Jpn. J. Oral Biol., 45: 437-444, 2003.
ORIGINAL
Alteration
of the Expression
of A 2 a Adenosine
Receptor
and
Toll-like
Receptor
4 in Macrophage
Cell Lines
Kyoko Watanabe*,
Yasutaka
Azuma**, Shinya Shirasu*,
Michiharu
Daito* and Kiyoshi Ohura**
*Department of Pediatric Dentistry
, Osaka Dental University
**Department of Pharmacology
, Osaka Dental University
*,**8-1 Kuzuhahanazono -cho
, Hirakata, Osaka 573-1121, Japan
〔Received on June 19,2003; Accepted on September 22,2003〕
Key words: A 2 a adenosine receptor/toll-like receptor 4/adenosine/ATP/LPS
Abstract: Macrophages are essential for controlling the majority of infections, and are mediators of natural immunity. During an infection, lipopolysaccharide (LPS) stimulates macrophages to produce pro-inflammatory cytokines. Recently, it has been shown that A 2 a adenosine receptor (A 2 aR) is a critical part of the physiological negative feedback mechanism for the limitation and termination of tissue-specific and systemic inflammatory responses. It was useful and meaningful to gain information about interaction between LPS, which generates the inflammation, and adenosine receptors, which terminate the inflamma-tion. However, very little, if anything, is known about the effect of bacterial LPS on the expression of A 2 aR during an infection of bacteria. The aim of this study is to evaluate the effects of adenosine, ATP and LPS on the expression of A 2 aR and toll-like receptor 4 (TLR 4), which is a receptor for LPS, in the mouse macrophage cell line RAW 264. Adenosine and ATP failed to affect proliferation in RAW 264 cells, whereas LPS increased proliferation. Adenosine significantly potentiated the expression of TLR 4, but not of A 2 aR. ATP and LPS markedly potentiated the expression of A 2 aR and TLR 4, respectively. Moreover, adenosine and ATP did not affect the expression of A 2 aR and TLR 4 in the presence of LPS, respectively. This study revealed that adenosine, ATP and LPS affect the expression of A 2 aR and TLR 4 in macro-phages.
抄 録:マ ク ロ フ ァー ジ は ほ と ん ど の 感 染 制 御 に 不 可 欠 で あ り,自 然 免 疫 機 構 を 担 っ て い る 。 感 染 時 に は,
lipopolysaccharide (LPS)が マ ク ロ フ ァー ジ を 刺 激 し,炎 症 性 サ イ トカ イ ン を産 生 す る 。 近 年,A 2 a adenosine
receptor(A 2 aR)が,組 織 特 異 的 お よ び 全 身 性 炎 症 応 答 を抑 制 し終 結 させ る 負 の生 理 的 フ ィ ー ドバ ッ ク機 構 に
重 要 な 役 割 を 果 た し て い る こ とが 示 され た 。炎 症 を惹 起 す るLPSと,炎 症 を終 結 させ るadenosine受 容 体 との 相
互 作 用 に つ い て の情 報 を得 る こ と は有 用 か つ 有 意 義 で あ る。 しか し な が ら,細 菌 感 染 時 に お け るA 2 aR発 現 に対
す る細 菌 由 来LPSの 影 響 に つ い て は ほ と ん ど解 明 さ れ て い な い。本 研 究 の 目 的 は,マ ウ ス マ ク ロ フ ァー ジ様 細 胞
株 で あ るRAW 264を 用 い て,A 2 aRお よ びLPSの 受 容 体 で あ るToll-like receptor 4 (TLR 4)の 発 現 に対 す
る,adenosine, ATPお よ びLPSの 影 響 を 検 討 す る こ とで あ る。 Adenosineお よ びATPはRAW 264の 増 殖 能
に は 影 響 を与 え な か っ た が,LPSは 増 殖 能 を 増 強 させ た 。 AdenosineはTLR 4発 現 を 有 意 に増 強 さ せ た が,
A 2 aR発 現 に は 影 響 を 与 え な か っ た 。 ATPお よ びLPSは,そ れ ぞ れA 2 aRお よ びTLR 4の 発 現 を有 意 に増 強
さ せ た 。 さ ら に,LPS存 在 下 に お い て, adenosineお よ びATPはA 2 aRあ る い はTLR 4発 現 の い ず れ に も影
響 を与 え な か っ た 。 以 上 の 結 果 よ り, adenosine, ATPお よ びLPSは,マ ク ロ フ ァー ジ に お け るA 2 aRお よ び
438 JPn. J. Oral Biol., 45: 437-444,2003.
Introduction
Macrophages are commonly the first cells of the immune system to encounter invaders such as bacteria and fungi, and are ready to leave circulation and attack intruders at any place and at any time. During an infection, lipopolysaccharide (LPS) , a predominant glycolipid in the outer membrane of Gram-negative bacteria, stimulates macrophages to produce pro-inflammatory cytokines. LPS induces cellular responses by its complexing with circulating LPS-binding protein and the subsequent LPS-binding to CD 14 that, in turn, facilitates the interaction of LPS with signaling molecules belonging to toll-like receptor 4
(TLR 4)1,2).
Adenosine is a purine nucleoside that is released from a variety of cells in response to metabolic stress or from the sympathetic nervous system, and occupies various adenosine receptor subtypes on target cells. Adenosine is known to bind four different types of G-protein-coupled cell surface receptors: A 1, A 2 a, A 2 b and A 3 adenosine receptors3). In addition, the accumulation of extracellular adenosine in inflamed and damaged tissues4-8) and the immunosuppressive properties of cAMP-elevating adenosine receptors indicate that signaling by A 2 a adenosine receptor (A 2 aR) on immune cells is a possible natural mecha-nism of inhibition and/or termination inflamma-tion9-15). Recently, it has been shown that A 2 aR is a critical part of the physiological negative feedback mechanism for the limitation and termination of tis-sue-specific and systemic inflammatory responses16). It was useful and meaningful to gain information about the interaction between LPS and adenosine receptors; the former generates the inflammation and the latter terminates the inflammation. However, very little, if anything, is known about the effect of bacterial LPS on the expression of A 2 aR during an infection of bacteria. To gain information, we sought to evaluate the effect of LPS on the expression of A 2 aR in macrophages. In addition, we also inves-tigated the effect of adenosine on the expression of TLR 4 in macrophages. It has been shown that extracellular ATP is not stable, and that ATP is
readily degraded by ectonucleotidases to adenosine. Here, we also examined the effect of ATP on the expression of A 2 aR and TLR 4 in macrophages.
Materials and Methods
1. Materials
Adenosine, ATP and LPS were provided by Sigma (St. Louis, MO, USA). Goat polyclonal antibodies against A 2 aR and TLR 4 were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA). A fluo-rescence-conjugated anti-goat antibody was obtained from Wako Purity Chemical Industries (Osaka, Japan).
2. Cell culture
Mouse macrophage cell line RAW 264 cells (Riken Gene Bank, Tokyo, Japan) were maintained in DMEM supplemented with 10% FBS, 100units/ml of penicil-lin and 100ƒÊg/ml of streptomycin.
3. Proliferation assay
Cell proliferation was evaluated by CellTiter 96(R)
AQueous One Solution Cell Proliferation Assay (Promega Corporation; Madison, WI, USA)17). Brief-ly, cells (6•~103) were incubated with adenosine, ATP or LPS in 96-well culture plates for 24 or 72h. After incubation,
1.9mg/ml[3-(4,5-dimethylthiazol-2-yl)-
5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2 H-tetrazolium, inner salt] (MTS) and 300μM
phen-azine ethosulfate (MTS solution) were added to the culture plates. The absorbance at a wavelength of 490 nm was measured at 2h after the addition of MTS
solution, and reference absorbance was 690 nm.
4. Assessment of receptor expression Cells (3•~105) were incubated with adenosine
, ATP or LPS for 24 or 72h. After being washed with PBS, cells were incubated with goat polyclonal antibody against A 2 aR or TLR 4 for 30min at 4℃. Cells were then washed with PBS, followed by incubation with a fluorescence-conjugated anti-goat antibody for 30 min at 4℃ in the dark. Then, cells were again washed with PBS, and stained cells were analyzed on a flow cytometer (Becton Dickinson, Mountain View, CA,
K. Watanabe, et al.: Alteration of A 2 aR and TLR 4 in Macrophages 439
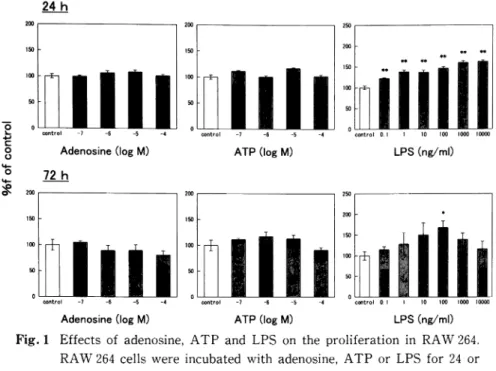
Fig. 1 Effects of adenosine, ATP and LPS on the proliferation in RAW 264.
RAW 264 cells were incubated with adenosine, ATP or LPS for 24 or
72h. Cell proliferation was determined by colorimetric MTS assay. The
data from four to six different specimens are shown. *p<0.05, **<0.01,
when compared with untreated cells.
USA)18). Data were expressed as mean fluorescent intensity for each sample as calculated by the Cell QuestR software (Becton Dickinson).
5 Statistical analysis
Results were expressed as the mean•}SE. Statisti-cal analysis was determined by one-way ANOVA for non-repeated to detect differences between multiple groups. Differences between groups were determined by Dunnett's test or the Student-Newman-Keuls
(SNK) test. Differences were considered to be signifi-cant when the p value was<0.05.
Results
1. Effects of adenosine, ATP and LPS on eration
We first assessed the effects of adenosine, ATP and LPS on proliferation in RAW 264 cells. Addition of increasing concentrations of LPS significantly in-creased the proliferation at 24h after incubation (Fig.
1, upper right panel). At 72h after incubation, in contrast, LPS at only 100ng/ml markedly increased
the proliferation (Fig. 1, lower right panel). However, adenosine and ATP at concentrations of up to 10-4 M failed to affect the proliferation, irrespective of incu-bation time (Fig. 1, left and middle panels). Based on
the result of Fig. 1 and the physiological concentration of LPS, we used LPS at concentrations of up to 100 ng/ml in the following experiments.
2. Potentiation of the expression of A 2 aR and TLR 4 by adenosine, ATP and LPS
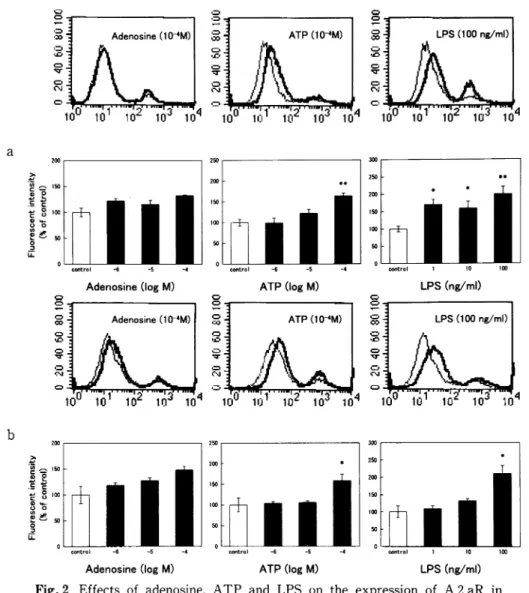
Next, we wanted to examine whether adenosine, ATP and LPS affect the expression of A 2 aR and TLR 4 in RAW 264 cells. In the subsequent experi-ments, we detected the expression of these receptors by using flow cytometry, but not immunoblotting. At 24 h after incubation, adenosine had failed to affect the expression of A 2 aR at concentrations of up to 10-4 M (Fig. 2a). ATP significantly increased the expression of A 2 aR at 10-4 M. LPS increased the expression of A 2 aR at a concentration range of l to 100ng/ml. At 72h after incubation, adenosine had not affected the expression of A 2 aR (Fig. 2b). ATP and LPS markedly potentiated the expression of A 2 aR at
440 Jim. J. Oral Biol., 45: 437-444, 2003.
Fig. 2 Effects of adenosine, ATP and LPS on the expression of A 2 aR in RAW 264. RAW 264 cells were incubated with adenosine, ATP or LPS for 24h (a) or 72h (b), followed by staining with anti-A 2 aR antibody. Representative histograms of RAW 264 treated with each drug at the highest concentration examined, as compared with untreated RAW 264, are shown in the upper panels. The thin line shows untreated cells, and the thick line shows cells treated with each drug. Fluorescent intensities are provided in the lower panels where values are the mean•}SE from 3 to 8 for (a) or 4 for (b) different specimens. *p<0.05, **<0.01, when compared with untreated cells.
the highest concentration examined.
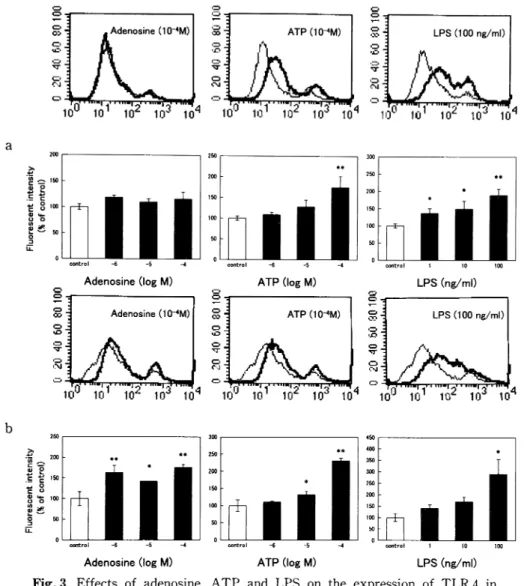
Regarding the expression of TLR 4, adenosine had not had an effect on expression at 24h after incuba-tion, whereas adenosine at a concentration range of 10-6 to 10-4 M had increased the expression at 72h after incubation (Fig. 3). ATP had significantly in-creased the expression at 10-4 M after incubation of
24h, and at 10-5 and 10-4 M after incubation of 72h. LPS had potentiated the expression of TLR 4 at a concentration range of 1 to 100ng/ml after incubation of 24h, and at 100ng/ml after incubation of 72h.
The above analyses suggested the importance of adenosine and ATP in addition to LPS during inflam-matory responses. To determine the effect of a
combi-K. Watanabe, et al.: Alteration of A 2 aR and TLR 4 in Macrophages 441
Fig. 3 Effects of adenosine, ATP and LPS on the expression of TLR 4 in RAW 264. RAW 264 cells were incubated with adenosine, ATP or LPS for 24h (a) or 72h (b), followed by staining with anti-TLR 4 antibody. Representative histograms of RAW 264 treated with each drug at the highest concentration examined, as compared with untreated RAW 264, are shown in the upper panels. Fluorescent intensities are provided in the lower panels where values are the mean±SE from 4 to 10 for (a)or 3 for (b) different specimens. *p<0.05, **<0.01, when compared with untreated cells.
nation of adenosine and LPS, or ATP and LPS on the expression of these receptors, RAW 264 cells were treated with adenosine or ATP in the presence of LPS at 100ng/ml for 72h. As shown in Fig. 4, left panel, neither adenosine at 10-4 M nor ATP at 10-4 M significantly affected the LPS-potentiated expression of A 2 aR. Similarly, neither adenosine at 10-4 M nor ATP at 10-4 M significantly affected the
LPS-Potentiated expression of TLR 4 (Fig. 4, right panel). Discussion
The importance of the results described in this paper is that adenosine, ATP and LPS evidently potentiated the expression of A 2 aR and TLR 4 in RAW 264 cells. This paper also revealed that A 2 aR
442 Jpn. J. Oral Biol., 45: 437-444, 2003.
Fig. 4 Effects of adenosine (Ade) and ATP in the presence of LPS on the expression of A 2 aR and TLR 4 in RAW 264. RAW 264 cells were incubated with adenosine or ATP in the presence of LPS for 72h, followed by staining with anti-A 2 aR or anti-TLR 4 antibodies. The data from four different speci-mens are shown. *p<0.05, **<0.01, when compared with untreated cells.
and TLR 4 are expressed on macrophages. Notably, adenosine did not affect the expression of A 2 aR among its own receptors, indicating that the effect of adenosine differs from the effect of LPS. Namely, LPS potentiated the expression of its own receptor, TLR 4. However, the effect of LPS on the expression of TLR 4 has been the object of seemingly conflicting results. LPS up-regulates TLR 4 in human monocytes and neutrophils19-21), but down-regulates TLR 4 in mouse peritoneal macrophages22,23). In the present study focused on mouse macrophage cell line RAW 264 cells, we observed that LPS up-regulates the expression of TLR 4. The question of whether treatment with LPS participates in the up-regulation or down-regulation of the expression of TLR 4 remains to be addressed before drawing any conclu-sions.
An interesting finding of this paper is that adenosine potentiated the expression of TLR 4, and LPS potentiated the expression of A 2 aR. In a previ-ous study, LPS increased A 2 aR mRNA in monocytes24). Importantly, ATP potentiated the expression of both A 2 aR and TLR 4. As described in the Introduction, more recent study using animals deficient in A 2 aR indicated that A 2 aR has an impor-tant role in the attenuation of inflammation and tissue damage in vivo16). Indeed, A 2 aR is a critical part of the physiological negative feedback mechanism for
the limitation and termination of both tissue-specific and systemic inflammatory responses. The uniqueness of the adenosine receptors may lie in the physiology of the accumulation of abundant and ubiquitous purine nucleosides25,26) in the local inflammatory environ-ment4-8).
Inappropriate or prolonged inflammation is the main cause of many diseases. It is important to under-stand the physiological mechanisms that terminate inflammation. Naturally, potentiation of the expres-sion of TLR 4 resulted in deterioration of inflamma-tion. In contrast, potentiation of the expression of A 2 aR resulted in termination of inflammation. An issue that is raised by the current work is whether the roles of the potentiated-expression of A 2 aR and TLR 4 are related to their roles in inflammatory responses. Because many types of cells are related to inflammatory responses, the alteration of macrophage responses is not necessarily related to regulation of inflammation. An alternative hypothesis is that our results could reflect a new interaction between A 2 aR and TLR 4 in inflammatory responses.
On the other hand, adenosine and ATP did not affect the proliferation of RAW 264 cells. In contrast, LPS had increased the proliferation at all concentra-tions examined at 24h, whereas, it increased the proliferation at only 100ng/ml at 72h. The increase of cells by treatment with LPS resulted in the increase
K. Watanabe, et al.: Alteration of A 2 aR and TLR 4 in Macrophages 443
of the number of all receptors. Under this condition of 72h incubation, we have demonstrated that adenosine and ATP did not significantly affect the expression of A 2 aR and TLR 4 in the presence of LPS, respective-ly. It is likely that the expression of A 2 aR and TLR 4 is also potentiated in the presence of adenosine and LPS, as well as adenosine alone and LPS alone .
This study revealed that adenosine, ATP and LPS may potentiate the expression of A 2 aR and TLR 4 in RAW 264 cells, and also indicates that the release of adenosine and ATP during inflammatory responses leads to potentiation of the expression of A 2 aR and
TLR 4.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (15591987) and a Grant-in-Aid for Encouragement of Young Scientists (B)
(15791232)from the Japan Society for the Promotion of Science of Japan.
References
1) Wright, S.D., Ramos, R.A., Tobias, P.S., Ulevitch, R.J. and Mathison, J.C.: CD 14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249: 1431-1433, 1990. 2) Wright, S.D., Ramos, R.A.,
Hermanowski-Vosat-ka, A., Rockwell, P. and Detmers, P.A.: Activation of the adhesive capacity of CR 3 on neutrophils by endotoxin: dependence on lipopolysaccharide
bind-ing Protein and CD 14. J. Exp. Med. 173: 1281-1286, 1991.
3) Ralevic, V. and Burnstock, G.: Receptors for pur-ines and pyrimidpur-ines. Pharmacol. Rev. 50: 413-492, 1998.
4) Winn, H.R., Rubio, R. and Berne, R.M.: Brain adenosine concentration during hypoxia in rat. Am. J. Physiol. 241: H 235-H 242, 1981.
5) Van Belle, H., Goossens, F. and Wynants, J.: For-mation and release of purine catabolites during hypoperfusion, anoxia, and ischemia. Am. J.
Physiol. 252: H 886-H 893, 1987.
6) Rudolphi, K.A., Schubert, P., Parkinson, F.E. and Fredholm, B.B.: Neuroprotective role of adenosine in cerebral ischaemia. Trends Pharmacol. Sci. 13:
439-445, 1992.
7) Marquardt, D.L., Gruber, H.E. and Wasserman, S.I.: Adenosine release from stimulated mast cells. Proc. Natl. Acad. Sci. USA 81: 6192-6196, 1984. 8) Filippini, A., Taffs, R.E. and Sitkovsky, M.V.:
Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc. Natl. Acad. Sci. USA 87: 8267-8271, 1990.
9) Cronstein, B.N.: Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 76: 5-13, 1994.
10) Cronstein, B.N.: A novel approach to the develop-ment of anti-inflammatory agents: adenosine release at inflamed sites. J. Invest. Med. 43: 50-57,
1995.
11) Firestein, G.S., Boyle, D., Bullough, D.A., Gruber, H.E., Sajjadi, F.G., Montag, A., Sambol, B. and Mullane, K.M.: Protective effect of an adenosine kinase inhibitor in septic shock. J. Immunol. 152: 5853-5859, 1994.
12) Huang, S., Apasov, S., Koshiba, M. and Sitkovsky, M.: Role of A 2 a extracellular adenosine receptor-mediated signaling in inhibition of T-cell activation and expansion. Blood 90: 1600-1610, 1997. 13) Eigler, A., Greten, T.F., Sinha, B., Haslberger, C.,
Sullivan, G.W. and Endres, S.: Endogenous adenosine curtails lipopolysaccharide-stimulated tumor necrosis factor synthesis. Scand. J. Immunol. 45: 132-139, 1997.
14) Sullivan, G.W., Sarembock, I.J. and Linden, J.: The role of inflammation in vascular diseases. J. Leukoc. Biol. 67: 591-602, 2000.
15) Hoskin, D.W., Reynolds, T. and Blay, J.: Adenosine as a possible inhibitor of killer T-cell activation in the microenvironment of solid tumors. Int. J. Cancer 59: 854-855, 1994.
16) Ohta, A. and Sitkovsky, M.: Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414: 916-920, 2001.
17) Azuma, Y. and Ohura, K.: Alteration of con-stitutive apoptosis in neutrophils by quinolones. Inflammation 27: 123-130, 2003.
18) Azuma, Y. and Ohura, K.: Endomorphin-2 lates productions of TNF-α, IL-1β, IL-10 and 12, and alters functions related to innate immune of macrophages. Inflammation 26: 223-232, 2002. 19) Muzio, M., Natoli, G., Saccani, S., Levrero, M and Mantovani, A.: The human toll signaling
path-444 Jpn. J. Oral Biol., 45: 437-444, 2003.
way: divergence of nuclear factor (B and JNK/ SAPK activation upstream of tumor necrosis factor
receptor-associated factor 6 (TRAF 6). J. Exp. Med.
187: 2097-2101, 1998.
20) Muzio, M., Bosisio, D., Polentarutti, N., D'amico, G., Stoppacciaro, A., Mancinelli, R., Veer, C.V.,
Penton-Rol, G., Ruco, L.P., Allavena, P. and
Mantovani, A.: Differential expression and
tion of toll-like receptors (TLR) in human
cytes: selective expression of TLR 3 in dendritic
cells. J. Immunol. 164: 5998-6004, 2000.
21) Visintin, A., Mazzoni, A., Spitzer, J.H., Wyllie, D. H., Dower, S.K. and Segal, D.M.: Regulation of
toll-like receptors in human monocytes and dritic cells. J. Immunol. 166: 249-255, 2001. 22) Nomura, F., Akashi, S., Sakao, Y., Sato, S., Kawai,
T., Matsumoto, M., Nakanishi, K., Kimoto, M.,
Miyake, K., Takeda, K. and Akira, S.: Endotoxin
tolerance in mouse peritoneal macrophages
lates with down-regulation of surface toll-like
ptor 4 expression. J. Immunol. 164: 3476-3479,
2000.
23) Medvedev, A.E., Kopydlowski, K.M. and Vogel, S. N.: Inhibition of lipopolysaccharide-induced signal
transduction in endotoxin-tolerized mouse
phages: dysregulation of cytokine, chemokine, and
toll-like receptor 2 and 4 gene expression. J.
munol. 164: 5564-5574, 2000.
24) Bshesh, K., Zhao, B., Spight, D., Biaggioni, I., kistov, I., Denenberg, A., Wong, H. R. and Shanley,
T.P.: The A 2 A receptor mediates an endogenous
regulatory pathway of cytokine expression in 1 cells. J. Leukoc. Biol. 72: 1027-1036, 2002. 25) Fredholm, B.B., Arslan, G., Halldner, L., Kull, B.,
Schulte, G. and Wasserman, W.: Structure and
function of adenosine receptors and their genes.
Naunyn Schmiedebergs Arch. Pharmacol. 362:
364-374, 2000.
26) Burnstock, G.: Purine-mediated signalling in pain and visceral perception. Trends Pharmacol. Sci. 22: 182-188, 2001.