INTRODUCTION
In the management of HNSCC, a surgical resection often involves reconstructive or func-tional difficulties (loss of voice, compressive changes of the face etc.), which severely influ-ence the patient’s quality of life. In contrast,
chemoradiotherapy (CRT) with 5-fluorouracil (5-FU) may be an efficient modality of treat-ment, because of a relatively high radiosensitivi-ty and both cosmetic and functional benefits. However, in some cases tumor cells may show a poor response to CRT or recur after a complete response. In these cases, additional chemother-apy may be ineffective, and radiotherchemother-apy is not applicable because of dose limitations, so a sal-vage operation would become an alternative treatment of choice.
5-FU is an effective and widely used
Chemoradiotherapy with 5-fluorouracil Inhibits the Expression of
Thymidylate Synthase and Dihydropyrimidine Dehydrogenase in
Head and Neck Squamous Cell Carcinoma
Takanobu S
HIMADA1), Shigetoshi H
ORIGUCHI2), Yoshitaka O
KAMOTO2),
Zennsei M
ATSUZAKI1), and Keisuke M
ASUYAMA1)1)Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine,
University of Yamanashi, Yamanashi 469-3898, and
2)Department of Otolaryngology, Graduate School of Medicine,
Chiba University, Chiba, Japan
Abstract: In order to examine the effect of chemoradiotherapy (CRT) with 5-fluorouracil (5-FU) on
thymidylate synthase/dihydropyrimidine dehydrogenase (TS/DPD), the expressions and activities of TS/DPD of the tumors in head and neck squamous cell carcinoma (HNSCC) were examined and evaluated in tumors from the subjects with HNSCC before treatment, in residual tumors after CRT, and in recurrent tumors after a complete response (CR) with CRT. Thirty tissue samples were excised from 26 patients with HNSCC. TS/DPD activities were determined with a tritium release-assay and a radio-enzymatic assay, and the protein contents were evaluated by immunohistochemistry. The mean TS/DPD activities of the residual tumors after CRT were significantly lower than those of the before-treatment and recurrent tumors. No significant difference was observed between these values in the before-treatment and recurrent tumors. Furthermore, observations obtained from the same subjects with HNSCC before and after CRT demonstrated the TS/DPD enzymatic expressions to be sup-pressed by CRT with 5-FU; however, they returned to the previous levels when the tumor recurred. These results indicate that 5-FU chemotherapy immediately after CRT may therefore be an effective treatment for residual tumors.
Key words: thymidylate synthase, dihydropyrimidine dehydrogenase, 5-fluorouracil, head and neck
squamous cell carcinoma
Correspondence to: Keisuke Masuyama, Department of Otorhinolaryngology, Head and Neck Surgery, Fac-ulty of Medicine, University of Yamanashi, 1110 Shimokato, Chuo-shi 469-3898, Yamanashi, Japan. Received December 7, 2007
Accepted January 23, 2008
Original Article
chemotherapeutic agent for HNSCC. Sensitivity to 5-FU is influenced by two factors; thymidylate synthase (TS) and dihydropyrimidine dehydro-genase (DPD)1-3). TS is the key enzyme in the de
novo pathway of deoxythymidylate (dTMP) and
catalyzes the conversion of deoxyuridylate (dUMP) and 5,10-methylene tetrahydrofolate (CH2-THF) to dTMP and dihydrofolate (DHF). 5-FU is converted to fluoro-deoxyuridylate (FdUMP), which then binds TS with CH2-THF irreversibly to form a ternary complex, thus inhibiting the catalytic activity of TS4).
On the other hand, DPD catalyzes the conver-sion of 5-FU to 5-fluoro-β-alanine (F-BAL), and the concentration of F-dUMP is thought to be influenced by DPD activity. Since the TS activity has been shown to correlate with the number of sites bound to F-dUMP5), a higher concentra-tion of cytosolic F-dUMP is thus required to sup-press the catalysis of TS in tumors with a high TS activity. Furthermore, in tumors with a high DPD activity, the percentage of 5-FU converted to F-dUMP decreases. These facts indicate that sensitivity to 5-FU tends to be lower in tumors with high TS and DPD activities.
TS/DPD expressions have been reported to be high in cancer cells treated with 5-FU in
vitro6,7). Accordingly, 5-FU exposure may induce
an increase in TS/DPD expressions and lower the sensitivities to 5-FU in residual or recurrent tumors after CRT with 5-FU. However, whether TS/DPD expressions are increased or not in residual or recurrent tumors after CRT with the use of 5-FU has not yet been clarified. In order to address these issues, we obtained tissue sam-ples of HNSCC, 1) before treatment, 2) 3-6 weeks after finishing CRT, and 3) at the time of recurrence after CRT-induced complete remis-sion, and estimated the levels of TS/DPD expressions among these specimens through assays of TS/DPD activities and
immunohisto-chemistry (IHC). Furthermore, the influence of CRT on the TS/DPD expressions is discussed.
MATERIALS AND METHODS
Patients and samples
This study was approved by the Ethics Com-mittee of the University of Yamanashi and per-formed with the written consent of all partici-pants. Thirty samples of HNSCC obtained from 26 patients were examined in this study. The mean age of the subjects was 64.2 ± 11.6 years. Of the cases, 23 were males and three were females.
The tumor samples were divided into three groups: Group A (n=20), which consisted of samples obtained from the subjects who were initially treated by surgery and had not previ-ously received radiotherapy and/or chemother-apy; Group B (n=5), which contained samples that were surgically removed from residual tumors 6 weeks after CRT,; and Group C (n=5), which consisted of samples obtained from recurrent tumors redeveloping after CRT. In three cases, paired normal mucosa and tumor tissues were obtained and preserved. In order to evaluate the TS/DPD expressions in all the tis-sue samples, the tistis-sues were cut in half, and then one half was stained with immunohisto-chemistry, and the other half was used to deter-mine the TS/DPD activities, respectively.
Tritium release assay
The TS activity was determined with a tritium release assay reported by Spear8)with modifica-tions. Briefly, the frozen samples were homoge-nized in 50mM Tris-HCl buffer (pH 7.3) and centrifuged, and the cytosol fraction was sepa-rated. After determining the protein content, 0.62µM 5,10-methylene-tetrahydrofolate (CH2-THF) and 1µM 5-3H-dUMP were added and
incubated at 37˚C. After 10, 20, or 30 minutes, reactions were terminated by the addition of 10% activated neutral charcoal with 4% trichloroacetate. The samples were centrifuged at 14,000 × g for 5 min. The 3H-radioactivity of the separated supernatants was measured, and the time-reaction curve was determined, while the enzyme activities were also calculated.
Radioenzymatic assay
The DPD activity was determined with a radioenzymatic assay reported by Diasio9) with modifications. Briefly, the frozen samples were homogenized and centrifuged at 105,000 × g at 4˚C for 60 min and the cytosol fraction was sepa-rated. After determining the protein contents, 6.25 mM NADPH and 125µM 3-3H-5-FU were added and the samples were incubated at 37˚C. After 10, 20, or 30 minutes of incubation, equal volumes of hydrogen perchloride were added to the samples, and diluted two-fold with 20 mM sodium dihydrophosphate (pH 3.5) and cen-trifuged. The radioactivities of the supernatant fractions of 5-FU, DHFU, FUPA and FBAL were examined, the time-reaction curve was deter-mined, and then the enzyme activities were cal-culated.
Immunohistochemistry
RTSSA, an human TS polyclonal anti-body, which was used as the primary antianti-body, was prepared from the serum of a rabbit sensi-tized with recombinant human TS, which was kindly supplied by Taiho Pharmaceutical Co., Ltd., Tokyo. We used the Envision-kit (DAKO) for the second antibody and detection system.
All samples were fixed in 4% paraformalde-hyde for 24 hr and embedded in paraffin-blocks. A transplantable tumor of a human col-orectal cancer, DLD-1/FdUrd, was used as posi-tive controls. As negaposi-tive controls, normal
rab-bit serum was applied to every specimen instead of the primary antibody. The blocks were sliced into 4µm sections and mounted on silan-coated glass slides. The sections were deparaffinized in xylene and then were rehydrated in decreasing ethanol concentrations and then autoclaved. After cooling to room temperature (RT), the sections were incubated in 0.3% hydrogen-per-oxide in methanol for 20 min and washed in deionized water (dH2O) and Tris-buffered saline with 0.1% Tween 20 (TBS-T). Protein block solution (DAKO) was applied to the sec-tions and incubated for 5 min at RT. After removing the excess protein solution, 100-times diluted primary antibody was added and incu-bated for 60 min at RT. After washing, the sec-ond antibody was added and incubated for 30 minutes at RT. After washing, DAB-solution was applied and incubated for 4 min at RT. After washing, the sections were counterstained with hematoxylin, dehydrated and mounted with malinol (Muto-kagaku, Tokyo). Three high power fields were examined by two observers who did not know the patients’ backgrounds and immunoreactivity was evaluated and classi-fied using a visual grading system based on the cytoplasm staining as negative(-), weak(+), mod-erate(++), and strong(+++)10).
Statistical analysis
The regularity of distribution was analyzed with using the Chi-square test. Any correlations between TS and DPD were analyzed with Pear-son’s parametric correlation test. Differentia-tion between TS/DPD in every group was ana-lyzed with Student’s t-test (non-paired) when comparing two groups and with one-way analy-sis of variance when comparing in three groups. P values of less than 0.05 were considered to be significant.
RESULTS
Enzymatic activity of TS/DPD
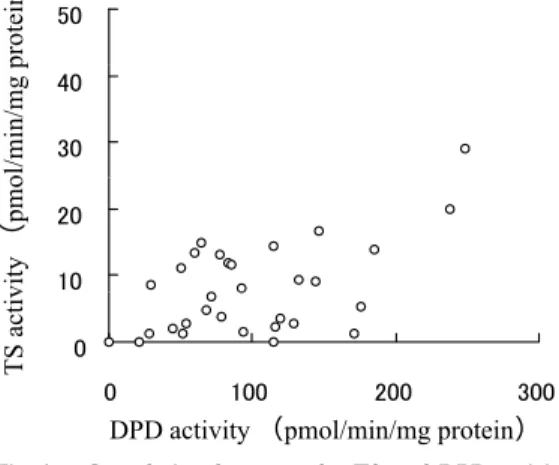
A weak but significant correlation was observed between the TS and DPD activities of the head and neck tumors (r=0.457, p=0.04, Fig. 1).
The mean TS activities of the tumors and nor-mal mucosa were 10.786 ± 9.517 (n.s.-19.9) and 1.833 ± 0.850 (1.2-2.8) pmol/min/mg protein,
respectively, whereas the mean DPD activities of the tumors and normal mucosa were 108.1 ± 59.0 (n.s.-249) and 66.3 ± 24.0 (79-171) pmol/min/mg protein, respectively. The activities of both TS and DPD in the tumor sam-ples tended to be higher than those of the nor-mal mucosa, but not by a significant amount (Fig 2-A, B, p=0.06, p=0.12). In addition, in paired samples of tumors and normal mucosa obtained from the same subjects, the tumor DPD activity was significantly higher in compari-son to the normal mucosa (p=0.033). The tumor TS activity was higher than the normal mucosa but the difference was not significant(p=0.12).
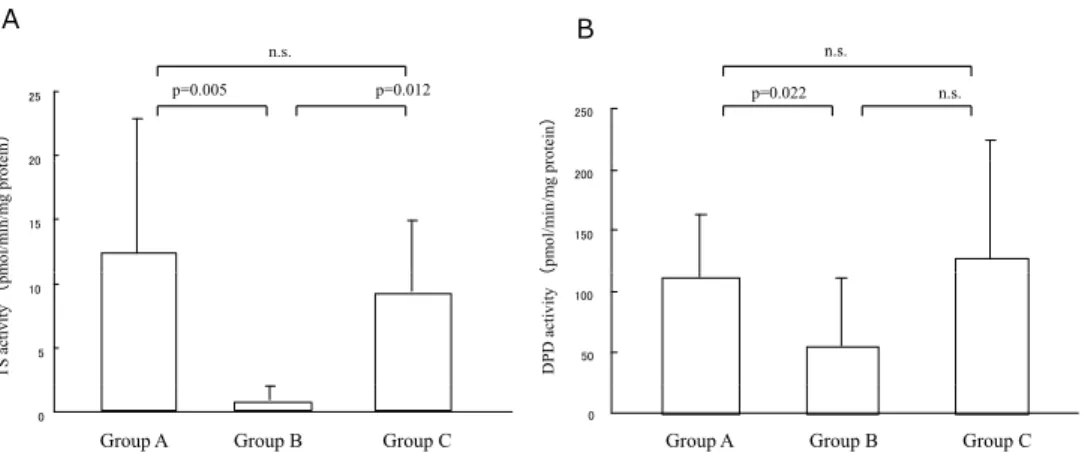
The TS activities of the tumors before treat-ment (Group A), the residual tumors after CRT (Group B) and the recurrent tumors obtained following a complete response after CRT( Group C) were 12.2 ± 10.4, 0.7 ± 1.0, and 9.06 ± 5.58 pmol/min/mg protein, respective-ly, whereas the DPD activities of these groups were 111.8 ± 51.5, 56 ± 55.3, and 127.8 ± 96.3 pmol/min/mg protein, respectively. Both the TS and DPD activities of Group B were sig-nificantly lower in comparison to those of Fig. 1. Correlation between the TS and DPD
activi-ties of the head and neck cancer cells. A weak but significant correlation (r=0.457, p=0.04) was observed between the TS and DPD activities in the head and neck cancer cells.
Fig. 2. The mean TS and DPD activities of the cancer cells and normal mucosa.
The mean TS (A) and DPD (B) activities of the cancer cells tended to be higher in comparison to the normal mucosa; however, not by a significant amount. ns = not significant.
Group A (Fig. 3. p=0.005, p=0.022). In addition, the TS activity of Group B was significantly lower than that of Group C (p=0.012), however, the DPD activity of Group B tended to be lower than that of Group C but the difference was not significant (p=0.100). In contrast, no significant differences were observed between the TS and DPD activities of Groups A and C (p=0.245, p=0.314).
In addition, we were able to examine the tumor tissues before and after various treatment modalities from each of three subjects. In case 1, the tumor was surgically removed without postoperative CRT following the recurrence. The TS and DPD activities of the recurrent tumor were likely to be higher than those of the primary tumor. In case 2, the patient was initial-ly treated with surgery followed by CRT and a recurrence developed. The recurrent tumor showed a lower DPD activity, although the TS activity was unchanged in comparison to the primary tumor. In case 3, the patient was treat-ed with preoperative CRT followtreat-ed by surgery,
and 12 months after the recurrence a salvage operation was performed. The TS and DPD activities of the recurrent tumor were remark-ably higher in comparison to the tumor tissue obtained after CRT.
Immunohistochemistry
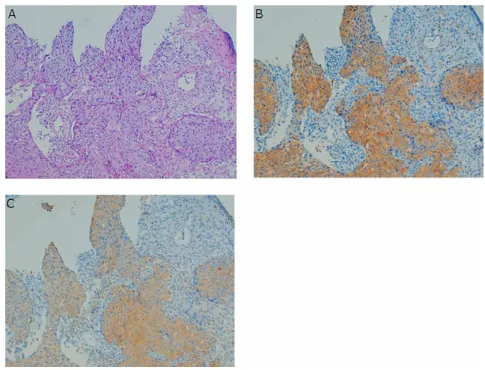
TS and DPD staining was observed in the cytosol and the results were consistent with previ-ous reports (Fig. 4). The grades of immunoreac-tivity in the tumor tissues were stronger in com-parison to the normal mucosa, although weak staining in the superficial or intermediate layers of the normal mucosa were observed with these antibodies (data not shown). Serial sections revealed that both TS and DPD were co-expressed in the tumor cells of all groups (Fig. 5). Secondly, the relationship between the TS/DPD activities and grades of immunostain-ing was examined. No significant difference was observed in the TS and DPD activities between the stronger stained and weaker stained speci-mens (data not shown).
Fig. 3. Mean TS and DPD activities of the tumor tissues obtained before treatment (group A; n=20), from the residual tumor after CRT (group B; n=5), and from the recurrent tumor which showed complete response after CRT (group C; n=5).
Both the TS and DPD activities of Group B were significantly lower in comparison to those of Groups A (p=0.005, p=0.022). The TS activity of group B was also significantly lower than that of group C (p=0.012).
Fig. 4. Immunohistochemical staining of TS (A) and PDP (B) in head and neck cancer cells. Both TS (A-1) and DPD (B-1) staining were observed in the cytosol of the cancer cells. A-2, B-2 = negative controls. Magnification × 100
Fig. 5. Colocalization of TS and DPD immunoreactivity using a serial section. H-E stain-ing (A), TS immunoreactivity (B), and DPD immunoreactivity (C). Magnification × 40
DISCUSSION
In this study, we found that the TS/DPD activ-ities of the residual tumors just after CRT were significantly lower in comparison to the primary tumors before treatment. The TS activities of the residual tumors just after CRT were also sig-nificantly lower than those of the recurrent tumors. However, their DPD activity tended to be lower in comparison to the recurrent tumors after CRT. We had expected higher expressions of TS/DPD after CRT; however, the reverse was the case.
Peters et al6)has shown that the sensitivity to 5-FU decreases when tumor cells are cultured with 5-FU and the TS activity of these cells increases. It has also been demonstrated that the TS expression is regulated at a translational level, and TS mRNA has a binding site to which TS binds to inhibit TS translation11). When F-dUMP forms a ternary complex with TS and then irreversibly inhibits TS activity, free TS concentrations decrease. This decrease in turn reoperates TS mRNA translation, thus inducing the upregulation of TS expression11-13). Further-more, the tumor DPD activity has been shown to increase after treatment with Tegafur and Uracil (UFT) in urinary tract cancer patients7). These reports suggest that the low sensitivity to 5-FU of tumors treated with 5-FU may be due to the elevated expressions of TS/DPD. The rea-son for the discrepancy between our results may be that there seems to be a balance between the TS/DPD expressions triggered with either chemotherapy (inductive effects) or radiothera-py (inhibitory effects). Therefore, when the inhibitory effects of radiotherapy are stronger than the inductive effects of 5-FU, TS/DPD expressions may decrease. Our results suggest that radiotherapy might show stronger effects than chemotherapy in terms of TS/DPD
expres-sions in the case of concurrent chemoradiother-apy with 5-FU. Further studies may be necessary to address these issues.
We have also demonstrated that the TS/DPD activities of the recurrent tumors were not sig-nificantly different from those of the untreated primary tumors. Clinically, chemotherapy is sometimes not effective for recurrent tumors, which seems to be due to the higher expres-sions of TS/DPD activities in recurrent tumors. However, no significant differences in these val-ues have been found between untreated prima-ry and recurrent tumors. These results indicate that recurrent tumors’ resistance to 5-FU may be due to other mechanisms besides higher TS/DPD expressions. In order to confirm these facts, more data will have to be collected and examined.
The problem with this study is whether or not the tumor cells were viable in the tissues obtained from the samples after CRT. When tumor cells become necrotic after chemoradio-therapy, the percentage of viable tumor cells may not be high enough to correctly estimate the TS/DPD enzymatic activity. In order to care-fully evaluate the TS/DPD expressions in the tissue samples after CRT, the tissues were cut in half, and then one half was stained with immunohistochemistry to confirm if enough viable tumors cells were present. These results demonstrated that more than 60% viable tumor cells were obser ved in ever y specimen of Groups A, B, and C and no difference was found among the samples.
Studies from three cases imply that primary surgery had no effect on the TS/DPD activities in the tumor cells at all. However, the decrease in those values observed after CRT seemed to recover or increase when a tumor recurred. These results suggest that CRT inhibits the TS/DPD expressions in HNSCC to the levels of
normal mucosa at first, but these enzymatic activities can reactivate afterward during tumor regrowth. Further studies, which compare the values of TS/DPD between before and after CRT in the same subjects, have to be necessary in order to show the direct evidence of inhibito-ry effect of CRT on TS/DPD activities.
A salvage operation is the preferred treat-ment when tumors remain after CRT comple-tion. However, this study suggests that sensitivity to 5-FU may increase just after CRT due to the suppressed TS/DPD activities. It is thus conceiv-able that 5-FU chemotherapy immediately after CRT may be an alternative treatment to surgery for residual tumors. This is the first report to examine the ST/DPD activities in tumors both after CRT and at the time of recurrence. These findings may help create a novel therapeutic approach for the treatment of residual tumors after CRT in HNSCC.
REFERENCES
1) Nita ME, Tominaga O, Nagawa H, Tsuruo T, and Muto T: Dihydropyrimidine dehydrogenase but not thymidine synthase expression is associated with resistance to 5-fluorouracil in colorectal cancer. Hepato-Gastroenterol 45, 2117-2122, 1998.
2) Beck A, Etienne MC, Chèradame S, Fischel JL, Formento P, Renèe N, et al.: A role for dihy-dropyrimidine dehydrogenase and thymidylate synthase in tumor sensitivity to fluorouracil. Eur J Cancer 10, 1517-1522, 1994.
3) Ishikawa Y, Kubota T, Otani Y, Watanabe M, Ter-amoto T, Kumai K, et al.: Dihydropyrimidine dehydrogenase activity and messenger RNA level may be related to the antitumor effect of 5-fluo-rouracil on human tumor xenografts in nude mice. Clin Cancer Res 5, 883-889, 1999.
4) Hartmann K-U, and Heidelberger C: Studies on fluorinated pyrimidine XIII. Inhibition of thymidylate synthethase. J Biol Chem 236, 3006-3013, 1961.
5) van Triest B, Pinedo HM, van Hensbergen Y, Smid K, Telleman F, Schoenmakers PS, et al: Thymidylate synthase level as the main predictive parameter for sensitivity to 5-fluorouracil, but not for folate-based thymidylate synthase inhibitors, in 13 nonselected colon cancer cell line. Clin Cancer Res 5, 643-654, 1999.
6) Peters GJ, Backus HHJ, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, et al.: Induction of thymidylate synthase as a 5-fluo-rouracil resistance mechanism. Biochemi Bio-phys Acta 1587, 194-205, 2002.
7) Yano A, Hashimoto K, Okuchi T, Mizoguchi H, Mutaguchi K, Nakamoto T, et al.: Dihydropyrimi-dine dehydrogenase activity in urothelial cancer-Influence of UFT administration on DPD activi-ty. Jpn J Cancer Chemother 28, 1877-1883, 2001 (in Japanese).
8) Spears CP, and Gustavsson BG: Methods for thymidylate synthase pharmacodynamics; serial biopsy, free and total TS, FdUMP, and dUMP and H4-Pteglu and CH2-H4Pteglu assays. In:
Rus-tum Y, McGuire JJ, eds. The Expanding Role of Folates and Fluoropyrimidine in Cancer Chemotherapy. Plenum Press, New York: 97-104, 1988.
9) Diasio R B, Beavers TL, and Carpenter JT: Famil-ial deficiency of dihydropyrimidine dehydroge-nase. J Clin Invest 81, 47-51, 1988.
10) Huang CL, Yokomise H, Kobayashi S, Fukushima M, Hitomi S, and Wada H: Intratumoral expres-sion of thymidylate synthase and dihydropyrimi-dine dehydrogenase in non-small cell lung can-cer patients treated with 5-FU-based chemothera-py. Int J Oncol 17, 47-54, 2000.
11) Chu E, Voeller D, Koeller DM, Drake JC, Taki-moto CH, Maley GF, et al.: Identification of an RNA binding site for human thymidylate syn-thase. Proc Natl Acad Sci USA 90, 517-521, 1993. 12) Chu E, Koeller DM, Casey JL, Drake JC, Chabner
BA, Elwood PC, et al.: Autoregulation of human thymidylate synthase messenger RNA translation by thymidylate synthase. Proc Natl Acad Sci USA
88, 8977-8981, 1991.
13) Chu E, Koeller DM, Johnston PG, Zinn S, and Allegria CJ: Regulation of thymidylate synthase in human colon cancer cells treated with 5-fluo-rouracil and interferon-gamma. Mol Pharmacol