九州大学学術情報リポジトリ
Kyushu University Institutional Repository
化学選択的触媒的共役付加反応の開発
李, 釗
http://hdl.handle.net/2324/1866280
出版情報:Kyushu University, 2017, 博士(創薬科学), 課程博士 バージョン:
権利関係:Public access to the fulltext file is restricted for unavoidable reason (3)
Development of Catalytic Chemoselective Conjugate Addition Green Pharmaceutical Chemistry 3PS14027K Zhao Li
Introduction
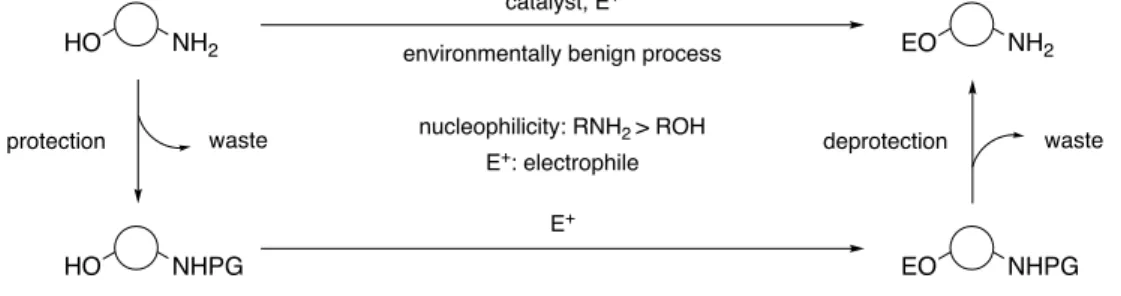
Amino groups and hydroxy groups were widely found in natural and artificial molecules.
Because the innately high nucleophilicity of amino groups than hydroxy groups, several chemoselective reactions of amino groups over hydroxy groups were reported. However, chemoselective reactions of hydroxy groups over amino groups still depended on the use of protecting groups, which would cause more unwanted steps for the installation and the removal, at the same time, accompanying more unwanted waste (Figure 1). Therefore, much attention was focused on catalytic chemoselective reactions of hydroxy groups over amino groups, which would offer new opportunities for the minimal reliance on protecting groups even in the presence of innately more reactive functionalities, amino groups, contributing to both the atom economy and the step economy.
Although excellent works on the development of catalytic chemoselective reactions of hydroxy groups over amino groups were reported recent years, these successful examples were highly limited to arylation
[1]and transesterification,
[2]and scope of amino alcohols remains unexplored.
Moreover, these reported reactions included the inevitable formation of stoichiometric amounts of unneeded co-products, such as inorganic salts and alcohols, which would reduce the reaction efficiency. Therefore, our group focused on the conjugate addition, which was of high atom- economy and without formation unneeded co-products.
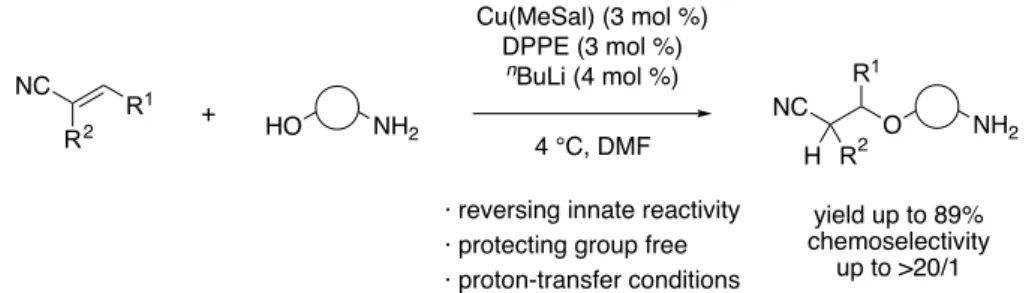
Our group first reported the catalytic hydroxy group-selective conjugate addition over amino groups to unsaturated nitrile compounds using soft Lewis acid/hard Brønsted base cooperative catalyst system in 2014 (Figure 2).
[3]Under the optimized conditions, innately less reactive hydroxy groups were selectively functionalized with wide substrate scope including β-amino
HO NH
2HO NHPG EO NHPG
EO NH
2catalyst, E
+protection
environmentally benign process
deprotection
E
+E
+: electrophile waste
nucleophilicity: RNH
2> ROH waste
Figure 1. Catalytic hydroxy group-selective reactions
Experiments and Result
Though the nitrile functionality of hydroxy groups’ adducts could be transformed into versatile functionalities by multiple steps protocol, which would decrease the efficiency and the utility of catalytic hydroxy group-selective conjugate addition. In order to install lots of functional groups to hydroxy groups in the presence of amino groups directly and selectively, α,β-unsaturated sulfonyl derivatives were selected, where functional groups were installed readily. Basing on former research mentioned above, a catalytic chemoselective functional group installation method was developed.
[4]This catalytic method had a broader scope of both α,β-unsaturated sulfonyl compounds and unprotected amino alcohols. Functional groups of high utility value, such as azide, protected amino acid and dansyl group, were installed directly to the hydroxy group. For amino alcohols, pharmaceuticals and dipeptide were also applicable.
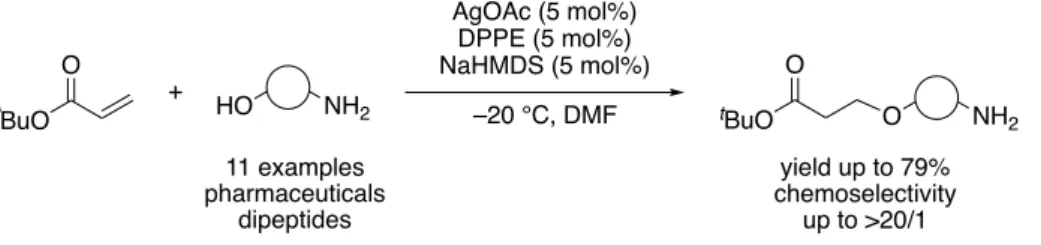
Although the use of α,β-unsaturated ester as an electrophile for catalytic conjugate addition reactions of alcohols was more advantageous for their further transformation, research was hammered by the difficulty in controlling chemoselectivity between conjugate addition (1,4- addition) and transesterification (1,2-addition). Therefore, a catalytic chemoselective conjugate addition using acrylates was also developed.
[5]Various amino alcohols, including unprecedented cyclic β-amino alcohol, were applicable to this catalysis.
Cu(MeSal) (3 mol %) DPPE (3 mol %)
n
BuLi (4 mol %) 4 °C, DMF NC
+
yield up to 89%
chemoselectivity up to >20/1
HO NH
2NC O NH
2R
1R
R
12
· reversing innate reactivity
· protecting group free
· proton-transfer conditions
H R
2Figure 2. Catalytic chemoselective conjugate addition to nitriles
S O O
HO NH
2S
R O O
O NH
2AgOAc (3 mol%) DPPE (3 mol%) KHMDS (4 mol%)
–20 °C, DMF +
R
yield up to 97%
chemoselectivity up to >20/1 21 examples
extended scope pharmaceuticals
dipeptides nucleoside derivatives
Figure 3. Direct catalytic chemoselective functional group installation 12 examples
azide etc.
Next, research interest moved to the development of catalytic chemo-, regioselective conjugate addition of amino diol. Besides the chemoselectivity between hydroxy groups and amino groups, this kind reaction faced one additional problem that was the regioselectivity between two hydroxy groups. To date, only one nonenzymatic catalytic regioselective acylation of primary hydroxy groups of protected amino diol was reported, which gave high yield and excellent regioselectivity.
The chemo-, and regioselective conjugate addition was successfully developed by using rac-BINAP as the ligand.
[4]The utility of this reaction was highlighted by the reaction showed in Figure 5, where dansyl group was chemo-, and regioselectively installed to the β-hydroxy group with good yield.
Besides these successful development of the catalytic conjugate addition of hydroxy groups in the presence of amino groups mentioned above, attempts were made to develop the catalytic strain- release reaction of hydroxy groups in the presence of amino groups, which would present a catalytic chemoselective cyclobutane motif installation method to hydroxy groups, which has not been developed.
HO NH
2O NH
2AgOAc (5 mol%) DPPE (5 mol%) NaHMDS (5 mol%)
–20 °C, DMF +
t
BuO
yield up to 79%
chemoselectivity up to >20/1 11 examples
pharmaceuticals dipeptides
Figure 4. Catalytic chemoselective conjugate addition using acrylate O
t
BuO
O
HO NH
2OH
electrophile AgOAc (10 mol%) rac-BINAP (10 mol%)
KHMDS (11 mol%) –10 °C, DMF, 9 h
N S O O S N
O O
N Me Me
O NH
2OH
68%
high regioselectivity >99/1 high chemoselectitivy >20/1
Figure 5. Catalytic chemo-, and regioselective reaction
Figure 6. Catalytic chemoselective cyclobutane motif installation method
HO NH
2+
yield up to 73% (2 steps) chemoselectivity up to
20/1 PhSO
2O NH
21). catalyst
2). deprotection
Discussion
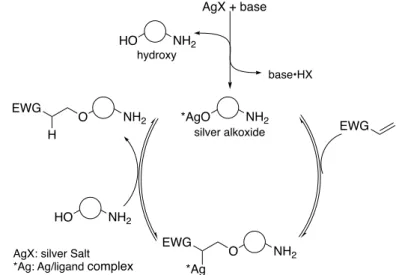
The plausible mechanism of catalytic chemoselective conjugate addition was showed in Figure 6, which was for both reactions using sulfonyl compounds and acrylates. The combined use of silver(I) complex with Brønsted base generated the actual catalytic species, the silver(I) alkoxide. After nucleophilic addition of silver alkoxide, subsequent protonation occurred with other
hydroxy group to give product with the regeneration of the silver(I) alkoxide. Additionally, for the reaction of acrylates, it was hypothesized that the generated nucleophilic soft metal alkoxide would be preferred the soft conjugate addition (1,4-addition) over undesired hard transesterification (1,2- addition).
For the catalytic chemo-, and regioselective conjugate addition, it was hypothesized that the hydroxy group at the β-position of a free amino group would be selectively activated through bidentate coordination to the Lewis acidic silver salt even in the presence of other distinct hydroxy group, where the free amino group would serve as the directing group.
Reference
[1] Selected example: D. Maiti, S. L. Buchwald, J. Am. Chem. Soc. 2009, 131, 17423.
[2] Selected example: Y. Hayashi, S. Santoro, Y. Azuma, F. Himo, T. Ohshima, K. Mashima, J. Am.
Chem. Soc. 2013, 135, 6192.
[3] S. Uesugi, Z. Li, R. Yazaki, T. Ohshima, Angew. Chem. Int. Ed. 2014, 53, 1611.
[4] Li, Z.; Yazaki, R.; Ohshima, T.; Org. Lett. 2016, 18, 3350.
HO NH
2AgX + base
base•HX
*AgO NH
2O NH
2EWG
*Ag
HO NH
2O NH
2EWG
EWG
AgX: silver Salt
*Ag: Ag/ligand
complex H
hydroxy
silver alkoxide
Figure 7. Plausible mechanism of catalytic chemoselective conjugate addition
OH NH
2HO
AgOAc (cat.) ligand (cat.) KHMDS (cat.)
OH NH
2O
aminodiolR S O O
Ln
OH NH
2O
Ag
Free NH2 serving as directing group