Serological
and
Immunohistochemical
Detection
of
a
Gastrointestinal
Cancer
-associated
Asialoglycoprotein
Antigen
of
Human
with
a Murine
Monoclonal
Antibody
Masaaki
ADAM,*1
Teruaki
SEKINE,*2,*3
Kohzoh
IMAI,*2
Akira
YACHT*2
and
Shigeaki
SATO*1
*1Biochemistry
Division
, National
Cancer
Center
Research
Institute,
1-1,
Tsukiji
5-chome,
Chuo-ku,
Tokyo
104 and
*2Department
of Internal
Medicine
(Section
I), Sapporo
Medical
College,
South
1, West
16, Chuo-ku,
Sapporo
060
A murine
monoclonal
antibody
(mAb),
NCC-AS
13 (IgM,ƒÈ),
was producedafter
immunization
with
ascites
from
a gastric
cancer
patient.
In
a sandwich
enzyme
immunoassay,
NCC-AS
13
reacted
with
the
sera
from
32%
of 58
gastric
cancer
patients,
44%
of 9 hepatocellular
cancer
patients
and
33%
of 6 colorectal
cancer
patients.
Immunohistological
analysis
showed
NCC-AS
13 to react
with
approximately
90%
of 51 gastric
carcinomas,
12 out
of 12 colon
carcinomas
and
3 out of 4 lung
carcinomas.
The
NCC-AS
13 defined
antigen
was
determined
to be a carbohydrate
without
terminal
sialic
acid
on macromolecular
glycoproteins.
This
mAb
NCC-AS
13, detecting
a
novel
antigen
associated
with
gastrointestinal
cancers,
may
have
potential
uses
for
tumor
im
munoscintigraphy
and
for
the therapy
of gastric
cancer
patients.
Key
words:
Asialoglycoprotein
-
Ascites
-
Monoclonal
antibody
There
are various
tumor
markers
of colon
cancer,1)
pancreas
cancer,2,3)
lung
cancer,4)
bladder
carcinoma5)
or
hepatoma,6)
all of
which
are good detectors.
However,
a good
marker
of gastric
cancer
has not been estab
lished,
although
this cancer
has a high inci
dence
in Japan.
We have previously
estab
lished in our laboratory
mAb NCC-ST-2707)
to human
gastric carcinoma
cell line, St-15.8)
The
NCC-ST
- 270 - defined
Ag
was
not
detected
in patients'
sera by radioimmuno
assay,
however,
and seems
to be similar
to
other
mAbs
reported
by various
research
workers.
We have recently
raised
a mAb,
NCC-AS
13, to a macromolecular
ascites fraction
from
a
gastric
cancer
patient.
The
molecular
weights
of most
tumor
markers
in sera are
known
to be extremely
high and, because
a
soluble
antigen
is desirable
for detecting
a
circulating
antigen
in sera,
the macromole
cular ascites fraction
was used as an immuno
gen. This report
describes
the detection
of the
NCC-AS
13-defined
antigen
in sera or tissues,
and preliminary
studies on its molecular
prop
erties.
MATERIALS AND METHODS
Preparation of Macromolecular Fraction Ascites from a human stomach cancer patient was cen trifuged at 17,700g for 20min. The supernatant (5ml) was applied to a Sepharose CL-6B column (1.6•~92cm) equilibrated in GF buffer, i.e. 10mM Tris.HCl buffer, pH 7.4, containing 0.5M NaCl, 5mM EDTA and 0.02% sodium azide. The void volume eluates were pooled and concentrated to 1.6mg protein/ml. This was designated the macromolecular fraction (MMF), and was used as the immunogen, for screening, and as a standard antigen in sandwich enzyme immunoassay (EIA). Ascites from a patient was kindly supplied by Dr. Hasegawa (Nihon University School), the patient being a 68-year-old female diagnosed as having stomach cancer (Borrmann 3 type, poorly differen tiatedadenocarcinoma). Upon serological exami nation, the levels of carcinoembryonic antigen and
ƒ¿ -fetoprotein were found to be within normal limits. The MMF was also obtained from sera (5 ml) from healthy donors.
Monoclonal Antibody Production A BALB/c mouse was intraperitoneally immunized twice with 0.2ml of the MMF and Freund's complete adju vant, over a three-month period, followed by a 0.2 ml boost of the immunogen without the adjuvant, 3 days prior to cell fusion. Spleen cells from the immunized mouse were fused with P3•~63Ag 8 U.1 cells using polyethylene glycol 4,000 (Wako
*3 To whom
communications
should
be addressed
.
ANTIBODY-DEFINED
ASIALOGLYCOPROTEIN
Chemical Industries, Ltd.), and hybrids selected in 10% fetal bovine serum (FBS) and RPMI 1640 containing hypoxanthine, aminopterin and thy
midine (HAT). Supernatants of these hybridomas were screened using EIA (antigen insolubilized on plates).
EIA (Antigen Insolubilized on Plates) Ninety-six-well microplates (Immunoplate ITM), Nunc, Den mark) were coated with 50ƒÊl of MMF (20ƒÊg/ ml)/well, washed and incubated with 300ƒÊl of 1% bovine serum albumin (BSA, Sigma, USA)/well in 0.01M phosphate buffer, pH 7.4, containing 0.15M NaCl (PBS) for 2 days at 4•‹. Culture supernatants were added to the plates and incubated for 1hr at room temperature. After washing of the plates with 0.05% Tween 20 in PBS (PBST), 50ƒÊl of peroxidase-conjugated, affinity-purified goat anti mouse immunoglobulins (H & L) (Cappel, USA), 1ƒÊg/ml, in PBS containing 20% normal horse serum, were added to each well, and the plates were incubated for 1hr at room temperature. After thorough washing of the plates, 100ƒÊl of freshly prepared substrate solution (50mM phosphate-25 mM citric acid buffer, pH 5.0, containing 1mg of o-phenylenediamine/ml and 0.01% hydrogen per oxide) was added. The reactions were stopped with 25ƒÊl of 2M H2SO4 after being incubated in the dark for 20min at room temperature. The resultant color was measured as optical density at 490nm. In addition, the reactivities of the culture superna tants with the MMF from the sera of normal sub jects were checked.
MAb NCC-AS 13 MAb NCC-AS 13 was selected because it reacted with the MMF from the ascites but not with that from normal subjects. The NCC-AS 13 isotype was determined by using Ouchterlony tests.
Preparation of Partially Purified NCC-AS 13 and Biotinylated NCC-AS 13 NCC-AS 13 hybridoma ascites were applied to a Sepharose CL-6B column (1.6•~42cm) equilibrated with GF buffer, and fractions found by the Ouchterlony test to have reacted with goat anti-mouse IgM (Cappel) were collected and used as a partially purified mAb. These fractions were concentrated and dialyzed overnight against 0.1M NaHCO3 containing 0.5M NaCl at 4•‹ and the mAb biotinylation was per formed as described by Bayer et al.9) Briefly, 22.5
ƒÊ g of N-hydroxysuccinimide biotin (NHS-biotin, Pierce, USA) in dimethyl sulfoxide (DMSO, Merck, West Germany) was mixed with 2.26mg of NCC-AS-13 and the mixture was incubated for 4 hr at room temperature, followed by excessive dial ysis against GF buffer at 4•‹.
Sandwich EIA Microplates were coated with 3ƒÊg of NCC-AS 13/ml in 0.1M carbonate buffer, pH 9.6, overnight at 4•‹. Coating solutions were re moved and the remaining protein-binding sites
blocked by adding 300ƒÊl of 1% BSA/well in PBS, followed by incubation for more than 2 days at 4•‹. The plates were washed 3 times with PBST, a 10ƒÊl sample and 40ƒÊl of 2% mouse serum in PBS (dilution buffer) were added to each well, and the plates were incubated for 2hr at 37•‹. They were then washed, and 2ƒÊg of biotinylated NCC-AS 13/ ml and 1.25ƒÊg of horseradish peroxidase-avidin
(HRP-avidin, Vector Lab., USA)/ml in dilution buffer were added to each well (50ƒÊl/well). The plates were incubated for 3hr at room tempera ture, washed, and their colors developed as described for the EIA (antigen insolubilized on plates).
Enzyme and Various Other Treatments The ascites MMF with which the plates were coated, was treated with various reagents. Neuraminidase
(Sigma; 80mU/ml in 0.2M acetate buffer, pH 5.0) digestion was performed for 1hr at 37•‹. Sodium
m-periodate (Wako; 5mM in 50mM acetate buffer, pH 5.0, with 100mM NaCl) oxidation was carried out for 1hr at room temperature in the dark. For mild alkali treatment, the plates were incubated with O.1N NaOH overnight at room temperature. Following the above regimens, EIA (antigen insolubilized on plates) was carried out. Non-specific binding of NCC-AS 13 could be dis regarded, since the optical density of the resultant color of non-coated wells was extremely low (i.e. less than 0.005 at 490nm). For heat treatment, the perchloric acid (PCA) extract of the ascites was boiled for 5min at 100•‹, then examined by using sandwich EIA.
Specificity of NCC-AS 13 Determined by Im munohistochemical Staining Formalin-fixed, paraffin sections including sections from 6 blood type A patients, 4 B patients and 9 O patients were stained with culture supernatants using the ABC method.10) Briefly, the sections were deparaffinized, hydrated, incubated with 0.3% H2O2 in methanol for 20min to inactivate endogenous peroxidase and overlaid with 10% bovine serum in PBS for 20min at room temperature to eliminate non-specific bind ing. After washing, the sections were incubated with the NCC-AS 13 supernatant for 90min at room temperature, washed, incubated with 7.5ƒÊg of biotinylated anti-mouse IgG (H+L) (Vector Lab.)/ml in PBS containing 10% FBS for 60min and washed. The sections were incubated with ABC reagents (Vector Lab.) for 30min, then washed, and their colors developed by incubating them with 0.01% H2O2 and 0.05% 3,3'-diamino benzidine tetrahydrochloride (Sigma).
Hemagglutination Test This was performed by following a standard procedure in conjunction with goat anti-mouse IgM antiserum (Cappel).
Western Blotting Analysis The soluble fraction of the ascites in 1M PCA was separated by
PAGE
on
5%
polyacrylamide
slab
gel,
and
the
transfer
to membranes
was
achieved
as described
by Towbin
et al.11) in
a chamber
(Bio
Rad
Lab.,
USA)
at 200mA
overnight,
using
25mM
iris/liter,
0.1%
SDS
and
192mM
glycine/liter
in 20%
meth
anol,
pH
8.3.
The
membranes
were
then
cut
verti
cally
into
strips,
incubated
in 10%
bovine
serum
in
PBS
at 4•‹ overnight,
incubated
with
2ƒÊg
of mAb
NCC-AS
13 or
mouse
IgM
(as
a control)/ml
at
room
temperature
for
1hr,
washed
and
then
treated
as described
in the
ABC
method.
RESULTS
Monoclonal
Antibody
Production
In
the
EIA
(antigen
insolubilized
on
plates),
20
out
of
384
wells
reacted
selectively
with
the
ascites
MMF
but
not
with
that
from
normal
subjects.
Repeated
cloning
resulted
in
4 clones
which
stably
secreted
antibodies.
One
of
these,
NCC-AS
13
(IgM, ƒÈ),
showed
the
desired
reactivity
in preliminary
studies
with
stomach
cancer
tissues
(data
not
shown)
and
was
characterized
further.
Sandwich
EIA
by
mAb
NCC-AS
13
Sand
wich
EIA
was
used
to
measure
the
level
of
NCC-AS
13 antigen
in body
fluids,
especially
sera.
All
samples
were
run
in
duplicate.
The
immunogen
was
used
as
a standard
antigen.
The
quantity
of
NCC-AS
13
antigen
in
a
sample
was
expressed
in
arbitrary
units/ml
with
reference
to the
standard
antigen
sample.
The
amount
of
NCC-AS
13 antigen
in
10ƒÊl
of
a 1:3200
dilution
of
standard
antigen
was
taken
to
be
25
units/ml.
In
order
to
establish
the
reproducibility
of
this
assay,
the
standard
antigen
was
assayed
5 times
during
a 3-week
period.
The
coefficient
of
variation
ranged
from
4.2%
at 3 units/ml
to 3.0%
at 25
units/
ml.
Detection
of NCC-AS
13 Antigen
in Sera
The
NCC-AS
13 antigen
levels
were
measured
by
using
sandwich
EIA
in
sera
from
healthy
donors,
from
patients
with
benign
disease
and
from
cancer
patients.
In
146
healthy
donors,
the
mean
antigen
level
was
0.89
units/ml
and
the
standard
deviation
(SD),
1.34.
The
assay
cut-off
value
was
taken
to
be
3.6
units/ml
(mean+2SD),
and
positive
sera
ratio
values
for
patients
with
various
diseases
vs.
healthy
donors
are
summarized
in Table
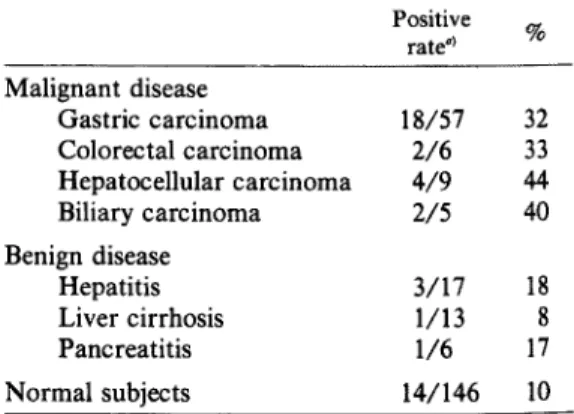
I. Out
of
57
gastric
cancer
patients,
18
(32%)
showed
levels
higher
than
the
cut-off
value
with
the
mean
value
of
64U/ml;
patients
with
colo
rectal, hepatocellular and biliary tract cancer
showed high positive percentages of 33 (the
mean value; 10.5U/ml), 44 (4.3U/ml)
and
40 (7.1U/ml),
respectively. Although four
out of 19 patients (21%) with chronic and
acute hepatitis showed an elevation in antigen
level, the mean value was not high (2.1U/
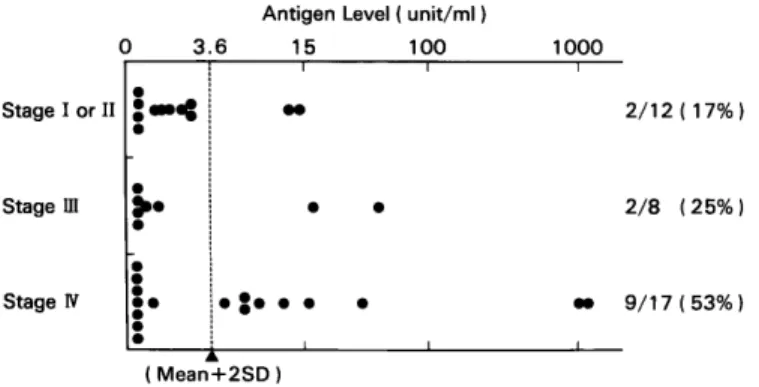
ml). The relation between antigen level and
clinical stage was evaluated for patients with
stomach cancer. Values higher than 15 units/
ml were not detected in stage I or stage II
patients, but were detected in 2 stage III cases
and 4 stage IV cases (Fig. 1), suggesting an
association between antigen level and tumor
burden.
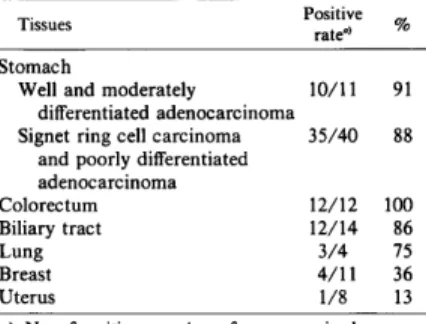
Reaction of mAb NCC-AS 13 with Various
Tumor Tissues Immunohistochernical stain
ing was used to examine the reactivities of
mAb NCC-AS 13 with malignant human
tissues (Table II). MAb NCC-AS 13 reacted
with approximately 90% of all the gastric
carcinomas examined without showing any
correlation to histological type. Representa
tive pictures are shown in Fig. 2. For other
malignant tissues, 12 out of 12 colon carcino
mas (100%), 12 out of 14 biliary tract carci
nomas (86%) and 3 out of 4 lung carcinomas
(75%) showed positive staining.
Reaction of mAb NCC-AS 13 with Non
cancerous Tissues The reactivities of mAb
NCC-AS 13 with various non-cancerous
human tissues are summarized in Table III.
The mAb NCC-AS 13 reacted with tissue
from the colon epithelium, biliary tract, pan
creatic duct, salivary gland, bronchial gland
Table
I.
Detection
Rate
of NCC-AS
13-defined
Antigen
in Sera
ANTIBODY-DEFINED ASIALOGLYCOPROTEIN
Fig. 1. NCC-AS 13 antigen levels and the clinical stages in stomach cancer patients.
Fig. 2. Sections of adenocarcinoma of the stomach stained by monoclonal antibody NCC-AS 13. The cytoplasmic regions of the well-differentiated adenocarcinoma penetrating the muscle layer (a) and signet-ring cell carcinoma (b) were stained. As representatives of antigen-negative and antigen-positive tissues, unstained stomach (c), and colon (d), respectively, were also examined. These sections were counterstained with hematoxylin. (a, b, c, d: •~50).
and skin. Some patients with intestinal meta
plasia of the stomach showed antigen expres
sion. It is noteworthy
that the antibody
reacted with non-cancerous cells of tissue sec
tions of either blood type A, B, O, Lea(+)
Leb(+)
or Lea(-)
Leb(-),
but did not
react with red blood cells or white blood cells
in the sections.
Hemagglutination
Test
The mAb
NCC-AS
13 did not react with red blood cells of A, B,
AB
or O.
This
was
confirmed
by
the
Table
II.
Immunohistochemical
Detection
of
NCC-AS 13-defined Antigen in Malignant Tissues
Table III. Immunohistochemical Detection of NCC-AS 13-defined Antigen in Normal Tissues
Fig. 3. Western blotting analysis (5% polyacryl
amide gel) of the NCC-AS 13 antigen prepared
form the soluble fraction of the ascites in 1MPCA.
The monoclonal antibody NCC-AS 13 was used for
the left lane and the myeloma protein (IgM) was
used for the right lane.
Table IV. Binding Ratio of mAb NCC-AS 13 to
the Ascites Antigen after Various Treatments
Assessed by EIA
ANTIBODY-DEFINED
ASIALOGLYCOPROTEIN
hemagglutination test with second antibodies
(i.e. goat anti-mouse IgM antiserum).
Biochemical Profile of NCC-AS 13 Antigen
The molecular profile of NCC-AS 13 antigen
in the immunogen was examined by Western
blotting analysis. MAb NCC-AS 13 reacted
with a molecule having an extremely high
molecular weight under reducing conditions
(Fig. 3). The biochemical nature of the anti
genic determinant was examined by enzyme,
chemical agent and heat treatments of the
MMF of the PCA extract of the ascites
(Table IV). The reactivity of the antigen
remained unchanged upon heat treatment,
and the antigen was soluble in 1 M PCA. The
antigen was resistant to neuraminidase but
sensitive to 0.1N NaOH and periodate oxida
tion. These findings indicated the epitope to
be an O-linked sugar chain without terminal
sialic acid.
DISCUSSION
This is the first report of mAb to ascites
MMF being established for the detection of
soluble antigen in the sera. Many antigens
detected by various mAbs in sera are known
to exist on high-molecular-weight glycopro
teins.In the present study, culture superna
tants of the hybridomas were selected if they
reacted with the ascites MMF from a gastric
cancer patient which had been prepared by gel
filtration of the ascites on Sepharose CL-6B
and used as an immunogen.
W
e confirmed the NCC-AS 13 antigen to be
different from the human ABO and the Lewis
blood group antigens, by means of immuno
histochemical studies and hemagglutination
tests. The antigen levels of sera from 146 nor
mal subjects were measured and the mean
was found to be 0.89 units/ml. Tentatively,
the cut-off value was set at 3.6 units/ml (0.89
+2 SD), and sera from patients with various
diseases were examined. It was apparent that
the antigen levels in cancer patients were
higher than those in normal subjects, and the
positive ratios for digestive-organ cancer pa
tients were 30-40% vs. 10% for normal sub
jects. Although the positive ratio was not so
high, it is noteworthy that mAb NCC-AS 13
could detect scirrhous gastric cancer patients
with the mean value of 4.3U/ml (2 out of 6
patients were positive). Furthermore, higher
antigen
levels
were
observed
in the
sera
from
gastric
cancer
patients
in stage
III
or
IV than
in
those
from
patients
with
early-stage
dis
ease.
These
data
suggest
that
NCC-AS
13-defined
antigen
levels
may
reflect
the
size
of
the
gastric
cancer.
Western
blotting
analysis
showed
the
mo
lecular
weight
of
the
NCC-AS
13
antigen
in
the
ascites
to
be
>200,000
daltons
under
reducing
conditions,
and
the
antigen
was
detected
in
void
volume
fractions
from
gel
filtration
on
a Sepharos
CL-6B
column.
These
data
suggest
the
epitope
of
NCC-AS
13 anti
gen
to exist
on
a protein(s)
with
an
extremely
high
molecular
weight.
In
addition,
the
bio
chemical
nature
of
the
epitope
was
examined
by
enzyme
and
heat
treatments.
It was
found
that
the
epitope
was
resistant
to
neuramini
dase
and
heat
treatments,
indicating
it to be
a
carbohydrate
without
terminal
sialic
acid.
The
antigen
was
detected
in the
spent
medium
of
cultured
SW
1116
cells
(human
colon
cancer
cell
line)
and
on
cell
membranes
(data
not
shown).
The
cells
were
cultured
with
or
without
0.5ƒÊg
tunicamycin/ml
for
48hr.
The
NCC-AS
13
antigen
levels
of
the
NP-40
cell
extract
were
not
inhibited
by
the
treatment,
suggesting
that
the
epitope
may
be
an
O-linked
oligosaccharide
of
the
cell-surface
glycoproteins
(unpublished
observation).
There
are
many
mAbs
which
react
with
carbohydrate
chains,
and
some
of
them
have
been
shown
to be
useful
for
detecting
cancer
-associated
antigens
in sera.
NS
19-9,2)
C 50,12)
DU-PAN-2,3)
OC
125,13)
115D8,14)
or
DF
3,15)
CSLEX1,16)
YH
2064)
and
FH-617)
have
been
reported.
All
of
these
epitopes,
however,
are
known
to
have
terminal
sialic
acid
in
the
carbohydrate
chain,
except
for
YH
206.
The
NCC-AS
13 antigen
epitope
has
not
yet
been
determined,
but
it
was
found,
based
on
its
resistance
to neuraminidase
treatment,
to pos
sess
no
terminal
sialic
acid.
Furthermore,
im
munohistochemical
analysis
of the
antigen
in
dicated
that
colon
cancer
cells
had
a large
amount
of the
antigen,
although
YH
206
anti
gen
was
not
detected
in
colon
cancer
cells.
These
data
suggest
that
NCC-AS
13 antigen
is
different
from
each
of
the
nine
antigens
listed
above.
The
reactivity
of
mAb
NCC-AS
13
with
various
cancer
tissues
was
examined
by
using
immunohistochemical
analysis.
It
was
found
that NCC-AS 13 reacted with approximately
90% of gastric carcinomas and also reacted
with other digestive-organ cancer tissues. The
antibody reacted with non-malignant tissues
to some extent, especially colon epithelium,
bile ducts, pancreatic ducts, bronchial glands,
salivary glands and basal skin cells. All these,
however, are kept away from the blood
stream by the basement membrane. A high
level of NCC-AS 13 antigen in sera would
seem to indicate, therefore, the destruction of
the basement membrane or the loss of its
normal construction.
It is known that the concentrations of
asialoglycoproteins are very low in sera, be
cause they are trapped by asialoglycoprotein
receptors on the membranes of hepatocytes
and disappear promptly from the plasma.
Lenten and Ashwell18) reported desialylated
ceruloplasmin to be cleared from the circula
tion within 10 to 15min after injection, in
contrast to the 25-hr half-life of fully sialyl
ated ceruloplasmin. This mechanism provides
one possible explanation for the low positive
ratios of NCC-AS 13 antigen in cancer
patients' sera compared to the high positive
ratios in cancer tissues. All four established
mAbs to the ascites in our laboratory were
found to recognize sugar residues without ter
minal sialic acid (to be published elsewhere),
suggesting that a reasonable amount of asialo
glycoproteins is contained in the ascites. It is,
therefore, unlikely that asialoglycoprotein an
tigens are not secrected from cancer cells.
We speculate that the appearance of asialo
glycoprotein antigens is the result of incom
plete synthesis of the carbohydrate chain, and
these products disappear promptly from the
plasma. This would have important biological
implications.
In conclusion, NCC-AS 13 antigen could be
a useful marker for the detection of the serum
antigen and for immunohistochemical analy
sis of gastric cancer. In addition, it has poten
tial uses for tumor immunoscintigraphy for
sero-negative cancer patients, since most gas
tric carcinomas are expected to possess
NCC-AS 13 antigen and the localization of
the antibody is not inhibited by free antigen
in plasma. Further studies on immunoscinti
graphy and therapy are under way in our
laboratory.
ACKNOWLEDGMENTS
This study was supported in part by a Grant-in-Aid from the Ministry of Health and Welfare for the Comprehensive 10-Year Strategy for Cancer Control, Japan. Masaaki Adachi is the recipient of a Research Resident Fellowship from the Founda tion for Promotion of Cancer Research, Japan.
(Received Aug. 7, 1987/Accepted Oct. 15, 1987)
REFERENCES
1) Imai, K., Moriya, Y., Fugita, H., Tsujisaki, M., Kawaharada, M. and Yachi, A. Immu nological characterization and molecular profile of carcinoembryonic antigen detected by monoclonal antibodies. J. ImmunoL, 132, 2292-2297 (1984).
2) Koprowski, H., Herlyn, M., Steplewski, Z. and Sears, H. F. Specific antigen in serum of patients with colon carcinoma. Science, 212, 53-55 (1981).
3) Metzgar, R. A., Rodriguez, N., Finn, O. J., Lan, M. S., Daasch, V. N., Femsten, P. D., Meyers, W. C., Sinler, M. S., Sandler, R. S. and Seigler, H. F. Detection of a pancreatic cancer associated antigen (DU-PAN-2 anti gen) in serum and ascites of patients with adenocarcinoma. Proc. Natl. Acad. Sci. USA, 81, 5242-5246 (1984).
4) Hinoda, Y., Imai, K., Endo, T., Yamashita, T. and Yachi, A. Detection of circulating adenocarcinoma-associated antigen in the sera of cancer patients with a monoclonal antibody. Jpn. J. Cancer Res. (Gann), 76, 1203-1211 (1985).
5) Masuko, T., Yagita, H. and Hashimoto, Y. Monoclonal antibodies against cell surface antigens present on human urinary bladder cancer cells. J. Natl. Cancer Inst., 72, 523-530 (1984).
6) Heyningen, V. V., Barron, L., Brock, D. J. H., Crichton, D. and Lawrie, S. Monoclonal antibodies to human ƒ¿-fetoprotein: analysis of the behaviour of three different antibodies. J. Immunol. Methods, 50, 123-128 (1982). 7) Sekine, T., Hirohashi, S., Kitaoka, H.,
Hirota, T. and Sugimura, T. A monoclonal antibody reactive with gastric carcinoma. Gann, 75, 106-108 (1984).
8) Shimosato, Y., Kameya, T., Nagai, K., Hirohashi, S., Koide, T., Hayashi, H. and Nomura, T. Transplantation of human tumors in nude mice. J. Natl. Cancer Inst., 56, 1251-1260 (1976).
9) Bayer, E., Skutelsky, E. and Wilchek, M. The avidin-biotin complex in affinity cytochemistry.