Title
Japanese Black Cattle with Orotic Aciduria Detected by Gas-
Chromatography/Mass-Spectrometry(Internal Medicine)( 本文
(Fulltext) )
Author(s)
OHBA, Yasunori; TAKASU, Masaki; NISHII, Naohito;
HOSODA, Iwai; KITOH, Katsuya; MATSUMOTO, Isamu;
ZHANG, Chunhua; KITAGAWA, Hitoshi
Citation
[The journal of veterinary medical science] vol.[69] no.[3]
p.[313]-[316]
Issue Date
2007-03-25
Rights
The Japanese Society of Veterinary Science (社団法人日本獣医
学会)
Version
出版社版 (publisher version) postprint
URL
http://hdl.handle.net/20.500.12099/28589
ABSTRACT. A 4-months-old calf of Japanese black cattle was diagnosed with orotic aciduria by gas-chromatography/mass-spectromerty (GC/MS). Until now orotic aciduria had not been reported in Japanese black cattle. The animal showed repeated diarrhea. The hema-tocrit was low, and microcytes and acanthocytes were observed in blood smears. The calf had lower serum total protein concentrations with a higher blood ammonia concentration. Needle-shaped crystals of orotic acid were observed in urinary sediments. Sequence homol-ogous analysis with cattle uridine monophosphate synthase DNA indicated silent mutation in the affected calf.
KEY WORDS: gas chromatography/mass spectrometry, Japanese black cattle, orotic aciduria.
J. Vet. Med. Sci. 69(3): 313–316, 2007
Orotic aciduria is a condition in which orotic acid is excreted into urine. In humans, slight to moderate increases have been observed in pregnancy and premature births and in patients with heart failure, hypertension, diabetes, malig-nancy, trauma, liver damage, and so on [14]. High urinary excretions of orotic acid were found in conditions that induce hyperammonemia and an accumulation of intramito-chondrial carbomyl phosphate [8]. Moreover, most cases of orotic aciduria arise from inherited defects of enzymes involved in the urea cycle after the synthesis of intramito-chondrial carbamoyl phosphate. Such defects include ornitine transcarbamylase (OTC) deficiency [2], arginine-mia, lysinuric protein intolerance [6], and argininosuccinic acid synthetase deficiency [9], as well as a deficiency of uri-dine monophosphate synthase (UMPS) [13]. In veterinary medicine, the hereditary orotic aciduria in Holstein and Red Holstein cattle has been made famous in cattle as the DUMPS (deficiency of UMP synthase) [1, 7]. Until now, however, orotic aciduria had not been reported in Japanese black cattle.
Gas chromatography/mass spectrography (GC/MS) is among the useful tools available for detecting metabolic dis-orders because of its higher accuracy and sensitivity. GC/ MS can detect a large number of metabolites at one time, and it is widely used in mass-screening tests for the detec-tion of metabolic disorders in newborn children [4]. We have applied GC/MS for the detection of metabolic disor-ders in frail and growth retarded Japanese black cattle, and have detected a calf with orotic aciduria.
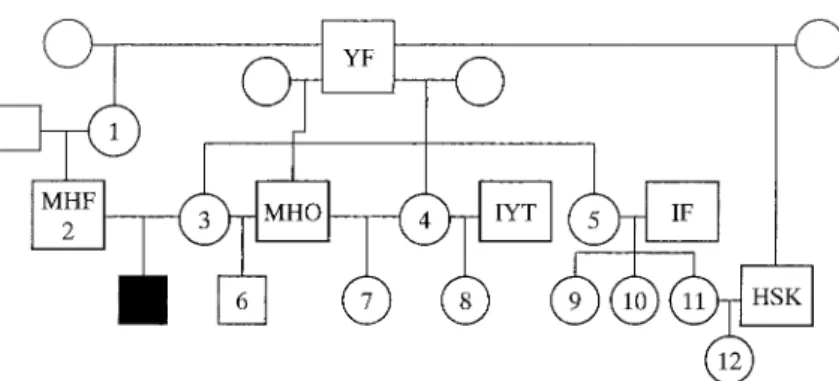
The affected calf was a 4.0-month-old male. Figure 1 shows the pedigree network of the affected calf including 12 consanguineous cattle examined. A GC/MS system
(QP5050, Shimadzu Corporation, Kyoto) with an Ultra Alloy Plus-5 column and a metal capillary column (30 mm × 0.25 mm, i.d., 0.25 µm) was used for analyses of the affected calf’s and 12 cattle’s urine. Results of the GC/MS of urine in normal, and affected calves are shown in Fig. 2. In a normal calf the peak of orotic acid was ambiguous (Fig. 2A). In the affected cattle, the peak was sprung (Fig. 2B). The normal calf is No.6 (Fig. 1). In the other 11 cattle as well as No. 6 the peak of orotic acid was ambiguous.
In addition, the calf’s serum specimen showed an obvious peak of orotic acid (data not shown).
The affected calf showed growth retardation and emacia-tion with consistently body weight, very low, i.e., 60 kg and 42 kg at 4.0 and 5.5 months of age, respectively. His rectal temperature was 39.0 centigrade, heart rate 78 beats/min, and respiratory rate 18 respirations/min. From birth to death, the calf suffered repeated aqueous diarrhea that showed no response to antibiotics or sulfa drugs. His appe-tite ranged from normal to sub-normal.
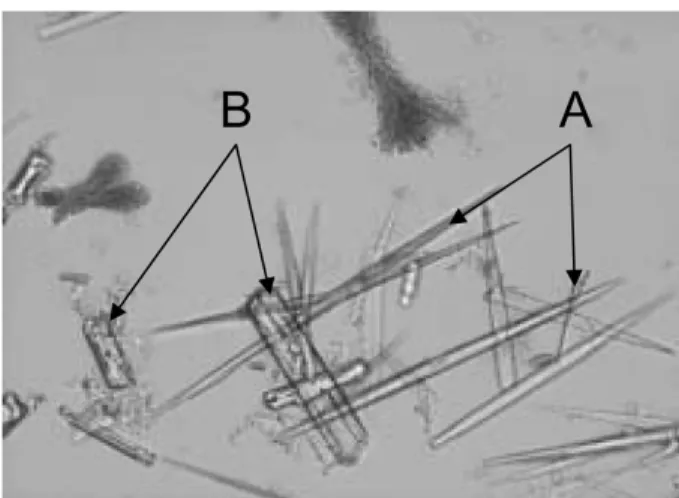
As shown in Table 1, the RBC, WBC and mean corpus-cular volumes were normal, and the hematocrit was slightly low. Microcytes and acanthocytes were observed in blood smears, but megaloblasts could not be detected. The calf had slightly high serum AST activity, and somewhat low concentrations of serum urea nitrogen, creatinine, total pro-tein, albumin, and glucose. At 5.5 months of age, the calf had hypoglycemia and hyper-ammoniemia. Serum insulin-like growth factor-1, insulin, total tri-iodothyronine, total thyroxine, and cortisol concentrations were low. In urinaly-sis, ketone body and protein showed positive with a dipstick test paper. The pH (8.5) and specific gravity (1.038) were normal. In urine sediments, needle-shaped crystals coin-cided with orotic acid crystals, while struvite (magnesium ammonium phosphate)-like crystals were also observed (Fig. 3). Genomic DNAs of the affected calf and 12 cattle were extracted from blood or sperm samples. All the region
* CORRESPONDENCE TO: KITAGAWA, H., Laboratory of Internal Medi-cine, Division of Veterinary MediMedi-cine, Faculty of Applied Bio-logical Sciences, Gifu University, 1–1 Yanagido, Gifu 501– 1193, Japan.
Y. OHBA ET AL.
314
of UMPS of the 13 cattle was amplified by PCR using of the data (Schoeber and others 1993) [10] and were sequenced by the dideoxy chain termination method with GeneRapid
automated DNA sequencer (Amersham Biosciences, Piscat-away, U.S.A.). Sequence homologous analysis with cattle UMPS DNA [10, 11] indicated silent mutation (ACT to
Fig. 1. Pedigree network of the family of calf with orotic aciduria. : Affected calf, : male, : female. Circles and squares with the number indicate 12 consanguine-ous cattle examined. Squares with capital letter indicate sire.
Fig. 2. Chromatograms in urine of normal calf (A) and the affected calf (B). The allow indicates high peak of orotic acid. The normal calf is No. 6 (Fig. 1). In the other 11 cattle as well as No. 6 the peak of orotic acid was ambiguous.
ACC at codon 135, threonine to threonine) in the affected calf and his parents. The others 10 cattle had only normal sequence (ATC).
The calf was euthanized because of severe prostration. At necropsy, muscle and adipose tissues were in very poor condition. Mucous membranes of the duodenum, jejunum, ileum and cecum were thin and fragile, and their chorions were edematous. Dropsy was noted in the pleura, perito-neum, mesentery, and pericardium. In the mesentery, some enlarged lymph nodes 3 to 4 cm in size were observed. In the right lung, cranial and middle lobes were hepatized. Histopathologically, the central veins were hemostatic, and the hepatocytes surrounding them were vacuolated in the liver. Histiocytes and fibroblasts were observed around Glisson’s capsule. In the spleen, the red pulp was atrophied and the trabeculae were clear. Using a hemosiderin-like dye, many macrophages were observed in the splenic sinus. Submucosa of the colon was edematous. In the renal cor-puscle, epithelial cells of glomeruli and distal tubules were premature with distinct nucleoli.
Breeding records of the family indicated no history of stillborn or mummified fetuses and no fetal deaths. Twelve related cattle received a clinical check, laboratory examina-tion and GC/MS analysis, but no clinical abnormalities such as growth retardation or emaciation were found. Moreover, no orotic acid crystals were found in urine nor any obvious peak of orotic acid in urine and serum specimens by GC/MS analysis.
Orotic aciduria is a disorder of pyrimidine biosynthesis de novo [3, 12]. The enzyme uridin-5-monophsophate
(UMP) synthase, which contains the activities of two enzymes in a single protein, phosphoribosyltransferase (OPRT) and orotidine-5’-monophosphatase (OMP), cata-lyzes the last two steps of UMP synthesis [14], a single gene codes for the protein [13]. DUMPS is an autosomal reces-sive hereditary disease also in cattle, for which nonsense mutation of UMP gene (CGA to TGA at codon 405, argin-ine to stop) is responsible [11]. Because high levels of orotic acid were detected in urine by GC/MS, the calf in the present study clearly suffered from orotic aciduria. How-ever, no meaningful mutation of the UMP gene other than silent mutation could be detected in the affected calf and his parents. Only one calf was affected in the family, so that it may be an orphan case of some hereditary disease, although genetic defects other than DUMPS have not been reported in cattle. Of course, there is always the possibility that the orotic aciduria is secondary to some other diseases men-tioned above. However, most cases of massive orotic aci-duria arise from inherited defects, and secondary orotic aciduria is rare in humans [8]. In any event, this may be the first report of orotic aciduria in Japanese black cattle.
GC/MS analysis of urine and serum can provide data in support of a diagnosis of inherited errors of metabolism, because the specimen contains numerous organic acids and other chemical groups of compounds at a variety of concen-trations [4, 5, 15]. Screenings for metabolic defects using GC/MS can detect diseases also in cattle early enough to devise adequate countermeasures.
REFERENCES
1. Harden, K. K. and Robinson, J. L. 1987. J. Inherit. Metab. Dis. 10: 201–209.
2 Hauser, E. R., Finkelstein, J. E., Valle, D. and Brusilow, S. W. 1990. New Engl. J. Med. 322: 1641–1645.
3. Huguley, C. M. Jr., Bain, J. A., Rivers, S. L. and Scoggins, R B. 1959. Blood 14: 615–634.
4. Matsumoto, I. 1995. pp. 3–14. In: GC/MS Practitional Chemi-cal Diagnosis (Matsumoto, I. and Sakamoto, S. eds.), Soft
Sci-Creatinine (mg/l) 0.9 0.7 Ammonia (mg/dl) ND 123 Total protein (g/dl) 5.0 3.4 Albumin (g/dl) 2.6 1.3 Glucose (mg/dl) 70 25 Total cholesterol (mg/dl) 120 40 Calcium (mg/dl) 10.9 10.7 Inorganic phosphorus (mg/dl) 6.8 5.3 Sodium (mEq/l) 145 136 Potassium (mEq/l) 4.5 4.1 Chloride (mEq/l) 102 96
Insulin-like growth factor-1 (ng/ml) ND 8.9
Insulin (mU/ml) ND 1.6
Total triiodthyronine (ng/dl) ND 49
Total thyroxine (mg/dl) ND 0.6
Cortisol (mg/dl) ND <1.0
Fig. 3. Urine sediments of the affected calf. A: Orotic acid crys-tals, B: struvite crystals.
Y. OHBA ET AL.
316
ence Publications, Tokyo (in Japanese).
5. Matsumoto, M., Zhang, C. -H., Kosugi, C. and Matsumoto, I. 1995. J. Vet. Med. Sci. 57: 205–211.
6. McManus, D. T., Moore, R., Hill, C. M., Rodgers, C., Carson, D. J. and Love, A. H. G. 1996. J. Clin. Pathol. 49: 345–347, 7. Robinson, J. L., Drabik, M. R., Dombrowski, D. B. and Clark,
J. H. 1983. Proc. Natl. Acad. Sci. U. S. A. 80: 321–323. 8. Salerno, C. and Crifò, C. 2002. J. Chromatography B 781: 57–
71.
9. Sass, J. O. and Skladal, D. 2000. Horm. Metab. Res. 32: 44–46. 10. Schoeber, S., Simon, D. and Schwenger, B. 1993. Gene 124:
307–308.
11. Schwenger, B., Schober, S. and Simon, D. 1993. Genomics 16: 241–244.
12. Smith, L. H., Sullivan, M. and Huguley, C. M. 1961. J. Clin. Invest. 40: 656–664.
13. Suchi, M., Mizuno, H., Kawai, Y., Tsuboi, T., Sumi, S., Oka-jima, K., Hodgson, M. E., Ogawa, H. and Wada, Y. 1997. Am. J. Hum. Genet. 60: 525–539.
14. Sumi, S., Kidouchi, K., Imaeda, M., Asai, M., Ito, T. and Wada, Y. 1997. Clin. Chim. Acta 266: 195–197.
15. Washizu, T., Washizu, M., Zhang, C., Matsumoto, I., Sawa-mura, M. and Suzuki, T. 2004. J. Vet. Med. Sci. 66: 701–703.