Oertner, T., G. & Svoboda, K. (2002). The life cycle of Ca2+ ions in dendritic spines.
Neuron, 33, 439–452.
Sawa, A., Snyder, S. H., (2002) Schizophrenia: Diverse approaches to a complex disease. Science, 296, 692-695.
Schiller, J., Helmchen, F., & Sakmann, B. (1995). Spatial profile of dendritic calcium transients evoked by action potentials in rat neocortical pyramidal neurones. J. Physiol., 487, 583–600.
39 pyramidal neurons. Nature, 404, 285-289.
Schneggenburger, R., Zhou, Z., Konnerth, A. & Neher, E. (1993) Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron, 11, 133–143.
Schoepp, D. D., Jane, D. E. & Monn, J. A. (1999) Pharmqcological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology, 38, 1431-1476.
Sem'yanov, A. V. (2005) Diffusional extrasynaptic neurotransmission via glutamate and GABA. Neurosci.
Behav. Physiol., 35, 253-66.
Shcheglovitov, A., Vitko, I., Lazarenko, R. M., Orestes, P., Todorovic, S. M. & Perez-Reyes, E. (2012) Molecular and biophysical basis of glutamate and trace metal modulation of voltage-gated Cav2.3 calcium
channels. J. Gen. Physiol., 139, 219-234.
Shigemoto, R., Kinoshita, A., Wada, E., Nomura, S., Ohishi, H., Takada, M., Flor, P. J., Neki, A., Abe, T., Nakanishi, S. & Mizuno, N. (1997) Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci., 17, 7503–7522.
Skeberdis, V. A., Chevaleyre, V., Lau, C. G., Goldberg, J. H., Pettit, D. L., Suadicani, S. O., Lin, Y., Bennett, M. V., Yuste, R., Castillo, P. E. & Zukin, R. S. (2006) Protein kinase A regulates calcium permeability of NMDA receptors. Nat. Neurosci., 9, 501-510.
Sobczyk, A., Scheuss, V. & Svoboda, K. (2005) NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocamapl dendritic spikes. J. Neurosci., 25, 6037-6046.
Sobczyk, A. & Svoboda, K. (2007) Activity-dependent plasticity of the NMDA receptor fractional Ca2+ current. Neuron, 53, 17-24.
Stuart, G., Spruston, N. & Hausser, M. (Eds) (2007) "dendrites " 2nd Ed. , Oxford University Press, USA.
Sugihara, H., Moriyoshi, K., Ishii, T., Masu, M. and Nakanishi, S. (1992) Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem. Biophys. Res. Commun., 185, 826-832.
40
Neurosci., 28, 521-534.
Tai, C., Kuzmiski, J. B. & MacVicar, B. A. (2006) Muscarinic enhancement of R-type calcium currents in hippocampal CA1 pyramidal neurons. J. Neurosci., 26, 6249-6258.
Takumi, Y., Ramírez-León, V., Laake, P., Rinvik, E. & Ottersen, O. P. (1999) Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat. Neurosci., 2(7), 618-624.
Thompson, C., L., Drewery, D., L., Atkins, H., D., Stephenson, F., A. & Chazot, P., L. (2002) Immunohistochemical localization of N-methyl-D-aspartate receptor subunits in the adult murine hippocampal formation: evidence for a unique role of the NR2D subunit. Brain Res. Mol. Brain Res., 102, 55-61.
Traynelis, S., F., Wollmuth, L., P., McBain, C., J., Menniti, F., S., Vance, K., M., Ogden, K., K., Hansen, K., B., Yuan, H., Myers, S., J. & Dingledine, R. (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev., 62, 405-496.
Visi, E.S. (2000) Role of high-affinity receptors and membrane transporters in nonsynaptic communication and drug action in the central nervous systems. Phramacol. Rev., 52, 63-90.
Watanabe, S., Hong, M., Lasser‑Ross, N. & Ross, W., N. (2006) Modulation of calcium wave propagation in the dendrites and to the soma of rat hippocampal pyramidal neurons. J. Physiol., 575, 455–468.
Waters, J., Schaefer, A. & Sakmann, B. (2005) Backpropagating action potentials in neurones: measurement, mechanisms and potential functions. Prog. Biophys. Mol. Biol., 87(1), 145-170.
Wei, D-S., Mei, Y-A., Bagel, A., Kao, J.P.Y., Thompson, S.M. & Tang, C-M. (2001) Compartmentalized and binary behavior of terminal dendrites in hippocampal pyramidal neurons. Science, 293, 2272-2275.
Yuste, R., & Denk, W. (1995). Dendritic spines as basic functional units of neuronal integration. Nature,
375, 682–684.