投与 ^<32>P,^<65>Zn の母体並に胎仔(児)に於ける移行分布に関する研究
全文
(2) Vol. 17, No. 1 Journal of Hokkaido University of Education (Section II B) September 1966. Studies on the Transfer and Distribution of Injected 32P and 65Zn in Maternal and. Fetal Tissues of Rabbits lchiro SASAKI. Kushiro Branch, Hokkaido University of Education. ^^-jiiP : ^^. 32P, 6SZn ®^^^^B^ff(J^)r^M-%. w^w^T-sm Summary. Placental transfer of Zn and its relation to fetal growth within 24 hours after dosage was studied in rabbits by means of double tracer techniques using 32P and 65Zn with the following results.. 1) In 24 hours after dosage, 65Zn was eliminated from the circulating system later than 82P, and only 2,1—2.2% of 65Zn remained in blood. 2) Urinary excretion of 65Zn was very late compared to that of 32P, and 0.2 % of 85Zn was excreted in the urine during the 24-hour evaluation period, while the value of 32P was 20 % of the dose. 3) Differences in transfer velocity and amount to the indivisual tissues were found. In 2 hours after dosage, 65Zn in the liver — about 45% of the dose — was gradually transferred to the various organs and tissues. 65Zn — about 8 % of the dose — was concentrated in the kidney more than in the urine, while 32P was in the urine more than in the kindney. 4) Deposition of both 85Zn and 3ZP in the uterus and ovary in 24 hours after dosage was slight. 5) Concentration of 65Zn in the lung in 1 hour after dosage was about 3 % of the dose, Comparison of the above value of 65Zn and that of Zinc complex — 50% in 45 minutes after dosage—shows that there is a difference between the behavior of transfer of 65Zn and that of Zinc complex. 6) 65Zn was transferred slightly but readily to the pancreas and reached the peak in 1 hour after dosage. 7) Transfer of both 65Zn and 32P to the brain was very late and only a trace, 8) Differences in elimination of both 65Zn and 32P from the circulating system in pregnant rabbits were of little significance. 9) For deposition of 85Zn in the liver of pregnant rabbits, the value for the middle pregnancy was higher than that for the late pregnancy. 10) In 1 hour after dosage, \~2 % of 65Zn was deposited in the maternal placentii, 5% in the fetal placenta and 4 % in the fetus and in 24 hours after dosage only about 1 % in. (34).
(3) Ichiro Sasakl the placenta and about 10 % in the fetus. 11) The tendency above was more significant for the late pregnancy than for the middle pregnancy. This seems to indicate that considerable amounts of 65Zn originally deposited in the liver were removed for uptake in the placenta and fetus in view of the higher liver accumulations for the middle pregnancy than for the late pregnancyand the about constant accumulations for the other tissues. 12) Though the completion of pancreas in fetus is late comparatively, and transfer of insulin across placenta to fetus from mother is yet undecided, it seems to be difficult to conclude that fetal metabolism is under preference of anti-insulin system in view of the mass transfer of 65Zn and more Zn-content in the some tissues of the fetus or new-born than in that of the mother.. Contents Page I . Introduction .•.....•..•............•.............<........•........................................... 35. n. Materials and Methods ........................................................................... 33 IH. Results ................................................................................................... 39. ]V. Summary and Considerations'""--"-"""'-"""""-""--------""---.-".-'"--.-" 44 V. Conclusions ............................................................................................. 45. V[. Acknowledgements ................................................................................. 47. I, Introduction It has been confirmed that the Zn-hunger animals show serious symptoms such as growth stop, motor disturbance, etc., and at last are put to death.7'' As a result of the investigations of the wide distribution of Zn throughout the entire bodies of normal animals, it has been found that Porcine Parakeratosis37? is due to Zinc deficiency and that Postalcoholic cirrhosis of the liver38? is caused by abnormal metabolism of this element. Furthermore, it has been observed that Zinc concentration in blood decreases in case of some illness,21? > 2A~>> 27~> and that 12% of Zn content of 700~800 /-(g/100 ml in normal human blood exists in albumin, 3 % in leukocyte, and remaining 85% in red blood cell,21)' 2Z)> 23>>24)' 265>39) and no significant differences of this ratio are observed in pregnancies.21^ >Z2) Approximately 34 % of the Zn in serum exists as "firmly" bound Zinc and remaining 66 % as " loosely" bound Zinc. It was reported that a large proportion of the intravenously injected 85Zn was eliminated within 24 hours, and 20—30 % of the 65Zn orally administrated to a dog was deposited in the serum albumin, 20—30 % in the a-i- and a^~ globulin, 20~30 % in the /3i- and r-globulin. Moreover, it has been confirmed that both of the " firmly " and " loosely " bound Zinc exsist in all these fractions.24) It goes without saying that studies on the transfer and distribution of Zn not only in blood but also in body are essential to the biochemistry, physiology and pathology of Zn. Now, it originated from the finding of the demand of Zn for the growth of Aspergillus (.35).
(4) Studies on the Transfer and Distribution of Injected 3ZP and 65Zn in Maternal and Fetal Tissues of Rabbits. niger by Raulin41) that the role Zinc plays in the animal and plant as the essential nutritive element was found. Raoult and Breton40> have reported first that Zn exists in the human liver, and in 1934 it has been known for the first time that Zn is essential to the development of normal animals.42)' i3~>' 45^ Furthermore, Carbonic anhydrase containing 0.33 % of Zn in a part of the molecular structure was separated and refined by Keilin and Mann,44) and it has been found that the mechanism of action of this element is essential to the activity of enzyme concerning the activation of carbonic anhydrase and metabolism of C0^7^>U1' la~>' l8~>' fl~> Thence-. forth, the variety of the role Zinc is playing in the metabolism of protein and hydrocarbon has been known. From such facts as were above mentioned, it would be easily realized that Zn would participate in the fetal growth, Guilbert'l suggested that Zn accumulations would be transferred through the placenta, in view of the higher accumulations of Zn in the infant and young rats and calves than in their mothers. Valee, Fluharty &nd Gibson2)'7?' is), 20), 25), 26), 27), 38) have demonstrated that ?Zn. passes through the placenta of a dog, and also J. P. Feaster et al.35 have observed placental transfer of 65Zn in a pregnant rat. However, least reports have been made concerning Zn content. in the placenta in pregnancies as well as in the fetus. Hashimoto46) has demonstrated Zn in the 7 out of 8 examples of the human placenta in normal labor and found that its distribution was localized to the deciduous cell of the placental septa projecting to, and involved with, the fetal placenta (placenta fetalis). It was, too, reported that no Zn was found in all 5 examples of human placenta in the 5th mouth of pregnancy and also none during the the first 15 days of fetal life in a rabbit, but it was found first after the 20 days, increasing until the end of gestation period, Also in this case, its distribution was limited to the border between the maternal and fetal placenta. Fujihara,G) too, demonstrated that Zn was found in the human placenta first in the 5th month of pregnancy. On the other hand, Takeuchi6? demonstrated 6.96 mg % of Zn already in the early stage of pregnancy reasoning that it was difficult for Zn to pass through the fetal placenta and the Zn content of the fetus was not significant in view of much lower Zn content in the cord blood than in non-pregnant normal mother blood especially in the second stage of labor. Besides, he reasoned that Zn content in blood was in inverse proportion to the activity of anti-insulin system from the view point that Zn at the oral administration of glucose had parallel relation to the variation of insulin in blood. In other words, Zn in the mother, intercepted by the placenta, is not transferred, and in the fetus growth promoting hormone of anti-insulin system urges the fetal development. Tracer technique is one of the most available means for the reserches of metabolism in a living body, and numerous studies using radioactive elements have been carried out. Using 32P, detailed studies concering placental transfer and distribution to fetal tissues have been performgd 16), 17), 18), 19), 26). Thus the author has intended to study the transfer and distribution of Zn to the indiviclual tissues of non-pregnant and pregnant rabbits within 24 hours with the object of knowing whether the developing fetus will demand Zn, and whether the fetus developing excellently in the special life environment will be in relative w;mt of Zn.. (.%>).
(5) Ichiro Sasaki. II. Materials and Methods 1) Experimental materials a) Non-pregnant rabbits : —used 28 (female, weighing 1.7—3.6 kg each) b) Pregnant rabbits : — used 38 (17 : 10~20 days of pregnancy, called "middle stage," and 21 : 21—30 days of pregnancy, called "late pregnancy" here.) c) Fetuses : — presented 399 in all. d) Feed stuff : — used mainly green grass, with some oats and wheat bran. e) Radioactive isotopes used Original solutions of carrier free 32P and 65Zn were nearly neutralized with dilute NaOH, and their activities were so adjusted as to reach 100/^c/ml with a physiological solution of sodium chloride, 2) Experimental methods a) Dosage of radioactive isotopes 100 ml per rabbit of the above mentioned solutions was intravenously injected. b) Non-pregnant rabbits At the intervals of 1, 2, 5, 10, and 24 hours after isotope-dosage, the rabbits were sacrificed. Individual tissues obtained by thoracotomy and abdominal hysterotomy were weighed after removing blood, water or dust adhered to them with filter paper. Thenceforth the individual tissues were uniformalized with the homogenizer for large samples and with the motor for small ones, and a part of each of them was presented for radioactive assays, c) Pregnant rabbits The fetuses were obtained by abdominal hysterotomy (without anethesia), and afterwards the samples of amnion fluid and allantoic fluid were taken, Next, the mixture of the uterine fetuses, total for the middle pregnancy and divided into the following five parts for the late pregnancy, was presented for assays : brain, heart and lung, liver, abdominal tissues excepting liver and sustentacular tissues (e. g. muscle, bone, skin, fat, etc.). The urine placentas, divided into two parts, namely maternal and fetal, were mixed together. d) Radioactivity measurements. i) 32P A part of the individual uniform samples described above which were wet-ashed by heating in sulfric acid, was diluted to the amount of 10 ml with distilled water, and its radioactivity of accurate 1 ml was determined with a Geiger-Muller counter. In these assays in which 32P and 85Zn were injected simultaneously, the radiation was filtered through alminium absorbers so that only Beta-rays of 32P were recorded.. ii) sszn The radiation of 65Zn was counted with a scintilation counter, when the samples were unashed and only weighed. In these counts, the radiation counts of 32P contained in the measured values were removed by plotting the attenuation curves of activities.. iii) A method of calculation The relative values of the activities (C cpm/ml or g) of 32P and G5Zn and distributions. (37).
(6) Studies on the Transfer and Distribution of Injected 32P and 65Zn in Maternal and Fetal Tissues of Rabbits. expressed by percentage of dose (D %) in individual tissues at each interval after isotope dosage were determined at the following formulae. detected activity (cpm/ml or g-sample) x weight of tissues amount of an injection (cpm) C = detected activity (cpm/ml or g-sample) x correct value for self-absorption*. body weight „ 6 x 106 (cpm) ***. 2** amount of an injection (cpm). *) In the measurements of the radiation of 32P in the wet ashed samples, experimental correct value for self-absorption (0.957 for urine, 0.829 for serum) was used to avoid uncertainties due to self-absorption.. **) Standard value of the body weight. ***) Standard value of the amount of an injection.. Whole capacity of serum and blood corpuscle was calculated from Brake's value (56 ml/kg-. body weight) for circulating blood in the rabbit and Hematocrit value (35 %),. III. Results A. Non-pregnant rabbits. 1) Distribution in the individual tissues (Table I) Data are presented in Table I for the concentration of 65Zn and 32P in the individual tissues, expressed by percentage of dose.. a) 1 hour after dosage The degree of up-take of 32P was found to be high in the following descending order : liver (highest, about 36 % of dosage), lung, kidneys and serum. Urinary excretion was about 7 % of the dose. In the other tissues, 32P was slightly distributed, showing under 1 % of the dose. The 65Zn, like 3!!P, was transferred to the liver in the highest amount (about 44 %). Above 10 % of the dose was detected in the serum, showing a greater proportion compared with the 32P. Urinary excretion was slight, and 2—3 % was detected in the kidneys, lung, blood corpuscle and very slightly in the other tissues. b) 2 hours after dosage About 15 % of 32P was transferred to the liver and lung, and 2~3 % to the kindneys, serum and urine. 65Zn was detected most in the liver (45.13 %) as in 1 hour after dosage. 7.74 % in the kindneys, 6 % in the serum, and 2 % in the blood corpuscles and lung. Difference compared with the values in 1 hour was that the difference between 3ZP and 65Zn detected in the serum became slight, but on the contrary great in the lung, especially in the liver. c) 5 hours after dosage Significant difference compared with the values in 2 hours was that both 32P and 65Zn were distributed almost equally in the liver, both showing about 40% of the dose. Urinary excretion of 32P was great in amount (about 20 % of the dose), while 65Zn was as slight as in 1 hour.. d) 10 hours after dosage Both 32P ('incl cilZn were detected aliout. 30% in the liver. The 32P in the lung clecreas(.38).
(7) Ichiro Sasakl > /. ed excessively compared with that in 5 hours, The values in the other tissues remained almost the same as in 5 hours. e) 24 hours after dosage The 32P and 65Zn in the liver decreased to about 20 % of the dose. Urinary excretion of 32P was about 17 %, and distribution in the other tissues showed significant decrease.. 2) Variation of distribution at different intervals after dosage. (Table I) As was shown in Table I, 32P decreased slowly in 1 hour after dosage in the serum, and in 2 hours in the kidneys and lung, while it continued to increase during 5~10 hours and decreased since then in the adrenals, spleen, liver and brain. Little variations were observed in the deposition of 65Zn in the uterus during the experimental period. In the kidneys and lung, 32P reached maximum deposition in 2 hours, on the contrary in the spleen, especially in the liver, it decreased remarkably during the same period, For e5Zn, it was shown that the deposition continued to be almost the same witli 32P during the entire period of observation in the kidneys, uterus, adrenals and in the liver except in case of 2 hours. However, there were reverse processes in the spleen and adrenals.. In the brain, 65Zn continued to increase during the entire period unlike 32P. As compared with 32P, little urinary excretion was detected and almost constant during the entire period. The 65Zn deposited in the lung was also constant during the entire period. The 65Zn in the blood corpuscle was shown to decrease more slowly than in the serum.. 3) Radioactivities detected in the individual tissues (Table II) The comparison of the radioactivities per 1 ml or 1 g-wet of tissues in the individual tissues was presented in Table II, a) 1 hour after dosage Activities of the 82P detected were maximum in the liver and spleen, and were second in the lung and kidney, showing about half of the value in the former. In the uterus, adrenals, urine and serum, were shown lower activities and lowest ones in the brain. The 8SZn was found to be highest in the liver, and to be high in the following descending order : kidneys, lung, spleen and adrenals, showing relatively high activities among the test tissues. Besides, the values in the other tissues were found to be high in the following descending order : ovarium, pancreas, serum, uterus, heart, blood corpuscle, and especially very low in the brain and urine. In the comparison of 32P and 65Zn, the activities of 32P were found to be higher in all tissues except the brain than in the serum, while the activities of 66Zn, were lower in the brain, blood corpuscle, uterus, heart and urine than in the serum. b) 2 hours after dosage The activities of 32P were found to be highest in the lung, and decreased after 1 hour in the liver and spleen, showing the values nearly equal to them in the kidneys and urine. The activity of 66Zn in the lung became smaller than that in 1 hour, c) 5 hours after dosage The activities of 32P detected in the liver and spleen were higher than in 1 hour, and were nearly equal in the lung, kidneys and urine, showing half of activity in the pancreas.. (3S).
(8) Studies on the Tsansfer and Distribution of Injected a2P and 65Zn in Maternal and Fetal Tissues of Rabbits. In the other tissues were the activities found to be high in the following descending order : adrenals, uterus, serum and brain. The activities of 6SZn in the lung, liver, kidneys and spleen decreased in the same order as in 2 hours, and were shown to be equal to the values in 1 hour in the adrenals, ovarium, uterus and heart. The activities of 65Zn in the serum and pancreas were very low and nearly equal to the value in the blood corpuscle, and were lowest in the brain and urine as in 2 hours. d) 10 hours after dosage The activities of 32P decreased in the urine and liver, and increased in the lung and spleen. Higher activities were shown in all the tissues than in the serum. The activities of 65Zn in the lung, uterus and blood corpuscle were found to be higher than in 5 hours, and the activities were little variations in the other tissues. e) 24 hours after dosage The activivities of 32P were found to be at their highst values in the liver, lung and spleen, and to be high in the following descending order : urine, kidneys, adrenals and uterus. The activity of 32P detected in the serum was lower than in the other tissues as in 10 hours. The activities of 65Zn were found to be high in the following descending order : liver, kidneys, spleen, adrenals, lung, pancreas, mammary glands, ovarium, uterus, bone marrow, heart, blood corpuscle, serum, brain, bone, muscle, and urine.. 4) The variations of activities at different intervals after dosage (Table II) The variations of activities detected in. the individual tissues at each interval after dosage are presented in Table II As was shown in the table, the activities of 32P decreased after 1 hour in the serum, and after 2 hours in the liver, lung and urine ; while they increased in 10 hours, and henceforth decreased in the kidneys, adrenals, spleen, uterus and brain. In these processes, the activities became remarkably low in the liver and pancreas in 2 hours, and somewhat low in the kidneys, uterus and brain, while they were very high in the lung and urine. On the other hand, the activities of 65Zn detected in the serum, liver and blood corpuscle decreased as in the case of 32P. In the uterus, lung and kidneys, the activities of 65Zn were slightly lower in 2 and 5 hours, but reached a peak in 10 hours, and henceforth decreased. Though the similar process was shown in the spleen, slightly different one was presented in the brain, showing slow increase during 10 hours as in the spleen, but being constant afterwards.. In the comparison of 32P and 65Zn, both showed approximately the same processes in the serum, uterus, kidneys and lung after 5 hours, in the liver after 10 hours. However, as was mentioned above, a significant variation of the activities of 32P was presented in the liver, lung and spleen, showing different processes from those of 65Zn. In the spleen and brain, 3ZP decreased rapidly after 10 hours, compared with 85Zn, and vice versa in the adrenals. B. Pregnant rabbits. 1) Distribution in the individual tissues (Table III) Data are presented in Table III for the distribution expressed by percentage of dose in the .individual tissues of middle and late pregnancies in 1, 10 and 24 hours after dosage. As. (40).
(9) Ichiro Sasaki was shown in the table, no variation of the distribution of 65Zn in the mother's tissues was <. presented.. a) 1 hour after dosage As was shown in the table, regarding the mother's tissues, in the liver 65Zn was shown to be high in the descending order : non-pregnancy, middle and late pregnancy, and in the serum and blood corpuscle to be neady equal in non-pregnancy and middle pregnancy, and lower in late pregnancy. On the contrary, the distribution in the kidneys of late pregnancy was slightly greater than in the others. In the other tissues, very little differences among non-pregnancy, middle and late pregnancy were presented with very small distribution. The distribution in the tissues except the kidneys of late pregnancy seemed to be smaller, while it was considerable in the placenta, especially in fetal placenta, and fetus. Of the five sections of fetal tissues, the greatest distribution was found in the liver as in the maternal liver, and next in the sustentacular tissues, which weighed most heavily, b) 10 hours after dosage 65Zn detected in the liver was more remarkable in late pregnency than in non- and middle pregnancy, and in the serum was rather smaller in late pregnancy. On the other hand, in the fetus was distributed a considerable amount of 65Zn, and to the sustentacular tissues and liver of the fetal sections was transferred a great proportion of 8SZn, c) 24 hours after dosage Of the maternal tissues, little differences of the distribution were found in non-pregnancy, middle and late pregnancy, For the fetus, 65Zn was distributed most in the sustentacular tissues and liver as in 1 and 10 hours, and in 24 hours distributed in the former more than in the latter.. 2) Variation of the distribution at different intervals after dosage (Table III) Data are presented in Table III for the distribution expressed by percentage of 65Zn at different intervals after dosage in the maternal and fetal tissues. For the maternal tissues, as was shown in the table, the processes were shown in non-preg-. nancy, middle and late pregnancy. On the other hand, for the placenta and fetal tissues, as was shown in the table, significant differences between the middle and late pregnancy were obtained. First, for the placenta, 85Zn was deposited in the maternal placenta of middle pregnancy more than in that of late pregnancy, and vice versa in the fetal placenta with the result that especially in 1 hour the examples of late pregnancy contained about 5 times as much 65Zn as those of middle pregnancy, Furthermore, for the total fetus, the 65Zn transferred to the fetus of late pregnancy was excessively greater than the 66Zn transferred to that of middle pregnancy according to the increase of fetal weight, showing rapid increase in 24 hours. 65Zn deposited in the amnion fluid and allantoic fluid of late pregnancy was slightly greater than in those of middle pregnancy. For the distrib,ution of 65Zn in the fetal tissues of late pregnancy, in the liver, amnion fluid and allantoic fluid, no variation was found during the entire periods of observation, while in the other tissues and total fetus the increase was shown during the same period.. (<?).
(10) Studies on the Transfer and Distribution of Injected 32P and 65Zn in Maternal and Fetal Tissues of Rabbits. 3) Radioactivities detected in the individual tissues (Table IV) Data are presented in Table IV for the activities expressed in cpm per ml or g-wet tissue in the mother and the five sections of the fetus in 1, 10 and 24 hours after dosage. a) 1 hour after dosage For the maternal tissues, slightly lower activities were found in the serum and blood corpuscle of late pregnancy, and vice versa in the kidneys, and this corresponed with the result of the distribution expressed by percentage. Very little differences among non-pregnancy, middie and late pregnancy were shown in the liver and the other tissues. For the placenta, the results were obtained that no differences of activities were detected between the maternal placenta of middle pregnancy and that of late pregnancy, and that slightly higher activities were found in the fetal placenta of late pregnancy than in that of middle pregnancy. Almost the same result was obtained in the total fetus. For the fetal five sections of late pregnancy, only in the liver was found high activity, and in the other tissues activities were high in the following descending order : abdominal organs, heart and lung, sustentacular tissues and brain. b) 10 hours after dosage For the maternal tissues, in the serum, blood corpuscle and brain very little differences of activities were found among non-pregnancy, middle and late pregnancy, much lower activity in the liver of late pregnancy than in those of non-pregnancy and middle pregnancy, and the result was the same in the ovarium. In the lungs and spleens of middle and late pregnancy were found lower activities than in those of non-preg nancy, and the result was the same in the kidneys. On the contrary, in the adrenals of middle and late pregnancy were found much higher activities than in that of non-pregnancy. For the placenta, activities in the maternal placenta of middle and late pregnancy were found to be nearly the same as in 1 hour, while in the fetal placenta of late pregnancy were shown slightly lower activities. Activities in the total fetus, amnion fluid and allantoic fluid of middle and late pregnancy were found to be nearly equal, c) 24 hours after dosage For the mother, very little differences were found among non-pregnancy, middle and late pregnancy except that higher activities \vere found in the ovarium of middle pregnancy and lower ones in the adrenals.. For the placenta, both in the maternal and fetal placenta, very little differences were found between middle and late pregnancy. In the total fetus of late pregnancy, slightly higher activities were found than in that of middle pregnancy, while in the amnion fluid and allantoic fluid no differences between middle and late pregnancy were found. In these fetal tissues were shown higher activities than in 10 hours with the same order as in 1 and 10 hours.. 4) Variation of radioactivities at different intervals after dosage (TableIV) Data are presented in Table IV for the variation of radioactivities detected in the inclividual maternal and fetal tissues. As shown in the table, it was found that the activities in the maternal and fetal tissues of non-pregnancy, middle and late pregnancy varied with almost the same processes. Especially. (42).
(11) Ichiro Sasaki the activities in the total fetus of both middle and late pregnancy were found to vary with almost the same processes, and it was found that the fetus was supplied with nearly equal amounts of 65Zn per unit weight despite its increase in weight, Further, the activities in the maternal placenta, amnion fluid and allantoic fluid were nearly constant during the entire period of observation, and in the fetal placenta they were highest in 1 hour and henceforth slowly decreased. The activities in the total fetus and the individual fetal tissues were found to increase slowly until 24 hours elapsed,. IV. Summary and Consideration It was observed that a great proportion of intravenously injected 65Zn was eliminated within 24 hours.2) Besides, in the present experiments the 65Zn value in blood in 24 hours was only 2.1~2.2 % of the close and the remainder was transferred to the individual tissues and sustentacular tissues.. In 1 hour after dosage, 44 % of 63Zn was transferred to the liver, 3 % to .the lung 5.6 % to the Iddneys, and simultaneously injected 32P was also transferred to the liver (36.3 %), lung (10 %}, kidneys and others (only a trace), only 2—3 % remaining in the blood. This shows that the remainder transferred from the circulating system was deposited in the sustentacular tissues containing the muscle, etc. The 65Zn eliminated from the circulating system was excreted into the urine later than 3ZP, showing that metabolism of 66Zn seemed apparently to be later than that of 32P. However, considering that 6SZn surpassed 32P in the liver and kidneys or that the 32P after 2 hours increased again in 5 hours after dosage, transfered to the individual tissues will be the problem not of transfer velocity, but of affinity related to metabolism in individual tissues. Urinary excretion of 85Zn was reported to be only about 2 % still in 7 days after dosagg^34),35) ^nd in this experiments only 0.186 % of the dose was excreted with little variation until 24 hours after dosage, and also only about 6.5 % after 5~7 days even through pancreatie juice32) and bile.33) From the facts above mentioned, it is assumed that a great proportion of ?Zn will remain in the body within 24 hours. Now, it is noticeable that 65Za/'has exceeded 32P in the kidneys in spite of low urinary excretion of esZn and vice versa for 32P. What was meant by the facts that 65Zn was deposited first after dosage in the liver and kidneys, and not in the other tissues, and it was not excreted to the urine ? Only about 3 % of :the 65Zn deposited in the kidneys and then in the lung was very slight, compared with about 50 o^t) 19) of intravenoasly injected Zinc carbonate, phosphate and dithizone complex. Variations of tlie distribution of 32P and 65Zn individual tissues will be due to the difference of uptake and loss in individual tissues excepting serum, gall bladder and urine. Hence, it is impossible to decide between the transfer to, and the elimination from, individual tissues only by the fluctuation of transfer ratio in course of time, 65Zn and 32P decreased in course of time until 24 hours after dosage, while Valee et al.2) have observed that 65Zn was eliminated completely from. the serum and afterwards deposited again in the serum, remaining there for long period. Very little variations of the 32P or 66Zn. (43).
(12) Studies on the Transfer and Distribution of Injected 32P and 65Zn in Maternal and Fetal Tissues of Rabbits. in the adrenals, uterus, ovarium and lung were shown within 24 hours. Moreover, the 32P in the liver increased slowly in 1 hour, and decreased in 2 hours and again increased in 5 hours, while in the lung it increased in 2 hours. The variation of uptake and loss of the 32P and e5Zn shown in the other tissues within 24 hours will be clue to the connection between the physiological functions of both elements and the individual tissues. However, it is of course impossible to reason the affinity of carrier free Zinc and individual tissues only from the uptake and loss of carrier free Zinc, considering, for example, the significant differences'0'19) between carrier free Zinc, and Zinc carbonate complex as is shown in the lung : in view of the increase of the 32P in the lung and the decrease in the liver during the same 2 hours after dosage, it was assumed that the 32P eliminated from the liver was removed for uptake by the lung through the liver veins, systemic veins and heart. Little urinary excretion of 65Zn was found and only 0.2 % of the dose in 24 hours. Besides, it was reported34'1'3B) that only about 1% of the dose was excreted after 7 days and 65Zn was early deposited in, but eliminated again from, the pancreatic juice and bile, and slightly in amount ; for example, 5~9 % after in the pancreatic juice. Hence, the variation of 65Zn within 24 hours would be due to mutual transfer among the individual tissues. It was assumed that e5Zn reaching a peak in about 2 hours was transferred mainly to the sustentacular tissues containing muscle, bone, skin, fat, etc. To consider that Zn is essential component in the structure and function of insulin, the problem of Zn level in pancreas and transfer of administrated 65Zn to pancreas is important.'0' io), ii), iz) Sheline et al.3'0 reported that 65Zn was transferred to pancreas in several hours after. dosage, and Birnstengle33) indicated that it was deposited in pancreatic juice and bile after 15' 70 minutes. Also in the present experiments, 65Zn seemed to reach its peak already in 1 hour after dosage.. Furthermore, it was remarkable that the 32P in the lung was excessively high in the degree of uptake in 2 hours. The urinary excretion of 32P in 2 hours was found to be higher than in more or less hours, showing that the consideration of the fluctuation of the urine volume will probably be necessary. For 65Zn, the 10 hour's value in the uterus was higher, while it was excessively lower in the adrenals. Very little 65Zn and 32P were transferred to the brain. To consider the transfer of 65Zn to the individual tissues and fetus of the pregnant rabbits, no significant fluctuations were observed in the elimination from the circulation system and the transfer to the kidneys, lung, etc,, but the degree of transfer to the liver was considerably low compared with that of the non-pregnant rabbits. This was more remarkable in the middle pregnancy than in the late pregnancy. The deposition of 65Zn in the placenta and fetus was also higher in the middle pregnancy than in the late pregnancy, resulting in the decrease in the mother's individual tissues, especially in the liver, It was noteworthy that the deposition of 65Zn in the fetus in 24 hours showing 10.4 % was about 2.2 times that in the mother of late pregnancy. For the five sections of the fetus, 5.2 % or half of the dose was transferred to the liver, 4.1 % to the sustentacular tissues and so on. The great proportion of 05Zn deposited in the fetal placenta decreased in course of time and was transferred to the fetus in 24 hours after. (.44).
(13) Ichiro Sasaki dosage.. In view of the higher Zn accumulation in maternal placenta of late pregnancy and the higher Zn content in umbilical blood than in mother blood, Takeuchi't7) has presented negative view-point concerning Zinc passage through fetal placenta. This point of view will be realized because of very little urinary excretion and high deposition in the kidneys and vice versa in the present experiments. However, judging from the results of the deposition of more 65Zn in the fetal placenta than in the maternal placenta and transfer to the fetus in short time in the present experiments and also Zn content in the fetus, Zn would be related closely to fetal metabolism. It was reported4^ that the Zn level in a new-born infant rat was 2~3 times that in the mother liver and decreased to the mother's level after 2 weeks. Slight deposition of both 32P and 65Zn in the amnion fluid and allantoic fluid was found. However, considering that it was not clear whether the isotopes were transferred from the fetus the isotopes transferred to the fetus will be difficult to be eliminated, 65Zn was difficult to be transferred to the brain, showing the lowest values in both the mother and the fetus during the observation period. Yoko-o36) measured lactic acid in amniotic fluid, false amniotic fluid, placenta, fetal liver, umbilical cord blood, and mother blood at each month of pregnancy. From this result, he concluded that the great amount of lactic acid in fetus was due to abnormal saccharometabolism caused by the active development of fetus under Og defficiency. Takeuchi, from this conclusion, assumed further that fetuses develop under preference of anti-insulin system or growth promoting hormones which are different from mother's, considering the different informations<t8) on the passage of insulin across the placenta. Though the perfection of pancreas in fetus is comparatively later, and transfer of insulin across placenta to fetus from mother is yet undecided, it seems to be difficult to conclude that fetal metabolism is under preference of anti-insulin system, in view of the mass transfer of 65Zn and Zn-content in some tissues of the new-born which is 2~3 times greater than in them of the mother,47). V. Conclusion Placental transfer of Zn and its relation to fetal development within 24 hours after dosage were studied in rabbits by method of double tracer technique using 82P and 65Zn with the following results. 1) In 24 hours after dosage, 85Zn was eliminated from the circulating system later than 82P, and only 2.1—2.2 % of 55Zn remained in the blood, 2) Urinary excretion of 65Zn was very late compared with that of 82P, and 0,2 % of 6SZn was excreted in the urine during the 24 hour evaluation period, while the value of 32P was 20 % of the dose. 3) Differences in transfer velocity and amount to the individual tissues were found. In 2 hours after dosage, 6SZn in the liver (45 % of the dose) was gradually transferred to the individual organs and tissues. 65Zn was concentrated in the kidney more than in the urine (about 8 % of the close), while 32P was in the urine more than in the kidney.. (45).
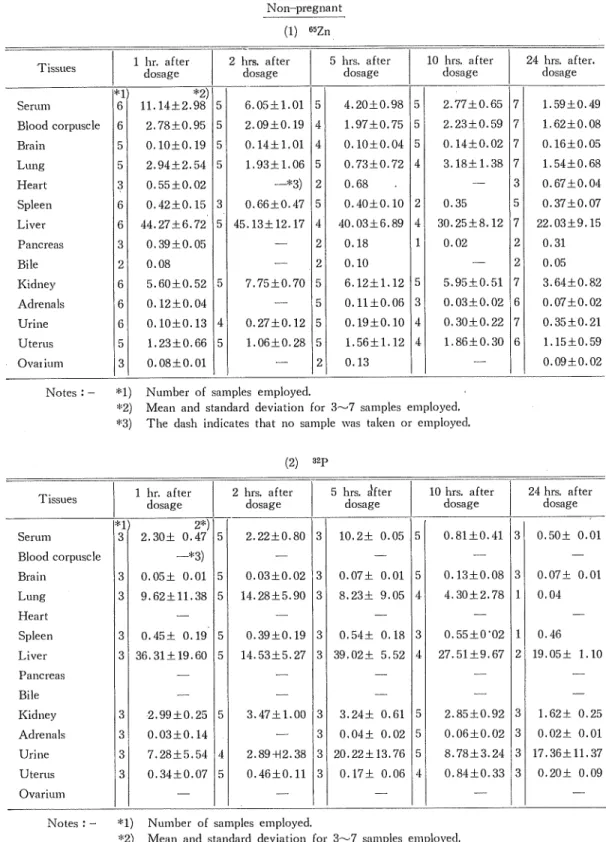
(14) Studies on tlie Transfer and Distribution of Injected 8ZP and 6'iZn in Maternal and Fetal Tissues of Rabbits. Table I. Distribution of isotopes in tissues of rabbits expressed as percentage of dose Non-pregnant. (1) liSZn. Serum. *2) "D 6 11.14±2.98 5. 6.05±1.01. 5. 4.20±0.98. 5 5. Blood corpuscle. 6I. 2.78±0.95. 5. 2.09±0.19. 4]. 1.97±0.75. Brain. 5. 0.10±0.19. 5. 0.14±1.01. 4. 0.10±0.04. Lung. 5. 2.94±2.54. 5. 1.93±1.06. 5. 0.73±0.72. Heart. 3. 0.55±0.02. 2I. 0.68. 0.42±0.15. Bile. 6I 6I 3I 21. Kidney. 6. 5.60±0.52. Adrenals. 0.12±0.04. Uterus. 61 61 5I. Ovai ium. 3. 0.08±0.01. Spleen Liver Pancreas. Urine. 44.27±6.72. —*3). 10 hrs. after dosage. 5 hrs. after dosage. 2 hrs. after dosage. 1 hr. after dosage. Tissues. 51 4]. 5. 0.40±0.10. 2. 5 45.13±12.17 4. 40.03±6.89. 4. 3. 0.66±0.47. 0.39±0.05 0.08. 5. 7.75±0.70. 2.77±0.65. 24 hrs. after. dosage. 7. 1.59±0.49. 2.23±0.59. 7. 1.62±0.08. O.U±0.02. 7. 0.16±0.05. 3.18±1.38. 7. 31. 0.35 30.25±8.12. 1.54±0.68 0.67±0.04. 5. 0.37±0.07. 7. 22.03±9,15. 1. 0.02. 21. 0.31. 2. 0.05. 6.12±1.12. 5I. 5.95±0.51. 7. 3.64± 0.82. 5. o.n±o.o6. 3. 0.03±0.02. 6. 0.07±0.02 0.35±0.21. 2I. 0.18. 2. 0.10. 5. 0.10±0.13. 4]. 0.27±0.12. 5. 0.19±0.10. 4. 0.30±0,22. 5I. 1.06±0.28. 5. 1.56±1.12. 41. 71. 1.23±0.66. 1.86±0.30. 6. 2I. 0.13. 1.15±0.59 0.09±0.02. Notes : - *1) Number of samples employed. *2) Mean and standard deviation for 3~7 samples employed. *3) The dash indicates that no sample was taken or employed.. (2). Serum. "D. 31. 5 hrs. sifter dosage. 2 hrs. after dosage. 1 hr. after dosage. Tissues. )2p. 2*). 2.30± 0.47. 5. 2.22±0.80. 3. 10,2± 0.05. 10 hrs. after dosage. 24 hrs. after dosage. 5. 0.81±0.41. 3. 0.50± 0.01. -*3). Blood corpuscle Brain. 3. 0.05± 0.01. 0.03±0.02. 3|. 0.07± 0.01. 5. 0.13±0.08. 3|. 0.07± 0.01. 31. 5I. Lung. 9.62±11.38. 5. 14.28±5.90. 3. 8.23± 9.05. 4. 4.30±2.78. 1. 0.04. 0.45± 0.19. 5. 0.39±0.19. 3. 0.54± 0.18. 3. 0.55±0-02. 1. 36.31+19.60. 5. U.53±5.27. 3. 39.02± 5.52. 4. 27.51 ±9.67. 2|. 5. 3.47± 1.00. Heart Spleen Liver. 31 31. 0.46 19.05± 1.10. Pancreas. Bile Kidney. 3. 2.99±0.25. Adrenals. 31. 0.03±0.14. Urine. 3. 7.28±5,54. Uterus. 31. 4. 0.34±0.07. 5. 31. 3.24± 0.61. 5. 2.85±0.92. 3. 1.62± 0.25. 3. 0,04± 0.02. 5. 0.06±0.02. 3. 0.02+ 0.01. 2.89+12.38. 3 20.22±13.76 5. 8.78±3.24. 3 17.36±11.37. 0.46±0.11. 3. 0.84±0.33. 3. 0.17± 0.06. 4. Ovarium Notes ; - *1) Number of samples employed. *2) Mean and standard deviation for 3~7 samples employed. *3) The dash indicates that no sample was taken or employed,. (4<?). 0.20± 0.09.
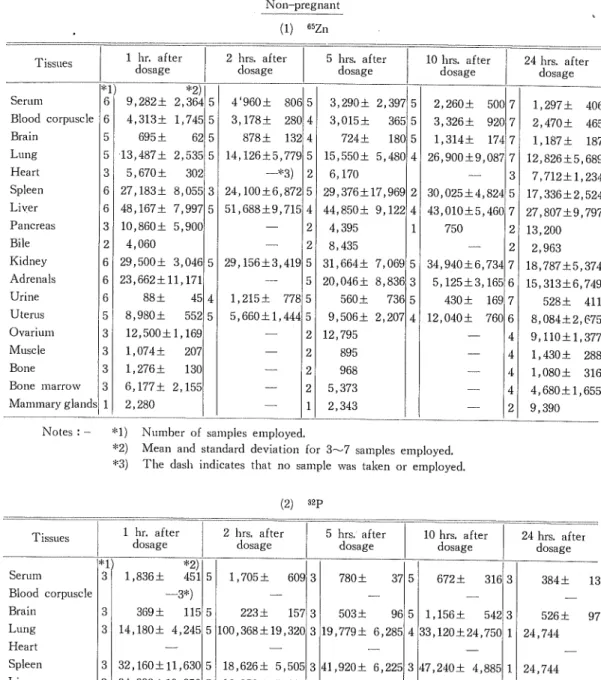
(15) Ichiro Sasaki. Table II. Distribution of isotopes in tissues of rabbits expressed as cpm. per ml. or g. -wet tissue Non-pregnant. (1). Serum Blood corpuscle Brain Lung Heart Spleen Liver Pancreas. Bile Kidney Adrenals Urine Uterus Ovarium Muscle Bone Bone marrow Mammary glands. 2 hrs. after dosage. 1 hr. after dosage. Tissues. *1) 6 6 5 5 3 6 6 3. *2). 9,282± 2,364 5. 4,313± 1,745 5. 695 ±. 62 5. 13,487± 2,535 5. 302. 5,670±. WZn. 27,183± 8,055. 5 hrs. after dosage. 4'960± 80£ 5. 3,290± 2, 397 5. 3,178 + 28C. 3,015±. 500 2,;260 ± 365 5 3,: , 326 ± 92C 878± 132 4 724 ± 180 5 ,314± 17-1 1,; 14,126±5,779 5 15,550± 5, 480 4 26,i ,900±9, 087 —*3). 31. 48,167± 7,997 5. 10 hrs. afte r dosage. 41 21. 24,100±6,872 5 51,688±9,71£. 41 2. 10,860± 5,900. 6,170 29,376±17, 969 2. 44,850± 9, 122 4. 1. 4,395. 2| 8,435 2 4,060 6 29,500± 3,04G 5 29,156±3,41£ 5 I 31,664± 5 I 20,046± 6 23,662±11,171 88 ± 560 ± 45 41 1,215 ± 77£ 5 6 552 5 5,660±l,44'i 5 9,506± 5 8,980± 12,500±1,169 2 12,795 3 895 1,074± 207 2 3 968 1,276± 2 3 13C 3 6,177± 2,15E 2| 5,373 1 2,343 1 2,280. 24 hrs. after dosage. 7 1,297 ± 406 7 2,470 ± 465 7 1,187± 187 7 12,826±5,689. 3|. 30,( ,025±4, 824 5 ,010±5, 43,1. 750. 7,712±1,234 17,336±2,524. 46C 7 27,807±9,797. 7, 069. 5 34,!,940±6, 73-1 ,125±3, 165 5,: 8, 836 3 73£ 5 430 ± 16S , 040 ± 76C 2, 207 4 12,1. 2| 21. 7 6 7 6. 41 41 41. 4| 2. 13,200 2,963 18,787±5,374 15,313±6,749. 528± 411 8,084±2,675 9,110±1,377 1,430 ± 288 1,080 ± 316 4,680±1,655 9,390. Notes : - *1) Number of samples employed. *2) Mean and standard deviation for 3—7 samples employed. *3) The dash indicates that no sample was taken or employed.. (2) 1. Tissues. Serum. 4 3. Lung. *2) 451 5. 1 , 836 ±. 1,705±. 5 hrs. after dosage. 609 3. 780 ±. 10 hrs. after dosage. 37 5. 672± 316 3. 3 3. 369 ± 14 ,180±. Spleen. 3 32 ,160±ll,,63C 5 3 34 ,332±19,:,35C 5. 18,626± 5, 50E 3 H,920± 6, 22E 3 47,240± 4,885 1. 16,850± 5, 91E 3 55,120±16, 64C. 41 43,290 ±15,860 2. Pancreas. Blle Kidney Adreneals Urine Uterus. 384 ±. 223 ± 157 3 115 5 503 ± 9£ 5 1,156 ± 542 3 526 ± 5 100,368±19, 32C 3 19,779± 6, 28E 4 33,120 ±24,750 1 24,744. 4,;,245. Heart Liver. 24 hrs. after dosage. —3*). Blood corpuscle Brain. 2 hrs. after dosage. hr. after dosage. 3 3 3 3. 15 ,560± 5 , 700 ± 4 ,863± 4 ,292 ±. 2,,858. 171. 5. 14,182± 4, 74C 3 17,708± 4, 99C 5 16,812± 6,735 3. 3 7,131± 2, 25; 5 10,806± 3,15£ 3 4 21,240± 6, 95C 3 20,032±15, 50C 5 10,l60± 2,69C 3 885 5 2,410 12E 41 5,239± 1,02C 3 3 3,200±. 2,,71S.
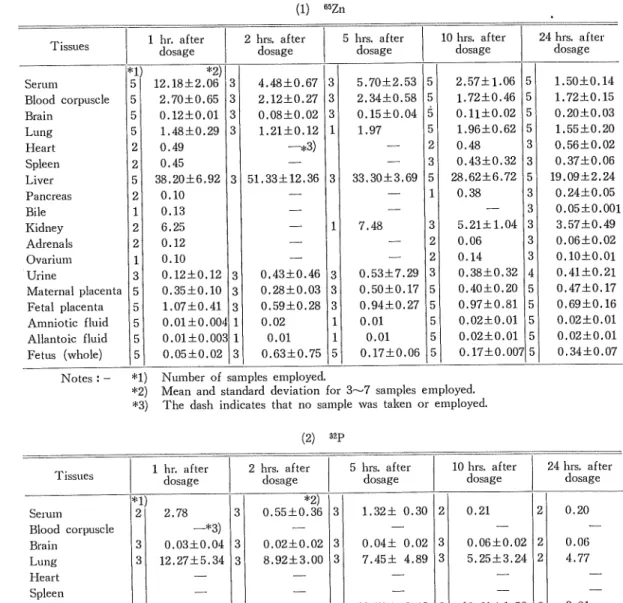
(16) Studies on the Transfer and Distribution of Injected 32P and 85Zn in Maternal and Fetal Tissues of Rabbits Table III. Distribution of isotopes in tissues of rabbits expressed as percentage of dose. Middle Pregnancy. (1) 1 hr. after dosage. Tissues cl. Serum Blood corpuscle Brain Lung Heart Spleen Liver Pancreas. Bile Kidney Adrenals Ovarium Urine Maternal placenta Fetal placenta. Amniotic fluid Allantoic fluid Fetus (whole). 5 5 5 5 2 2 5 2 1 2 2 1 3 5 5 5 5 5. 5Zn. 2 hrs. after dosage. *2). 3 3 0.12±0.01 3 1.48±0.29 3. 12.18±2.06. 4.48±0.67. 2.70±0.65. 2.12±0.27 0.08±0.02. 1.21±0.12. 31 31 31 11. —*3). 0.49 0.45. 38.20±6.92. 3 51.33±12.36 31. 0.10 0.13. 11. 6.25 0.12 0.10. 3 0.35±0.10 3 1.07±0.41 3 0.01±0.00' 1 0.01±0.00; 1 0,05±0.02 3 0.12±0.12. 10 hrs. after dosage. 5 hrs. after dosage. 0.43±0.46 0.28±0.03. 0.59±0.28 0.02 0.01 0.63±0.75. 3]. 31. 31. 11 11. 5. 5 5 0.15±0.04 5 5 1.97 2 3 33.30+3.69 5 1 5.70±2.53. 2.57±1.06. 2.34±0.58. 1.72±0.46. 7.48. 0.53±7.29 0.50±0.17 0.94±0.27 0.01 0.01. 0.17±0.06. 0.11±0.02. 1.96±0.62 0.48. 0.43±0.32 28.62±6.72 0.38. 3 2 2 3 5 5 5 5 5. 5.21±1.04 0.06 0.14 0.38±0.32 0.40±0.20. 0.97±0.81 0.02±0.01 0.02±0.01 0.17±0.00'i. 24 hrs. after dosage. 5 5 5 5 3 3 5 3 3 3 3 3 4 5 5 5 5 5. 1.50±0.14 1.72±0.15 0.20±0.03. 1.55±0.20 0.56±0.02 0.37±0.06 19.09±2.24 0.24±0.05 0.05±0.001 3.57±0.49 0.06±0,02 0.10±0.01 0.41±0.21 0.47±0.17. 0.69±0.16 0.02±0.01 0.02±0.01. 0.34±0.07. Notes : - *1) Number of samples employed. *2) Mean and standard deviation for 3~7 samples employed. *3) The dash indicates that no sample was taken or employed.. (2). ^1. 2. Serum Blood corpuscle Brain Lung. 2 hrs. after dosage. 1 hr. after dosage. is. 2.78 —*3). iq. -*2)~. 10 hrs. after dosage. 5 hrs. after dosage. 3. 0.55±0.36. 3. 1.32± 0.30. 2. 0.21. 24 hrs. after dosage. 2|. 0.20. 3 3. 3 3. 0.02±0.02. 7.45± 4.89. 3 3. 0.06±0.02. 8.92±3.00. 3 3. 0.04± 0.02. 12.27±5.34. 5.25±3,24. 2| 21. 2. 14.18. 3. U.31±5.09. 3. 12.78± 6.12. 3. 10.61±1.53. 2|. 9.01. 0.03±0,04. 0.06. 4.77. Heart Spleen Liver Pancreas. Bile. Kidney Adrenals Urine Ovarium Maternal placenta Fetal placenta. Amniotic fluid Allantoic fluid Fetus (whole). 3. 2.98±1.20. 3. 10.45±6.27. 3. 19.65±15.35. 3. 4.61±4,33. 2|. 5.81. 3 3 3 3 3. 0.47±0.09. 3 3 0.04±0.04 3 0.04±0.03 1 0.09+0.05 3. 0.53±0.17. 3 3 2 3 3. 0.65±0.31. 21 2| 21 21 21. 0.48. 1.33±0,96. 3 3 2 1 3. 0.62± 0.13. 0.49±0.04. 0.00 0.03 0.37±0.27. 0.60± 0.15 0.00 0.00 0.18± 0.07. 0.42±0.05 0.00 0.01±0.01 0.13±0.02. Notes : - *1) Number of samples employed. *2) Mean and standard deviation for 3~7 samples employed. *3) The clash indicates that no sample was taken or employed.. (48). 0.53 0.09 0.02. 0.17.
(17) ^. .*.. .*?). Tract & etc.. 4. 4 3 3 5. 3 3 3 5 5 4 4 4. 5. 0.28±0.09. 3.36±2.24. 0.10±0.06. 0.02±0.01. 1.30±0.27. 3.75±1.48. 0.03±0.01. 0.04±0.01. 4.91±1.63. 0.27±0.16. 0.15±0.04. 0.13±0.05. 0.12±0.01. 8.11±1.84. 34.38±7.68. 0.57±0.35. 0.50. 5. 41 51 41. 6. 21. 6 1. 41 41. 6 5 6 6. 65Zn. 6. 6 6 6 6. 6 6 6 6 6. 4|. 6. 41. 6. 3 5 6 1. 6I. 6 6 6. 0.73±0.58. 3.97±2.15. 0.29±0.10. 0.20±0.25. 5.18±2.88. 10.40±5.61. 0.03±0.01. 0.04±0.02. 1.32±0.45. 0.29±0.15. 0.13±0.04. 1.08±0.64. 0.09±0.04. 4.45±1.31. 0.39. 19.39±7.02. 0.34±0.10. 0.66±0.15. 1.95±0.89. 0.17±0.02. 1.90±0.49. 1.64±0.47. 24 hrs. after. *3) The dash indicates that no sample was taken or employed.. *2) Mean and standard deviation for 3- -7 samples employed.. 0.58±0.49. 4.38±4.07. 0.22±0.16. 0.19±0.36. 3.11±2.87. S.84±7.23. 0.02±0.01. 0.02±0.01. 1.70±0.62. 0.28±0.10. 0.10±0.01. 0.93±0.63. 0.11±0.05. 5.85±1.26. 0.06. 0.27. 18.50±3.05. 0.32±0.04. 0.66±0.13. 1.47±0.45. 0.12±0.03. l.Sl±0.39. 2.0S±0.45. 10 hrs. after. Notes : - *1) Number of samples employed.. Digestive organ' Genito-urinary. Liver & Gallbladder. Heart & Lung. Brain. Muscle, Bone, Skin, Fat & etc.. Fetal :. Fetus (whole). Allantoic fluid. Amniotic fluid. Fetal placenta. Maternal placenta. Ovarium. Urine. Adrenals. Kidney. Bile. Pancreas. Liver. Spleen. Heart. 2.13±0.82. 0.11±0.03. Lung. 5 2 5 5. 5. Brain. 8.31±2.39 1.87±0.37. iserum. Blood corpuscle. *1) I. 1 hr. after. 5 5. Maternal :. Tissues. Late Pregnancy. 0.37. 1.04. 2. 2I. 0.34. 0.05. 7.14. 8.95. 0.18. 0.65. 2.91. 1.48. 1.48. 5.53. 11.81. 0.18. 21.45. 0.10. 2.96. 2. 2I. 2. 21 2I 2I. 21. 2. 2I. 2. 2. 1. 2| 11. 2| —*3). 1 hr. after,. 21. 2I. 2. 21 21. 21. 1 1. 21 21. 1. 21 21 21. 2. 1. 2| 2|. 2|. 1.13. 2.69. 0.48. 0.07. 26.02. 30.41. 0.01. 0.27. 1.90. 0-22. 6.94. 0.07. 5.14. 0.04. 32.13. 0.34. 0.12. 0.10. 0.59. 10 hrs. after. 32p. i. 3. 3I. 3|. 3I. 31. 3 3. 31. 31. 3. 31. 21. 21 3I. 2. 3|. 3. 1.07± 0.64. 1.65± 0.56. 0.31± 0.03. 0.19± 0.09. 15.7G± 8.52. 19.07± 9.90. 0.04± 0.02. 0.10± 0.07. 0.16± 0.26. 0.20± 0.11. 12.37± 10.33. 0.06± 1.01. 2.31. 18.76± 2.59. 0.36. 2.73± 2.08. 0.11. 0.78± 0.28. 24 hrs. after. C/l. 0.
(18) Studies on the Transfer and Distribution of Injected 32P and 65Zn in Maternal and Fetal Tissues of Rabbits Table IV. Distribution of isotopes in tissues of rabbits expressed as cpm. per ml. g. -wet tissue. Middl Pregnancy. (D 1 hr. after dosage. Blood corpuscle Brain Lung Heart Spleen Liver Pancreas. Bile Kidney. Adrenals Urine Ovarium Maternal placent; Fetal placenta. Amniotic fluid Allantoic fluid Fetus (whole). Muscle Bone. Bone marrow Mammary g lands. 5 hrs. after dosage. 2 hrs. after dosage. *2). :1 Serum. iZn. 9,078± 1,896 5 4,178± 1,05C 1,005 ± 19£ 12,752± 2,467 2 6,282 2 24,360 5 55,480±15,59C 2 2,445 1 18,070 2 31,460 ± 10'; 2 16,170± 1,63(. 473 ± 49E 3 1 16,450 5 2,866 ± W 5 14,412 ± 82-, 235± 22C 5 160± 5-i 5 5 1,112± 14; 88S 2 2 1,015 2 7,050 2 9,885. 3 3 3 3. 4,632± 1,101 31 4,697± 1,686 3,148 ± 33£ 3 3,585± 887 709 ± 15-i 3 1,205± 152 9,490 ± 852 n 18,400. 5 5 5 5 —*3) 2 3 3 74,033 ±20,17', 3 50,800±4,350 5 1 1 39,600 3. 6,998± 6,72-i. 3 2.775± 54£ 3 11,740± 67C 1 108 1 126 3 1,642± 50?. 31. 1,293±4,769. 3 4,623± 16C 3 15,533±1,40E 1 181 1 331. 3I. 4,510±1,011. 3 2 3 2 5 5 5 5 5 2 2 2 1. 2,117± 873 2,649 ± 768 1,002± 137 13,422±2,546 5,800 19,350±1,540 39,920 ±13,950 11,300 24,850±3,007 13,950. 905 ± 942. 11,800 3,718±1,10E 10,835±2,458. 342± 20E 337 ± 221. 4,452±1,20C. 925. 2,183 8,160 10,100. Notes : - *1) Number of samples employed. *2) Mean and standard deviation for 3~7 samples employed. *3) The dash indicates that no sample was taken or employed.. (2) 32P 2. 1 hr. after dosage Serum Blood corpuscle Brain Lung. "1. 2. 2,142. 3. 5 hrs. after dosage. hrs. after dosage. 481 ±. ~*2). 261 3. —* 3) 3 301 ± 325 3 3 71,968±61, 30C 3. 66,,920 ±14 ,55C. 2 22,542. 20,,320± 6 ,83C. 231 rfc. 1,003±. 200. 93 305 ± 17£ 3 3 31,280±10, 430. Heart Spleen Liver. 3. 3. 18,352± 4, 840. Pancreas. Bile Kidney Adrenals Urine Ovarium Maternal placenta Fetal placenta. Amniotic fluid Allantoic fluid Fetus (whole). 3 17,852±11, 36E 3 137,372±92,50C 3 19,600±17, 690 3 3 3 3 3. 4,600±. 124 3. 8,756± 1, 261 3 1,269± 1, 555 3. 493 ±. 1,853±. 356 1 257 3. 5,,244± 1 ,su. 18,,164±16 ,13E. 808 ± 1 ,14-i 418. 4,,256± 1 ,80'!. 3 6,024± 1, 3 10,184± 1, 0 2 0 1 3 4,788±. 24 hrs. after dosage. 10 hrs. after dosage. 5 5 5 5 3 3 5 3 3 3 3 4 3 5 5 5 5 5 3 3 3 3. 1,240± 116 2,628± 226 1,499 ± 156 12,864±1,764 7,240 ± 618 18,587± 150 27,080±4,150 4,800±1,772 4,335 ± 853 19,450±1,379 10,093± 540 356 ±1,019 13,570±1,321 4,581±1,438 9,748±1,525. 568 ± 188 250 ± 114. 5,398±1,116 2,550±1,132 3,370±1,441 6,990 ± 668 9,570 ± 392.
(19) ^. 01. Notes : -. Bone marrow Mammary glands. Bone. Maternal : Muscle. tract & etc... *1) *2) *3). 1 1. 1. 1. 4. 4 3 3 5. 5 3 3 3 5 5 4 4 4. 2 5 5. 5. 5 5. 5. *1) *2). 724± 658. 6 5 6 6 4 4 6 1 2 6 4 5 4 6 6 5 5 5 5,125± 1,198. 314± 228 124± 54. 9,330^ 1,500. 3,608± 1,388. 2,811± 3,875 9,768± 1,130. 15,934± 4,042. 1,520 10,600 14,750. 978. 1,975 ± 273. 24 hrs. after. 1,460 ± 275 1,250 ± 564 6,060±:1,783. 1,020± 57 6 1,596± 487 3 6,927± 810 3 9,470± 1,390 3. 4 4 3 3. The dash indicates that no sample was taken .or employed.. . Mean and standard deviation for 3—7 samples employed.. 11,040±. 4,720. 6,872± 3,043. 3. 4,668± 2,299. 6. 6 4,872± 1,367 6 1,902 ±. 420 6 5,933± 1,564 6 39,700± 8,000. 4 18,575± 4,330 6 1,693± 973 4 8,968± 2,966 6 3,830± 1,331 6 8,768± 1,815 6 240 ± 88 6 2U± 98 6 7,713± 2,045. 23,287± 6,440. 6 1,325± 3,860 6 2,936 ± 318 6 1,489 ± 204 6 17,657± 9,480 3 7,460± 1,021 5 19,246+1 6,285 6 32,192±14,450 1 15,200. 2,830 28,642± 5,480 6. 7,120. 7,785± 1,570 18,117± 1,732 24,228± 5,820. 1,087 ± 126 14,412± 3,753. 2,726 ± 554. 1,703 ± 372. 10 hrs. after. e5Zn. 982 ± 138 6 2,636 ± 450 891 ± 238 325 ± 51 6 1,358 ± 169 6 3,818± 1,435 18,752 ±10,569 6 27,883± 9,587. 2,127 ± 803. 591 ± 501 509 ± 667. 18,630± 3,076. 2,536± 1,039. 12,897± 966. 41,930± 8,600 21,997± 3,852. 49,380 ±13,520. 5,975 35,138 ±21,360. 18,696± 5,220. 971 ± 179. 2,914± 651. 6,922± 1,900. 1 hr. after. Number of samples employed.. Digestive organ, Genito—urinary. Liver & Gallbladder. Heait & Lung. Brain. Muscle, Bone. Fat, Skin. & etc.. Fetal :. Amniotic fluid Allantoic fluid Fetus (whole). Fetal placenta. Maternal placenta. Ovarium. Urine. Adrenals. Kidney. Bile. Pancreas. Liver. Spleen. Heart. Lung. Brain. Blood corpuscle. Serum. Maternal :. Tissues. Late Pregnancy. 2,286. 2,992. 2. 2. 2 2 2. 2,535. 7,164. 3,984. 654. 6,522. 2 3,852 2 10,032 2 850 2 804 2 5,682. 2. 2 30,420. 1 13,680 2 14,478. 2 1,008 1 214,800. 2 —"3'j. 1 hr. after. 646. 14,034. 361. 2. 2. 2,577. 12,732. 2 10,542 2 1,023 2 5,097. 2 18,465 2 6,720 586 1 221 1 2 9,570. 2 20,310 2 9,408 2 3,552. 1 22,500 2 39,780. 2. 2. 2. 10 hrs. after. 32p. 597± 216. 3. 7,588± 3,225. 3 14,347± 6,020 3 3,661± 1,853 3 5,620± 1,308 3 13,168± 5,595. 3 2,610± 443 3 7,704± 2,168 375± 130 3 414± 104 3 3 12,436± 5,940. 2 13,824 3 13,040± 1,240 3 17,668±10,350. 2 26,238 3 33,990± 4,052. 2 1,016 3 25,564 ±19,080. 3. 24 hrs. after. en. 0. t~t. sr.
(20) Studies on the Transfer and Distribution of Injected 32P and 65Zn in Maternal and Fetal Tissues of Rabbits 4) Deposition of both 65Zn and 32P in the uterus and ovarium in 24 hours after dosage was slight. 5) Concentration of 66Zn in the lung in 1 hour after dosage was about 3 % of the dose. The comparison of the above value of 6SZn and that of Zinc complex (50 % in 45 minutes after dosage) showed that there was difference between the behavior of transfer of 65Zn and that of Zinc complex. 6) 65Zn was transferred slightly but rapidly to the pancreas, and reached a peak in 1 hour after dosage. 7) Transfer of both 65Zn and 32P to the brain was very late and only a trace. 8) Differences in the elimination of both 65Zn and 32P from the circulating system in pregnant rabbits were of little significance. 9) For the deposition of 66Zn in the liver of pregnant rabbits, the value for the middle pregnancy was higher than that for late pregnancy. 10) In 1 hour after dosage, 1~2 % of 65Zn was deposited in the maternal placenta, 5 % in the fetal placenta and 4 % in the fetus, and in 24 hours after dosage only about 1 % in the placenta and about 10 % in the fetus. 11) The tendency above mentioned was more significant for the late pregnancy than for the middle pregnancy. This seems to indicate that considerable amounts of 65Zn originally deposited in the liver were removed for uptake by the placeata and fetus, in view of the higher liver accumlations for the middle pregnancy than for the late pregnancy and the approximately constant accumulations for the other tissues. 12) Considering that the perfection of pancreas in fetus is comparatively late, and transfer of insulin across placenta to fetus from mother is yet undecided, it seems to be difficult to conclude that fetal metabolism is under preference of antiinsulin system, in view of the mass transfer of 65Zn and nore Zn content in ths soms tissaes of ths fetus or ths new-born than in those of the mother,. VI. Acknowledgements The author wishes to express his cordial thanks to Prof. Genichi Ogawa, Prof. Keizo Homma and Assist. Prof. Osamu Nishikaze for their kind guidance and reading of the manuscript, and also to Prof. Shinji Ito who has given facilities for the use of Isotope Laboratory during the entire period of this work,. References 1) Guilbert, F. B. : Mineral nutrition of plants and animals, Norman, Okla. : Unlv. Oklahoma Press,. 1948 (ref. Am. J. Physiol., 181, 291, 1955). 2) Valee, B. L., R. G. Flularity and J. G. Gibson. 11.: Acta Univ. Intern. Contra. Cancrum, 6, 869, 1949. 3) Peaster, J. P., Sam L. Hanclsancl, J. T. McCall and G. K. Davis : Am. J. PhysioL, 181, 291, 1955. .4) Banks, T. E., R. L. F. Tupper iincl A. Wormnll : Biochem. .1, 47, 466, 1950.. 5) SiltHJEHN-: MtwMWm, 8 , 197, 195B.. 6) W-^lK,^QM±, 2824 afc^|2||B(t{i).. (.52).
(21) Ichiro Sasaki 7) Valee, B. L. and M. D. Altschule : Physiol. Rev., 29,370, 1944. 8) Scott, D. A. : Biochem. J., 28, 1952. 9) P. 164, 1934.. 9) wws--w^v>^wm^.. P. i64, no?;, 1951. 10) Okamoto, K. : Acta Scholae Medicinalis Universitatis in Kioto, Vol. XXVII, P. 43-65, 1949.. 11) ^.M^- •• WP&^±^&^, 4, 1, 1945.. 12) ^.t^m •• ttPE^?^fi^. 4, 24, 1953. 13) Berfenstam, R. : Acta Paedatrica, 41, 32, 1952. 14) Keilin, D and T. Mann : Nature, Lond., 144, 442, 1939. 15) Keilin, D and T, Mann : Biochem. J., 34, 1163, 1940. 16) Blrnstengel, M., B. Stone and V. Richards : Am. J. PhysioL, 186, 377, 1956.. 17) JEa-J!P: B-WW^mm., 32, 206, 1956. 18) Nagasawa, K., G. Nakayama, Y. Kido and K. Kametani ; Eisei Shikenjo Hokoku, No. 75, 1957. 19) B. L. Valee and R. G. Fluharity : J. Glin. Invest., 26, 1199, 1947. 20) B. L. Valee and R. G. Fluharity ; Nuclear Sci. Abstracts., 4, 155, 1950. 21) Vikbladh, I. : Scand. J. Clin. and Lab. Invest,, SuppL, 2, 143, 1950. 22) Vikbladh, I. : Scand. J. Clin. and Lab. Invest., SuppL, 2, 1951. 23) Wolff, H. : Deut. Aich. Koin. Med., 197, 163, 1950. 24) Wolff, H. : Klin. Wochschr., 34, 409, 1956. 25) Valee, B. II and J. G. Gibson, II : J. Biol. Chem,, 176, 435, 1948. 26) Valee, B. L. and R. G. Fluharity : J. Clin. Invest., 26, 1199, 1947. 27) Valee, B. L. and J. G. Gibson, II, : J. Biol. Chem., 176, 445, 1948. 28) Helmberg, C. G, : Biochem., J., 33, 1901, 1939. 29) Praetorius, E. : Biochem. Biophys. Acta., 2, 590, 1948. 30) Roche, J., Nguyen-VanaThoai, Marcelet, J. Desruisseaux, G. & Durand, S. ; Bull. Acad. Med., 130,294,. 1946. 31) Roche, J., Come, L, Destuisseaux, G., Baudoin, N. & Long. Soc. Biol., Paris, 141, 1251, 1947. 32) Montgomery, M. L., G. H. Sheline and I. L. Chaikoff : J. Exp, Med., 78, 151, 1943. 33) Birnstengel, M., B. Stone and V. Richards : Am. J. PhysloL, 186, 377, 1957. 34) Sheline, G. E., I. L. Chaikoff. H. B. Jones and M. L. Montgomery : J. Biol. Chem., 174, 409, 1943. 35) Salant, W., J. B. Rieger and E. L. P. Treuthardt : J. Biol Chem., 34, 447, 1918.. 36) m^:k--^Wam., 3, 2, 1952. 37) Tucker, H. F. and W. D. Salmon: Biol. Med., 88, 613, 1955. 38) Valee, B. L,, W. E. C. Wacker, A. F. Bartholomay and F. L. Hoch : New Eng. J. Med., 257, 1055,. 1957, 39) H. Wolff : Deut. Arch. Klin. Med,, 197, 263, 1950. 40) Raoult, F. and H. Breton: Compt. Rend. Acad. Sci., 85, 40, 1877. 41) Paulin, J. : Ann. Sci. Nat. Botan. et Biol. Vegetable., 11, 93, 1869. (ref. Physiol. Rev. 39, 443, 1959). 42) Berttand, G. and R. C. Bhattacherjee : Compt. Rend. Acad. Sci., 198, 1823, 1934. 43) Bertrand, G. and R. C. Bhattacherjee : Ann, Inst. Pasteur., 55, 265, 1935. 44) Keioin, D. and T. Mann : Nature, 153, 107, 1944. 45) Fuji;, T., S. Utida and T. Mizuno : Nature, 176, 1068, 1955.. 46) %* : ^H • WK.fmW&±, 2824 (-^^@»?). 47) Bergil, F., A. J. L. Everett, J. B. Martin and J. S. Webb : J, Pharm, and PharmacoL, 9, 522, 1957. 48) Rupp ; Arch. F. Gynaek, 43, 143, 1930.. 49) ^», et al., : 70, 429, 1956. 50) gSia—^lJXg,^., 70, 429, 1956.. (53).
(22)
図

関連したドキュメント
In Section 13, we discuss flagged Schur polynomials, vexillary and dominant permutations, and give a simple formula for the polynomials D w , for 312-avoiding permutations.. In
Analogs of this theorem were proved by Roitberg for nonregular elliptic boundary- value problems and for general elliptic systems of differential equations, the mod- ified scale of
Then it follows immediately from a suitable version of “Hensel’s Lemma” [cf., e.g., the argument of [4], Lemma 2.1] that S may be obtained, as the notation suggests, as the m A
Definition An embeddable tiled surface is a tiled surface which is actually achieved as the graph of singular leaves of some embedded orientable surface with closed braid
Correspondingly, the limiting sequence of metric spaces has a surpris- ingly simple description as a collection of random real trees (given below) in which certain pairs of
[Mag3] , Painlev´ e-type differential equations for the recurrence coefficients of semi- classical orthogonal polynomials, J. Zaslavsky , Asymptotic expansions of ratios of
The theory of log-links and log-shells, both of which are closely related to the lo- cal units of number fields under consideration (Section 5, Section 12), together with the
We relate group-theoretic constructions (´ etale-like objects) and Frobenioid-theoretic constructions (Frobenius-like objects) by transforming them into mono-theta environments (and