Available online at
www.sciencedirect.com
journal homepage:
www.elsevier.com/locate/radcr
Case
report
Dysostosis
in
mucopolysaccharidosis
type
2:
A
case
of
longitudinal
follow
up
and
literature
review
✩
Tomoaki
Sasaki,
MD,
PhD
a,∗,
Miki
Ogata,
MD
b,
Aya
Kajihama,
MD
c,
Kouichi
Nakau,
MD
c,
Atsutaka
Okizaki,
MD,
PhD
baDepartmentofRadiologicalTechnology,GraduateSchoolofHealthSciences,OkayamaUniversity,2-5-1,
Shikata-cho,Kita-ku,Okayama700-8558,Japan
bDepartmentofRadiology,AsahikawaMedicalUniversity,Asahikawa,Japan cDepartmentofPediatrics,AsahikawaMedicalUniversity,Asahikawa,Japan
a r t i c l e
i n f o
Articlehistory:
Received25December2020 Revised29December2020 Accepted2January2021 Availableonline8January2021
Keywords:
Mucopolysaccharidosistype2 Dysostosis
Cranialhyperostosis
a b s t r a c t
Mucopolysaccharidosistype2isacongenitallysosomaldiseasecharacterizedby iduronate-2-sulfatasedeficiency,whichleadstoexcessiveaccumulationofglycosaminoglycansin tis-sue.Dysostosis,whichprimarilyinvolvesdecreasedbonemineralizationwith morpholog-icalchangesinthebone,isamajorskeletalconditioninmucopolysaccharidosis,butits pathophysiologyisnotwellknown.Here,wereportacaseofmucopolysaccharidosistype 2diagnosedattheageof2yearswithlongitudinalfollow-updataformorethan15years. Althoughthepatientunderwentbonemarrowtransplantation,thedevelopmentalquotient didnot improve,andcranialhyperostosisprogressedprominentlywithafaintlydilated perivascularspace.Otherdysostosesandcontractionofthejointswereobservedbutdid notimproveeither.
© 2021 The Authors.Published by Elsevier Inc.on behalf of UniversityofWashington. ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Introduction
Mucopolysaccharidosis(MPS)type2or Huntersyndromeis anX-linkedrecessivelysosomaldiseasecharacterizedby de-ficiency of iduronate-2-sulfatase, which leads to excessive accumulation of glycosaminoglycans (GAGs) such as der-matansulfate (DS)and heparansulfate (HS)intissue[1–4].
✩ Acknowledgments:ThisworkwassupportedbytheJapanSocietyforthePromotionofScienceGrant-in-AidforScientificResearch grantno.20K08136(T.S.).
∗Correspondingauthor.
E-mailaddress:tomoaki3est@gmail.com(T.Sasaki).
MPS can affect various organs, especially the central ner-voussystemand skeletalsystem.Inaddition,it maycause hepatosplenomegaly, retinal degeneration, corneal opacity, obstructivepulmonarydisorder,andvulvardisease[5].Onthe basisofGAGoveraccumulation,symptomsmaybeclassified asfollows:(1)symptomscausedbyoveraccumulationofGAGs itself,suchashepatosplenomegalyandcornealopacity,and (2)thosecausedbyinterferenceoftheexcessiveGAGswith
https://doi.org/10.1016/j.radcr.2021.01.003
1930-0433/© 2021The Authors.Publishedby ElsevierInc.on behalfof UniversityofWashington.Thisisanopenaccessarticleunderthe CCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/)
themetabolismofglycosylationaswellasthecollageninthe cytoplasmandextracellularmatrix.
Themajorskeletal finding inMPS can becharacterized asdysostosis,whichprimarilyinvolvesbonemineralization, thickened bone, and skeletal deformity [6,7]. However,the relationship between enzyme deficiency and dysostosis remains unknown. Here, we report a case of iduronate-2-sulfatase deficiency that presented with disproportionally worsenedcranialhyperostosisafterbonemarrow transplan-tationandreviewtheskeletalfindingswhilefocusingonthe pathophysiologyoftheenzymaticdeficiency.
Case
report
A2-year-old boywith a history of overgrowth (+1.5 SD in height),mildmacrocephaly,andrecurrentinguinalherniawas broughttoourhospital.Hisbirthweightwas3.512kg,height was51.5cm,headcircumferencewas34.2cm,andchest cir-cumferencewas33.0cmat40weeksofgestation.His devel-opmentquotient scorewas 56.Heshowedfacialdeformity, saddlenose,andmildlimitationsintherangeofmotionin multiplejoints,butnohepatosplenomegaly.Hisbrotherhad beendiagnosedwithHuntersyndromeandhaddiedasa re-sultofcomplicationsofbonemarrowtransplantation.
His urinary test revealed elevated DS and HS levels. Iduronate-2-sulfataseactivity wasundetectable, whichwas diagnosticforMPStype2.Subsequently,heunderwent allo-geneicbone marrow transplantation withmild normocytic normochromic anemiafor 3months; his hemoglobinlevel rangedfrom10to13g/dL.Hisiduronate-2-sulfataseactivity subsequentlyimprovedwithinnormallimits.
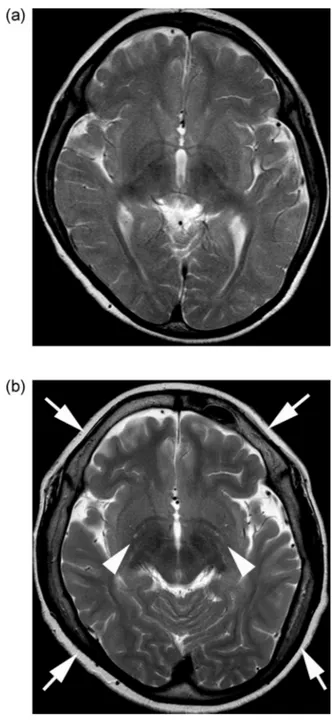
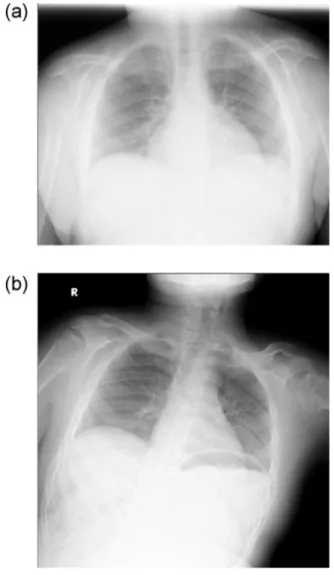
Thepatientdeveloped mitralvalvularregurgitation at3 yearsofage,and wasrepeatedlyhospitalizedforthe treat-mentofcardiovascularconditionsandevaluationof develop-mentandskeletalrehabilitation.BrainMRIshowedno appar-entabnormalityat6years ofage(Fig.1A).Attheageof12 years,hisheightwas134.4cm(-2.5SD),andprogressionof multiplejointcontractureswasnoted.Thedevelopment quo-tientwas36,whichwasequivalenttothatattheageof17 months.Follow-upbrainMRIrevealedprogressivecranial hy-perostosisandmildbrainatrophywithmildlydilated perivas-cularspacesattheageof12years(Fig.1B).Chest radiogra-physhowedbilateralhypertrophyintheribsandclaviclesat theageof9years(Fig.2A),which hadnotchangedinsize for8years(Fig.2B:at17yearsofage).Abilateralhand radio-graphobtainedat12yearsofageshoweddecreasedbone den-sity,delayedcarpalossification,shortdistalphalanges,and proximalpointingofthemetacarpals(Fig.3).At16yearsof age,ananterior–posteriorpelvicradiographrevealed scolio-sis,coxavalga,acetabulardysplasia,andwidenediliacwings (Fig.4A).Laterallumbar radiographyshowedposterior scal-loping,lowerlumbardislocation,andafan-shapeddeformity (Fig.4B).
Thepatient wasadmittedtothe hospitalforevaluation ofaorticregurgitationattheagesof14and15.The exami-nationrevealedcardiacfunctionwasstable.However,hewas followedupatanoutpatientclinicwithunchangedjoint con-tracture,bonedeformities,andmentalstatus.
Fig1– AxialT2-weightedimagingat(A)6yearsand(B)12 yearsofageshowingprogressivecranialhyperostosis (arrows)andworsenedcerebralatrophywithfaintlydilated
perivascularspaces(arrowheads).
Discussion
Thepatientinthe presentcasepresentedwithovergrowth in the early stage, but eventually showed short stature, disproportionalprogression ofhyperostosisin the cranium in comparison with the axial skeleton,and severe mental retardation,whichdidnotimproveafterbonemarrow trans-plantation.Thepatientalsodevelopeddysostoseswith degen-erationofthespineandhipjointsandvalvularinsufficiency. GAGmetabolismplaysanimportantroleintheCNS,skeletal
Fig.2– (A)Achestradiographshowingbilateral
hypertrophyintheribsandclaviclesat9yearsofage.(B)A follow-upchestradiographat17yearsofageshowingthat thefindingsdidnotprogress.
Fig.3– Ahandradiographat12yearsofageshowing decreasedbonedensity,delayedcarpalossification,and
proximalpointingofthemetacarpals.
Fig.4– (A)Ananterior-posteriorpelvicradiographat16 yearsofageshowingscoliosis,coxavalga,acetabular dysplasiawithdeformityoffemoralhead(arrows),and widenediliacwings.(B)Alaterallumbarradiographat16 yearsofageshowingdislocationofthelowerlumbaranda fan-shapeddeformity(arrows).
system,and cardiovascularsystem.Eventhoughbone mar-rowtransplantationwasperformedassoonaspossibleafter birth,thebrainandskeletaldamagehavenotimproved[8,9].
MPSisusuallyknowntocauseashortstature[10,11].Patel etal.demonstratedthatpatientswithMPStype2presented withovergrowthforthefirstseveralyearsandfinallyshowed ashortstaturebecausethegrowthratehaddecreasedfrom1 yearofage[11].Overgrowthinthefirstseveralyearscanalso beobservedinothertypesofMPS[12,13].Themechanism un-derlyingthisfindingisstillunknown,butitmayinvolvethe
interactionofDSwithfibroblastgrowthfactorsorothertypes ofgrowthfactors[14].Furthermore,overaccumulationofDS andHScouldaffectthequalityofcartilageandboneaswell ascollagen[15–18].Asaresult,thefragileligamentoustissue andosteochondraltissuemightnotbeabletotolerateweight gainwithgrowth,eventuallyresultinginashortstature.
Iduronate-2-sulfatasedeficiencyleadstotheaccumulation ofGAGs(DSandHS)inthelysosome.ExcessiveGAGscan grad-uallyaccumulateinjoints,ligaments,andcartilage,leadingto constrictionofjointsanddeformities[5,19,20].Inoncology, de-creasedactivityofiduronate-2-sulfatasehasbeenidentified asoneofthemechanismsunderlyingbreastcancer metas-tasis[21,22].IncreasedlevelsofDScanaffectnotonlytype1 collagenintheextracellularmatrixbutalsothecellular struc-ture[21,22].GAGs,includingglucuronicacidorsulfate,have ahighlynegativechargeandmighthavethepotentialto in-teractwithsurroundingproteins,suchasdecorinorcollagen
[14].ExcessiveGAGscanalsointeractwithcollagen compo-nentsthroughlysyloxidase,whichcatalyzespyridinolinefor collagencross-linking[23,24].Increasedlevelsoflysyloxidase orlysyloxidase-likeproteinscanpromotemetastasisand tu-morprogression,deterioratingthequalityoftheextracellular matrix,especiallycollagen,whichplaysanimportantroleas abarrier[25–28].
ExcessiveGAGscanalsointerferecathepsins,afamilyof proteaseswitheachtypelocatedinaspecifictissue[29].For theskeletalsystem,cathepsinKplaysanimportantrolein de-gradingcollagenandcartilageinosteoclasts[17,18].Decreased cathepsinKfunctioningcaninduceabnormalbone remodel-ing,leading tobonefragilityandfinallyresulting in dysos-tosis [17,18].Inthe cardiovascular areas,in additiontothe GAGdepositionintissues,MPSshowedanabnormalityinthe turnoverofcollagenandelastin[15,16] andinduced overex-pressionofcathepsinBinthefibroblastsoftheheart, vascu-larwall,andvalves,whichcanleadtodegradationof colla-genandelastinevenintheextracellularmatrix[30].Although cardiovasculareventscouldbefatalinMPS,enzyme replace-menttreatmentwithoptimaladministrationmightimprove theprognosis[31,32].
Thedysostosismultiplexgroupforcranialbonesandspine inMPSisbasedonanabnormalossificationprocesswith sec-ondarydegeneration,especiallyinthespine[6].InMPS,the skullmaybeenlargedordolichocephalic,showingpremature closureofthesutures,underdevelopedmastoidorsinuses, J-shapedsellaturcica,thickeneddura,andcranial hyperosto-sis[6,33].Defectivedevelopmentoftheanterosuperiorportion ofthevertebrae,scallopingofthevertebrae,instabilityofthe spine,andscoliosisorkyphosiswereobservedinthespine
[5,6].Withage,secondarydegenerativechangesmightdevelop intheloadedjoints,suchaslumbarorhipjoints,probablydue tothefragilityofthesurroundingligamentsandtendons.The spinalinstabilitymightleadtosecondaryspinalstenosisor spinalcompression[5].
Otherfeaturesofthisconditionincludeosteopeniaandan imbalanceinbonethickeningwithjointcontractureor dislo-cation.Patientsmayshowthickenedribsandclaviclesinthe axialskeleton[6],andthefindingsforthehandsinclude dif-fusedecreasedbonedensity,corticalthinning,ballet-shaped phalanges,proximalpointingofthemetacarpals,ordelayed ossificationinthecarpalbones[6,7].Coxavalgais
congeni-tallyobservedinthefemoralhead,anditmightresultin con-strictionofthejointsandvarusdeformities[6,7].Thepresence ofcoxavalgaafterbirthinMPSmightimplyincomplete col-lagenogenesis,whichalsogeneratesfragilecollagen.
In the central nervous system, excessive GAGs can ac-cumulate in the perivascular space, resulting in a dilated perivascular space [5]. Disease progression causes delayed myelination,demyelination,gliosis,andeventualbrain atro-phy[5].GAGsareamajorcomponentoftheextracellular ma-trixinthecentralnervoussystem.Theheterogeneityor va-rietyof3-dimensionalstructuresassitesforattachment of moleculesorreceptorsiswellcontrolledbyvariousenzymes ofglycosylation[34].Thus,unbalancedamountsofthese en-zymescouldleadtomorphologicalorfunctionalchangesin thebrain.InpatientswithMPS,thetherapeuticeffectsof en-zyme replacement therapy or bone marrow transplant are limitedbytherestricted entryoftheinfusedenzymes into thebrainviatheblood–brainbarrier[20].Althoughbone mar-rowtransplantationhasbeenshowntopreventthe progres-sionofskeletalfeaturesinmice[8],cranialhyperostosis pro-gressesdisproportionallyafterbonemarrowtransplantation withoutanyprogressionofskeletalthicknessintheribsand clavicles.Thisfindingsuggeststhatthecranialhyperostosis inMPSmightnotbeworsenedcranialGAGmetabolismbut ratherasecondaryreactiontoseveredisturbancesinCNS de-velopment.Inthispatient,theseverementalretardationdid notimprovedespitebonemarrowtransplantationat2years ofage.Cranialhyperostosiscandevelopduetothereduction ofintracranialvolumeorasevereMPSphenotype[33,35].
We encountered a case of type 2 MPS showing dispro-portionalprogressionofskeletalfeaturesafterbonemarrow transplantation.Thedeficiency ofiduronate-2-sulfatase via excessiveGAGsmighthaveinfluencedthemicroenvironment inthecytoplasmandextracellularmatrix,especiallythe colla-gen,cartilage,andGAGmetabolisminthecentralnervous sys-temandskeletalsystem.Thebrainandspinalsymptomsand featuresofMPSmightdevelopbasedonabnormalmetabolism ofboththecentralnervoussystemandskeletalsystem.
Patient
consent
Weobtainedthewritteninformedconsentfromthepatient’s parentsforpublication.
R E F E R E N C E S
[1] MoriniSR, SteinerCE, GersonLB.Mucopolysaccharidosis typeII:skeletal-musclesysteminvolvement.JPediatrOrthop B2010;19:313–17.
[2] LaoharaweeK, Podetz-PedersenKM, NguyenTT, EvenstarLB, KittoKF, NanZ, etal. Preventionofneurocognitivedeficiency inmucopolysaccharidosistypeIImicebycentralnervous system-directed,AAV9-mediatediduronatesulfatasegene transfer.HumGeneTher2017;28:626–38.
[3] Tylki-Szyma´nskaA.MucopolysaccharidosistypeII,Hunter’s syndrome.PediatrEndocrinolRev2014;12(Suppl1):107–13.
[4] WraithJE, JonesS.MucopolysaccharidosistypeI.Pediatr EndocrinolRev2014;12(1):102–6Suppl.
[5] Nicolas-JilwanM, AlSayedM.Mucopolysaccharidoses: Overviewofneuroimagingmanifestations.PediatrRadiol 2018;48:1503–20.
[6] McAlisterWH,, HermanTE.Osteochondrodysplasias, dysostoses,chromosomalaberrations,
mucopolysaccharidoses,andmucolipidoses.In:ResnickD, editor.Diagnosisofboneandjointdisorders.Philadelphia: Saunders;2002.p.4449–533.
[7] ChavhanGB, MillerE, MannEH, MillerSF.Twentyclassic handradiographsthatleadtodiagnosis.PediatrRadiol 2010;40:747–61.
[8] PievaniA, AzarioI, AntoliniL, ShimadaT, PatelP, RemoliC, etal. Neonatalbonemarrowtransplantationpreventsbone pathologyinamousemodelofmucopolysaccharidosistype I.Blood2015;125:1662–71.
[9] GuffonN, BertrandY, ForestI, FouilhouxA, FroissartR.Bone marrowtransplantationinchildrenwithHuntersyndrome: outcomeafter7to17years.JPediatr2009;154:733–7.
[10]ScarpaM.MucopolysaccharidosistypeII.In:AdamMP, ArdingerHH,PagonRA,WallaceSE,BeanLJH,StephensK, etal.,eds.GeneReviews(®).Seattle(WA):Universityof Washington,Seattle.
[11]PatelP, SuzukiY, MaedaM, YasudaE, ShimadaT, OriiKE, etal. GrowthchartsforpatientswithHuntersyndrome.Mol GenetMetabRep2014;1:5–18.
[12]TomatsuS, MontañoAM, OikawaH, GiuglianiR, HarmatzP, SmithM, etal. ImpairmentofBodyGrowthin
Mucopolysaccharidoses.In:PreedyVR,editor.Handbookof GrowthandGrowthMonitoringinHealthandDisease. NYNewYork:Springer;2012.NewYorkp.2091–117.
[13]MontañoAM, TomatsuS, BrusiusA, SmithM, OriiT.Growth chartsforpatientsaffectedwithMorquioAdisease.AmJ MedGenetA2008;146A:1286–95.
[14]TrowbridgeJM, GalloRL.Dermatansulfate:newfunctions fromanoldglycosaminoglycan.Glycobiology
2002;12:117R–125R.
[15]GonzalezEA, MartinsGR, TavaresAMV, ViegasM, PolettoE, GiuglianiR, etal. CathepsinBinhibitionattenuates cardiovascularpathologyinmucopolysaccharidosisImice. LifeSci2018;196:102–9.
[16]BaldoG, WuS, HoweRA, RamamoothyM, KnutsenRH, FangJ, etal. Pathogenesisofaorticdilatationin
mucopolysaccharidosisVIImicemayinvolvecomplement activation.MolGenetMetab2011;104:608–19.
[17]WilsonS, HashamiyanS, ClarkeL, SaftigP, MortJ, DejicaVM, etal. Glycosaminoglycan-mediatedlossofcathepsinK collagenolyticactivityinMPSIcontributestoosteoclastand growthplateabnormalities.AmJPathol2009;175:2053–62.
[18]LiZ, HouW-S, Escalante-TorresCR, GelbBD, BrömmeD. CollagenaseactivityofcathepsinKdependsoncomplex formationwithchondroitinsulfate.JBiolChem 2002;277:28669–76.
[19]MizumotoS, KoshoT, YamadaS, SugaharaK. Pathophysiologicalsignificanceofdermatansulfate proteoglycansrevealedbyhumangeneticdisorders. Pharmaceuticals(Basel)2017;10:34.
[20]TajimaG, SakuraN, KosugaM, OkuyamaT, KobayashiM. Effectsofidursulfaseenzymereplacementtherapyfor mucopolysaccharidosistypeIIwhenstartedinearlyinfancy: Comparisonintwosiblings.MolGenetMetab2013;108:172–7.
[21]SinghV, JhaKK, JKM, KumarRV, RaghunathanV, BhatR. Iduronate-2-sulfatase-regulateddermatansulfatelevels potentiatetheinvasionofbreastcancerepitheliathrough collagenmatrix.JClinMed2019;8:1562.
[22]Ko´zmaEM, WisowskiG, LatochaM, KuszD, OlczykK. Complexinfluenceofdermatansulphateonbreastcancer cells.ExpBiolMed(Maywood)2014;239:1575–88.
[23]BankRA, GroenerJE, vanGemundJJ, MaaswinkelPD, HoebenKA, SchutHA, etal. Deficiencyin
N-acetylgalactosamine-6-sulfatesulfataseresultsin collagenperturbationsincartilageofMorquiosyndromeA patients.MolGenetMetab2009;97:196–201.
[24]StevensonDA, RudserK, Kunin-BatsonA, FungEB,
ViskochilD, ShapiroE, etal. Biomarkersofboneremodeling inchildrenwithmucopolysaccharidosistypesI,II,andVI.J PediatrRehabilMed2014;7:159–65.
[25]SalvadorF, MartinA, López-MenéndezC, Moreno-BuenoG, SantosV, Vázquez-NaharroA, etal. Lysyloxidase-like proteinLOXL2promoteslungmetastasisofbreastcancer. CancerRes2017;77:5846–59.
[26]WangTH,HsiaSM,ShiehTM.Lysyloxidaseandthetumor microenvironment.IntJMolSci2016;18:62.
doi:10.3390/ijms18010062.
[27]ValletSD, MieleAE, Uciechowska-KaczmarzykU, LiwoA, DuclosB, SamsonovSA, etal. Insightsintothestructureand dynamicsoflysyloxidasepropeptide,aflexibleproteinwith numerouspartners.SciRep2018;8:11768.
[28]FisherKE, PopA, KohW, AnthisNJ, SaundersWB, DavisGE. Tumorcellinvasionofcollagenmatricesrequirescoordinate lipidagonist-inducedG-proteinandmembrane-typematrix metalloproteinase-1-dependentsignaling.MolCancer 2006;5:69.
[29]WangD, SuP, WangX, LiuK, LiC, GaoX, etal. Identification andcharacterizationofthelampreycathepsingenes. Immunogenetics2019;71:421–32.
[30]NygaardRH, JensenJK, VoermansNC, HeinemeierKM, SchjerlingP, HolmL, etal. Skeletalmusclemorphology, proteinsynthesis,andgeneexpressioninEhlers-Danlos syndrome.JApplPhysiol2017;123:482–8.
[31]KubaskiF,deOliveiraPoswarF,Michelin-TirelliK,MatteUDS, HorovitzDD,BarthAL,etal.MucopolysaccharidosistypeI. Diagnostics(Basel)2020;10:161.
doi:10.3390/diagnostics10030161.
[32]BiggPW, BaldoG, SleeperMM, O’DonnellPA, BaiH, RokkamVRP, etal. Pathogenesisofmitralvalvediseasein mucopolysaccharidosisVIIdogs.MolGenetMetab 2013;110:319–28.
[33]ManaraR, PrianteE, GrimaldiM, SantoroL, AstaritaL, BaroneR, etal. BrainandspineMRIfeaturesofHunter disease:frequency,naturalevolutionandresponseto therapy.JInheritMetabDis2011;34:763–80.
[34]SmithPD, Coulson-ThomasVJ, FoscarinS, KwokJC, FawcettJW.GAG-ingwiththeneuron":theroleof glycosaminoglycanpatterninginthecentralnervous system.ExpNeurol2015;274:100–14.
[35]MayH, MaliY, DarG, AbbasJ, HershkovitzI, PeledN. Intracranialvolume,cranialthickness,andhyperostosis frontalisinternaintheelderly.AmJHumBiol2012;24:812–19.