IRUCAA@TDC : Effects of stretching stimulation with different rates on the expression of MyHC mRNA in mouse cultured myoblasts
全文
(2) Biomedical Research 28 (1) 25-31, 2007. Effects of stretching stimulation with different rates on the expression of MyHC mRNA in mouse cultured myoblasts Katsuhide KUROKAWA1, Shinichi ABE2, 3, Koji SAKIYAMA2, Tomotaka TAKEDA1, Yoshinobu IDE2 and Keiichi ISHIGAMI1 1. Department of Sports Dentistry, 2 Department of Anatomy, and 3 Oral Health Science Center HRC7, Tokyo Dental College, Chiba, Japan (Received 10 November 2006; and accepted 30 November 2006). ABSTRACT In vivo studies have shown that changes in the characteristics of skeletal muscle fiber are determined by type of exercise or training. These earlier studies on mechanical stimulation, however, have all employed stimulation applied at a constant intensity, and no studies appear to have investigated change with variation of intensity of stimulation. In this study, we investigated the characteristics and differentiation of myoblasts stretched at different rates. Myoblasts were stimulated at 3 different rates, and the numbers of cells and nuclei on days 1, 3, and 5 were compared. The myosin heavy chain (MyHC) mRNA expression level was also compared. We investigated expression of MyHC-perinatal to determine speed of differentiation of myoblasts, and expression of MyHC2b, 2d, and 2a to ascertain muscle cell characteristics. Counting cells and nuclei of myoblasts revealed clear promotion of differentiation with stretching. With rapid stretching, expression of MyHC-perinatal was high at first, but then showed a decrease. In terms of effect on muscle fiber characteristics, MyHC-2b, MyHC-2d, and MyHC-2a were high with rapid, medium, and slow stretching, respectively. This indicated that myoblast differentiation was promoted regardless of difference in stretching speed, with the myoblasts acquiring the muscle-fiber characteristics appropriate to each rate of stretching.. Exercise and training are known to bring about changes in the characteristics of skeletal muscle fibers (26, 27). Numerous studies of the human (2, 3, 4, 12, 13, 17, 20, 24) have shown that skeletal muscle fiber characteristics change depending on the mode of exercise. Anaerobic exercise facilitates increases in fast-twitch muscle, while aerobic exercise facilitates increases in slow-twitch muscle. Studies using rats have reported that long-term low-intensity exercise increases slow-twitch muscle and decreases fast-twitch muscle (9, 15). In this manner, in vivo studies in the rat and the human have shown that Address correspondence to: Dr. Katsuhide Kurokawa Department of Sports Dentistry, Tokyo Dental College, 1-2-2 Masago, Mihama-ku, Chiba 261-8502, Japan Tel: +81-43-270-3605, Fax: +81-43-270-3609 E-mail: kurokawa@tdc.ac.jp. exercise changes muscle fiber characteristics. From the perspective of stress, severing a nerve controlling skeletal muscle not only decreases muscle weight, but also changes muscle fiber characteristics (7, 14). Studies in which muscle blood flow was regulated using devices such as a tourniquet have shown that even low-intensity training caused marked muscle hypertrophy and increased muscle force (25). However, few in vitro studies have been conducted to support and clarify these findings. In vitro studies have attempted to ascertain the effects of exercise and training by applying different stimuli. The first study (18) removed cells from rat hindlimb and soleus muscle and applied electrical stimuli via a culture solution to measure expression of myosin heavy chain (MyHC) isoforms. In the second study (11), osteoblasts were compressed using glass boards to ascertain morphological and bio-.
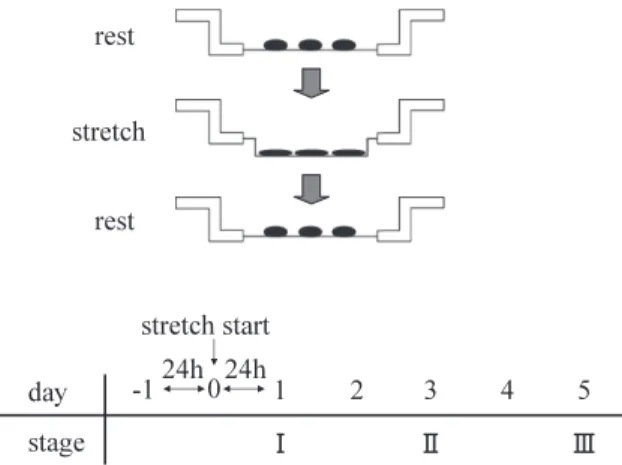
(3) K. Kurokawa et al.. 26. chemical changes. Magnetic stimuli were applied to rat hindlimb muscle to compare mRNA expression with that of controls (22). Furthermore, in the third study (21), cells were stretched to determine changes in number of cell and nuclei and muscle fiber characteristics. While some studies have analyzed morphological and biochemical changes, none appears to have investigated the effects of various stimulation intensities on muscle fiber differentiation and characteristics. Earlier in vivo studies have shown that training brings about changes in muscle characteristics. This in vitro study investigated the effects of different rates of stretching on muscle fiber differentiation and the characteristics of cultured myoblasts by microscope observation, cell count and cellular nuclei number and determination of mRNA expression in several MyHC isoforms. There are two ways to classify muscle contraction protein: according to stage of manifestation and contraction speed. Isoforms such as MyHC-perinatal is determined by the former method. Fast-twitch muscle contains MyHC2b, 2d, and 2a, and slow-type contains MyHC-1, as classified according to contraction speed. The functional roles of these four isoforms have been clarified. Therefore, the functions of muscles can be elucidated by determining the composition of these four isoforms (1, 5, 6, 10, 16, 19, 23, 28) (Table 1). In this study, we examined MyHC-perinatal to determine speed of differentiation of myoblasts, and MyHC-2b, 2d, 2a and 1 to ascertain muscle cell characteristics. MATERIALS AND METHODS Cell culture. C2C12 murine skeletal muscle myoblasts were used. C2C12 cells are an established cell line originating from murine skeletal muscle, and can be subcultured. Even after large-volume in-. cubation, proliferation and properties remain relatively stable (29). Furthermore, differentiation of C2C12 cells is induced in media containing relatively low serum concentrations, such as serum-free media and media containing 2% horse serum (8). Culture solution consisted of Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (FBS) (ICN Biomedicals, Ohio, USA) and penicillin (1000 units). Incubation was performed at 37°C under 5% CO2. Wells with a diameter of 25 mm were coated with type-I collagen. Each well was then inoculated with 2 × 105 C2C12 cells and 2.0 mL culture solution containing 10% FBS. Culture medium containing 2% FBS was used after allowing cells to adhere to the membrane for 1 day. The cells were then stretched according to the method of Sakiyama et al. (21). Mechanical stretching. The cells were mechanically stretched with the Flexercell® strain unit (Flexercell, Mckeesport, PA, USA). The stretch intensity was fixed to 15%, and the stretch rate was altered. The stretch rate was established using the stretch rate setting method of a Flexercell® strain unit. Since Sakiyama et al. (21) performed an experiment at a stretch rate of 0.5/sec in a previous report, this condition was adopted as the standard. This rate represents the distance of membrane movement per second: 1/sec indicates that a membrane stretches from a stationary state then relaxes to return to the original state in 1 second. At 0.5/sec, stretching requires 1 second followed by relaxation requiring 1 second. Three stretch rates, 0.1, 0.5, and 0.9/sec (designated as slow, medium and rapid stretch, respectively), were used. Stretching at each rate was continued for 1, 3, or 5 days. In addition, mRNA expression and differentiation were analyzed at 1, 3 and 5 days after stretching. Fig. 1 shows the stretch-. Table 1 Myosin heavy chain isoforms identified in skeletal muscle Designation. Nomenclature. Embryonic Neonatal Fast-twitch Fast-twitch Fast-twitch Fast-twitch Fast-twitch Slow-twitch. MHCemb MHCneo MHC-2b MHC-2d MHC-2a MHCeom MHC-2m MHC-1. Source: Brueckner et al. (1996). Distribution. }. Myobubes, intrafusal fibers, regenerating fibres Neonatal muscles, masseter, intrafusal fibres Fast-twitch isoforms in masseter of mice > contraction speed: 2b > 2d > 2a Super-fast fibers in extraocular muscles Super-fast fibers in muscles derived from first branchial arch Type I fibers.
(4) Effects of stretching on muscle fiber differentiation. ing device and stimulation periods. In the control group, cells were not stretched. Each experiment was repeated five times. Cell count and number of cellular nuclei. Morphological characteristics of cultured cells at each stage were observed using a phase-contrast microscope (Nikon, Tokyo, Japan). At each stage, cultured cells were immersed in trypsin-EDTA (Gibco BRL, Gland Island, NY) for 5 min. After detaching the cells, they were thoroughly soaked in culture solution, and number of cells was counted using a hemocytometer. Fused cells were counted as one. To ascertain the effects of stretching on cell fusion, the number of nuclei was counted in multinucleated muscle fibers. Detached cultured cells were stained using nuclear fast red (Funakoshi, Tokyo, Japan), washed using phosphate buffered saline, and then counted using the hemocytometer. We counted the number of cells and nuclei ten times. mRNA expression analyses. In order to investigate the effects of mechanical stretching at the mRNA level, the LightCycler™ (Roche Molecular Biochemicals, Mannheim, Germany) was employed to quantify mRNA expression using the QuickPrep micro mRNA Purification Kit (Amersham Pharmacia Biotech UK Ltd., Buckingham, UK). After establishing optimal conditions for all primers, mRNA expression was quantified according to the standard LightCycler™ protocol. As a hot start PCR solution for the LightCycler™, adjusted LC FastStart DNA Mastar SYBR Green 1 (Roche Molecular Biochemicals) was used. To each dilution of the PCR mixture, we added 2 μL LC FastStart DNA Mastar SYBR Green 1 containing λDNA (5 pg/μL) and SYBR Green 1 (1/60,000 dilution), and 1.6 μL. Fig. 1 Stretching stimuli and culture. 27. MgCl2 (25 mM) to 10.2 μL of sterile water. Furthermore, after adding 0.6 μL each of a forward primer (10 pmol/μL) and a reverse primer (10 mol/μL) prepared using Oligo 5 primer design (Biogene, Ltd., Kimbolton, UK), 5 μL of each diluted PCR product was added, bringing the final reaction volume to 20 μL. The primers for MyHC-perinatal, 2b, 2a and 2d were designed by selecting a unique sequence from the full DNA sequence of each isoform. The base sequences of the primers for each isoform were follows: MyHC-perinatal (Forward: 5’-GAGTCC CAGGTCAACAAGC-3’, Reverse: 5’-AACCCA GAGAGGCAAGTGAC-3’, Accession: M12289); MyHC-2b (Forward: 5’-ACAGACTAAAGT GAAAGCC-3’, Reverse: 5’-CTCTCAACAGAAA GATGGAT-3’, Accession: XM_126119); MyHC-2d (Forward: 5’-GACAAACTGCAATCAAAGG-3’, Reverse: 5’-TTGGTCACTTTCCTGCACTT-3’, Accession: AJ293626); MyHC-2a (Forward: 5’-CGAT GATCTTGCCAGTAATG-3’, Reverse: 5’-ATAACT GAGATACCAGCG-3’, Accession: NM-144961); and MyHC-1 (Forward: 5’-GTCCAAGTTCCG CAAGGT-3’, Reverse: 5’-CCACCTAAAGGGCT GTTG-3’, Accession: AY056464). In the PCR mixture, 2 μL LC FastStart DNA Mastar SYBR Green 1 containing λDNA (5 pg/μL) and SYBR Green 1 (1/60,000 dilution), 1.6 μL MgCl2 (25 mM), and 0.6 μL each of a forward primer (10 pmol/μL) and a reverse primer (10 pmol/μL) were added to 14.2 μL of sterile water. In addition, 1 μL target DNA was added to bring the final reaction volume to 20 μL. Each PCR mixture (20 μL) prepared in the above manner was added to the glass section of each capillary. PCR was performed at 95°C for 10 min, 95°C for 10 sec, 62°C for 10 sec and 72°C for 7 sec for a total of 50 cycles. Gene amplification was carried out according to a melting program of 70°C for 15 sec, with fluorescence continuously monitored at a rate of 0.1°C per second during the transition phase from 70 to 95°C. F1 (530 nm) was used as the fluorescent channel, and the gain indicated 89.9°C for MyHC-perinatal, 89.9°C for MyHC-2b, 89.6°C for MyHC-2d, 88.2°C for MyHC-2a, and 90.8°C for MyHC-1. The amount of each MyHC isoform calculated by the method described above was divided by amount of GAPDH, the house-keeping gene, to determine mRNA expression of each isoform. The base sequence of GAPDH was as follows: (Forward: 5’-TGAACGGGAAGCTCT CACTGG-3’, Reverse: 5’-TCCACCACCCTGTT GCTGTA-3’, Accession: NM_008084). Each PCR fragment was verified as part of an MyHC isoform using the ABI PRISM 310 Genetic Analyzer (Per-.
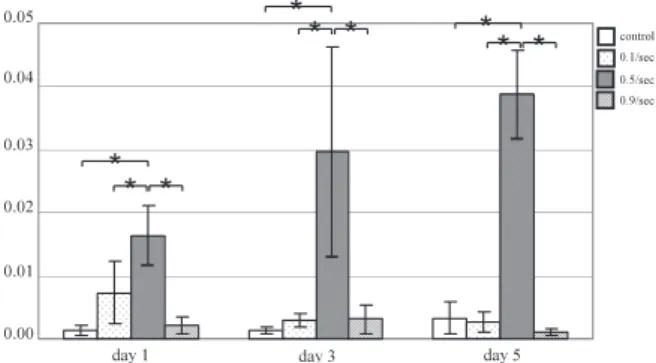
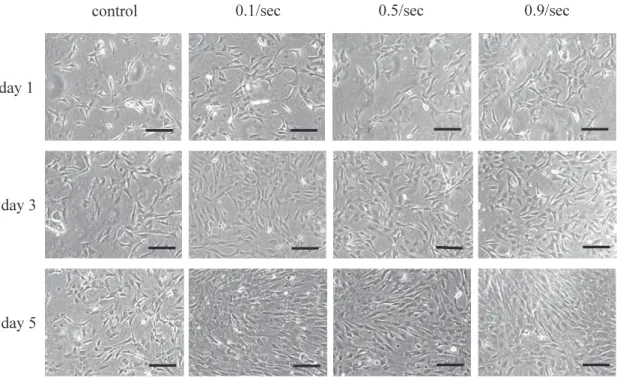
(5) K. Kurokawa et al.. 28. kin-Elmer Japan Applied Biosystem, Tokyo, Japan). Statistical comparisons were made using a oneway analysis of variance (ANOVA). Tukey’s multiple comparison tests was used for further comparisons between occlusal areas (P < 0.05), using SPSS® (SPSS Japan, INC., Tokyo, Japan). RESULTS Effect of stretching on morphological characteristics of cultured cells (Fig. 2) On days 1 and 3, no significant difference was noted between the control group and the three stretch groups. On day 5, number of cells showed a significant increase in all three stretch groups in comparison with the control group. Cells were not arranged in the control group, but slightly arranged in the 3 stretch groups. No multinucleated cells were noted. Effect of stretching on number of cells (Fig. 3) and nuclei (Fig. 4) First, number of cells was counted. In the control group, no major changes were noted from day 1 to day 3, but a significant increase was noted from day 3 and day 5. In the three stretch groups, although cell number tended to increase from day 1 to day 3, it showed little change from day 3 to day 5. Next, number of nuclei was counted. In the control group and three stretch groups, the nuclei continued in-. creasing on days 1, 3 and 5. There were no significant differences in the cell or nuclear count among the 3 stretch groups. mRNA expression of myosin heavy chain subtype Expression of MyHC-perinatal mRNA on days 1 and 3 was significantly higher for the 0.5/sec and 0.9/ sec groups than for the other groups. On day 5, expression for the 0.5/sec and 0.9/sec groups showed a marked decrease, while that for the 0.1/sec group showed an increase significantly higher than that seen in the 0.5/sec or 0.9/sec groups. Few changes were seen on days 1, 3 and 5 in the controls (Fig. 5). Expression of MyHC-2b mRNA was significantly higher in the 0.9/sec group than in the other groups throughout the experimental period. In the controls, little change was seen on days 1, 3 or 5 (Fig. 6). Expression of MyHC-2d on days 1, 3 and 5 was significantly greater in the 0.5/sec group than in the other groups, and tended to increase with time (Fig. 7). Expression of MyHC-2a mRNA on day 1 was significantly greater in the 0.1/sec and 0.5/sec groups than in the other 2 groups, but no significant difference was noted between the 0.1/sec and 0.5/ sec groups. On days 3 and 5, the expression was significantly higher in the 0.1/sec group than in the control, 0.5/sec or 0.9/sec groups, and expression of 0.1/sec group increased with time (Fig. 8). Expres-. Fig. 2 Phase contrast microscope image of myoblasts at each stage.
(6) Effects of stretching on muscle fiber differentiation. Fig. 3 Number of myoblasts at each stage (mean ± SD). Statistical analysis: *P < 0.05. It increased from day 3 to day 5 in the control group. On the other hand, it increased from day 1 to day 3 in the 3 stretch groups.. Fig. 5 MyHC-perinatal mRNA expression in mechanically stretched myoblasts by day. Statistical analysis: *P < 0.05. In comparison to the control group, significant increases were noted in the 0.5/sec and 0.9/sec groups on days 1 and 3 and 0.1/sec groups on day 5.. sion of MyHC-1 on days 1, 3 and 5 was significantly greater in the 0.1/sec group than in the other groups (Fig. 9). DISCUSSION Effects of mechanical stretching on cultured cells In the three stretch groups, although cell number showed little change from day 3 to day 5, number of myoblast nuclei was significantly greater in this study. This suggests that differentiation of myoblasts is promoted by stretching, that cell fusion becomes active, and that myoblasts differentiate to become myotube cells. Changes in the composition of each MyHC isoform in mechanically-stretched cultured cells MyHC-perinatal was investigated to clarify differences in myoblast differentiation. Expression of MyHC-perinatal was significantly increased on days 1 and 3 in the 0.5/sec and 0.9/sec stretch groups, and on day 5 in the 0.1/sec stretch group, compared to. 29. Fig. 4 Number of myoblast nuclei at each stage (mean ± SD). Statistical analysis: *P < 0.05. It slowly increased from day 1 to day 5 in the control and 3 stretch groups.. the control group, suggesting that stretch stimulation activated cell division and promoted cell proliferation. Expression of MyHC-perinatal was decreased on day 5 in the 0.5/sec and 0.9/sec stretch groups, suggesting that the cells had differentiated to myotube cells. These findings were consistent with the findings on the comparison of the cell and nuclear counts. Expressions of MyHC-2b and MyHC-2a mRNA were determined to ascertain differences in muscle fiber characteristics. Expression of MyHC-2b (fastest contraction rate) was high with rapid stretching, while expressions of MyHC-2a and MyHC-1 (slow contraction rate) were high with slow stretching. In previous studies on continuous training, Andersen and Henriksson (2, 3) instructed 12 healthy individuals to exercise 30 min/day at 80% intensity using an ergometer for 4 and 8 weeks, and reported that numbers of type IIb fibers decreased, while numbers of type IIa fibers and type I fibers increased. These type IIa and I fibers are categories based on tissue staining, and type IIa, IIb, and I fibers correspond to MyHC-2a, -2b, and -1, respectively. Baumann et al. (4) also instructed subjects to exercise 30 min/day at 90% intensity using an ergometer for 5 and 8 weeks, and likewise reported that numbers of type IIb fibers decreased, while numbers of type IIa fibers and type I fibers increased. Similar studies have been conducted using rats. Lauginbuhl et al. (15) made rats run on a treadmill at 30 m/min for 5 min/ day over 5 days/week for 12 weeks, and reported that numbers of type IIa fibers and type I fibers increased, while numbers of type IIb fibers decreased. Green et al. (9) obtained similar results by making rats run 15 min/day for 15 weeks. As to changes in muscle fiber characteristics caused by sprint training, Jacobs et al. (13) instructed human subjects to train on a treadmill for 6 weeks and reported that numbers of slow-twitch fibers decreased, while.
(7) K. Kurokawa et al.. 30. Fig. 6 MyHC-2b mRNA expression in mechanically stretched myoblasts by day. Statistical analysis: *P < 0.05. The value was increased in the 0.9/sec stretch group throughout the period.. Fig. 7 MyHC-2d mRNA expression in mechanically stretched myoblasts by day. Statistical analysis: *P < 0.05. The value was increased in the 0.5/sec stretch group throughout the period.. Fig. 8 MyHC-2a mRNA expression in mechanically stretched myoblasts by day. Statistical analysis: *P < 0.05. The value was increased on days 3 and 5 in the 0.1/sec stretch group.. Fig. 9 MyHC-1 mRNA expression in mechanically stretched myoblasts by day. Statistical analysis: *P < 0.05. The value was increased in the 0.1/sec stretch group throughout the period.. numbers of fast-twitch fibers increased. The present results for MyHC-2b, MyHC-2a, and MyHC-1 expressions support these earlier studies using humans and animals at the cellular level. MyHC-2d is considered to be a flexible isoform. Among the 3 rates of stretching used in the present study, expression was high with 1-sec stretching. MyHC-2d is considered to be an isoform that can change to either MyHC-2a or MyHC-2b, and the present results showed that 0.5-sec stretching increased expression of MyHC-2b, while 5-sec stretching increased expression of MyHC-2a. The increased expression of MyHC-2d of 0.5/sec stretching suggests that cells in this state are ready to allow differentiation to either 2a or 2b. The results of this in vitro experiment was clarified that stretch stimulation promoted cell differentiation regardless of the stretch rate. Also the characteristics of muscle fiber altered depending on type of stimulus. This suggests that muscle has the capability of responding to even small changes in its environment.. Acknowledgment This study was supported by grants-in-aid for scientific research (14704046, to S. Abe) from the Ministry of Education, Culture, Sports, Science and Technology, Japan and by Oral Health Science Center Grant HRC 7 (S. Abe) from Tokyo Dental College. REFERENCES 1. Abe S, Maejima M, Watanabe H, Shibahara T, Agematsu H, Doi T, Sakiyama K, Usami A, Gojyo K, Hashimoto M, Yoshinari M and Ide Y (2002) Muscle-fiber characteristics in the adult mouse tongue muscles. Anat Sci Int 77, 145–148. 2. Andersen P and Henriksson J (1977) Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol 270, 677–690. 3. Andersen P and Henriksson J (1977) Training induced changes in the subgroups of human type II skeletal muscle fibres. Acta Physiol Scand 99, 123–125. 4. Baumann H, Jaggi M, Soland F, Howald H and Schaub MC (1987) Exercise training induces transitions of myosin isoform subunits within histochemically typed human muscle fibres. Pflugers Arch 409, 349–360..
(8) Effects of stretching on muscle fiber differentiation 5. Brueckner J K, Itkis O and Porter PD (1996) Spatial and temporal patterns of myosin heavy chain expression in developing rat extraocular muscle. J Muscle Res Cell Motil 17, 297–312. 6. Doi T, Abe S and Ide Y (2003) Masticatory function and properties of masseter muscle fibers in microphthalmia (mi/ mi) mice during postnatal development. Ann Anat 185, 435– 440. 7. Geiger PC, Bailey JP, Zhan WZ, Mantilla CB and Sieck GC (2003) Denervation-induced changes in myosin heavy chain expression in the rat diaphragm muscle. J Appl Physiol 95, 611–619. 8. Goto S, Miyazaki K, Funabiki T and Yasumitsu H (1999) Serum-free culture conditions for analysis of secretory proteinases during myogenic differentiation of mouse C2C12 myoblasts. Anal Biochem 272, 135–142. 9. Green HJ, Klug GA, Reichmann H, Seedorf U, Wiehrer W and Pette D (1984) Exercise-induced fibre type transitions with regard to myosin, parvalbumin, and sarcoplasmic reticulum in muscles of the rat. Pflugers Arch 400, 432–438. 10. Hori A, Ishihara A, Kobayashi S and Ibata Y (1998) Immunohistochemical classification of skeletal muscle fibers. Acta Histochem Cytochem 31, 375–384. 11. Hoshina S, Matsuzaka K, Motoyoshi Y, Koike Y, Takeda T, Ishigami K and Inoue T (2004) Osteoblast-like cell behavior of rat bone marrow under continuous compressive force in vitro. Biomed Res 25, 109–117. 12. Howald H, Hoppeler H, Claassen H, Mathieu O and Straub R (1985) Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch 403, 369–376. 13. Jacobs I, Esbjornsson M, Sylven C, Holm I and Jansson E (1987) Sprint training effects on muscle myoglobin, enzymes, fiber types, and blood lactate. Med Sci Sports Exerc 19, 368– 374. 14. Jakubiec-Puka A, Ciechomska I, Joanna M and Matusiak A (1999) Contents of myosin heavy chains in denervated slow and fast rat leg muscles. Comp Bichem Physiol B Biochem Mol Biol 122, 355–362. 15. Luginbuhl AJ, Dudley GA, and Staron RS (1984) Fiber type changes in rat skeletal muscle after intense interval training. Histochemistry 81, 55–58. 16. Maejima M, Abe S, Sakiyama K, Agematsu H, Hashimoto M, Tamatsu Y and Ide Y (2005) Changes in tongue muscle fiber properties of mouse before and after weaning. Arch Oral Biol 50, 988–993.. 31 17. Malisoux L, Francaux M, Nielens H and Theisen D (2006) Stretch-shortening cycle exercises: an effective training paradigm to enhance power output of human single muscle fibers. J Appl Physiol 100, 771–779. 18. Naumann K and Pette D (1994) Effects of chronic stimulation with different impulse patterns on the expression of myosin isoforms in rat myotube cultures. Differentiation 55, 203–211. 19. Pette D and Staron RS (1990) Cellular and molecular diversitiexs of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol 116, 1–76. 20. Putman CT, Xu X, Gillies E, MacLean IM and Bell GJ (2004) Effects of strength, endurance and combined training on myosin heavy chain content and fibre-type distribution in humans. Eur J Appl Physiol 92, 376-384. 21. Sakiyama K, Abe S, Tamatsu Y and Ide Y (2005) Effects of stretching stress on the muscle contraction proteins of skeletal muscle myoblasts. Biomed Res 26, 61–68. 22. Sakuraba T, Shimada Y, Takahashi S, Matsunaga T, Itoi E and Kawatani M (2005) The effect of magnetic stimulation on unloaded soleus muscle of rat : changes in myosin heavy chain mRNA isoforms. Biomed Res 26, 15–19. 23. Schiaffino S and Reggiani C (1996) Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol Rev 76, 371–425. 24. Simoneau JA, Lortie G, Boulay MR, Marcotte M, Thibault MC and Bouchard C (1985) Human skeletal muscle fiber type alteration with high-intensity intermittent training. Eur J Appl Physiol Occup Physiol 54, 250–253. 25. Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y and Ishii N (2000) Effect of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol 88, 2097–2106. 26. Thomason DB, Herrick RE, Surdyka D and Baldwin KM (1987) Time course of soleus muscle myosin expression during hindlimb suspension and recovery. J Appl Physiol 63, 130–137. 27. Tsika RW, Herrick RE and Baldwin KM (1987) Interaction of compensatory overload and hindlimb suspension on myosin isoform expression. J Appl Physiol 62, 2180–2186. 28. Usami A, Abe S and Ide Y (2003) Myosin heavy chain isoform of the murine masseter muscle during pre- and post-natal development. Anat Histol Embryol 32, 244–248. 29. Yaffe D and Saxel O (1977) Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725–727..
(9)
(10)
図


関連したドキュメント
An easy-to-use procedure is presented for improving the ε-constraint method for computing the efficient frontier of the portfolio selection problem endowed with additional cardinality
If condition (2) holds then no line intersects all the segments AB, BC, DE, EA (if such line exists then it also intersects the segment CD by condition (2) which is impossible due
Many interesting graphs are obtained from combining pairs (or more) of graphs or operating on a single graph in some way. We now discuss a number of operations which are used
This paper is devoted to the investigation of the global asymptotic stability properties of switched systems subject to internal constant point delays, while the matrices defining
In this paper, we focus on the existence and some properties of disease-free and endemic equilibrium points of a SVEIRS model subject to an eventual constant regular vaccination
Then it follows immediately from a suitable version of “Hensel’s Lemma” [cf., e.g., the argument of [4], Lemma 2.1] that S may be obtained, as the notation suggests, as the m A
Classical definitions of locally complete intersection (l.c.i.) homomor- phisms of commutative rings are limited to maps that are essentially of finite type, or flat.. The
Yin, “Global existence and blow-up phenomena for an integrable two-component Camassa-Holm shallow water system,” Journal of Differential Equations, vol.. Yin, “Global weak