Japan Advanced Institute of Science and Technology
Title
Theoretical Assessment of FePt Nanoparticles as
Heating Elements for Magnetic Hyperthermia
Author(s)
Maenosono, Shinya; Saita, Soichiro
Citation
IEEE Transactions on Magnetics, 42(6): 1638-1642
Issue Date
2006-06
Type
Journal Article
Text version
publisher
URL
http://hdl.handle.net/10119/4654
Rights
Copyright (c)2006 IEEE. Reprinted from IEEE
Transactions on Magnetics , 42(6), 2006,
1638-1642. This material is posted here with
permission of the IEEE. Such permission of the
IEEE does not in any way imply IEEE endorsement
of any of JAIST's products or services. Internal
or personal use of this material is permitted.
However, permission to reprint/republish this
material for advertising or promotional purposes
or for creating new collective works for resale
or redistribution must be obtained from the IEEE
by writing to pubs-permissions@ieee.org. By
choosing to view this document, you agree to all
provisions of the copyright laws protecting it.
Description
Theoretical Assessment of FePt Nanoparticles as
Heating Elements for Magnetic Hyperthermia
Shinya Maenosono
1and Soichiro Saita
2Department of Chemical System Engineering, University of Tokyo, Tokyo 113-8656, Japan Mitsubishi Chemical Group Science and Technology Research Center Inc., Kanagawa 227-8502, Japan
FePt magnetic nanoparticles (MNPs) are expected to be a high-performance nanoheater for magnetic hyperthermia because of their high Curie temperature, high saturation magnetization, and high chemical stability. Here, we present a theoretical performance assess-ment of chemically disordered fcc-phase FePt MNPs. We calculate heat generation and heat transfer in the tissue when an MNP-loaded tumor is placed on an external alternating magnetic field. For comparison, we estimate the performances of magnetite, maghemite, FeCo, andL10-phase FePt MNPs. We find that an fcc FePt MNP has a superior ability in magnetic hyperthermia.
Index Terms—Bioheat transfer equation, cancer treatment, hyperthermia, iron-platinum nanoparticle.
I. INTRODUCTION
H
YPERTHERMIA is one of many techniques used in on-cology, based on heating tumors for therapeutic purposes, and usually used as an additive therapy with standard treatments, such as radiotherapy and chemotherapy. The principle of cancer treatment is to eliminate only cancer cells distinguishing from normal cells. They are distinguished by visual inspection in sur-gical procedure. However, cellular discrimination is difficult. Although various efforts have been made to attain tumor-selec-tive radiotherapy and chemotherapy, there remains considerable difficulty in these techniques. Hyperthermia is superior to other therapeutic techniques on this point. The blood flow is insuf-ficient in tumors and the inadequate blood flow makes tumors more acidic due to the lactic acid buildup in the tumor tissues from lack of oxygen. In general, cells are easy to die when the environment becomes acidic, because temperature-sensitivity of cell increases. Additionally, the temperature will rise easily when the blood flow is insufficient. Moreover, cancer cells have a lower thermal resistance than normal cells. In consequence, one can eliminate cancer cells selectively by rising the local temperature at the site of tumor.The magneto-thermo-cytolysis (or the magneto-thermoabla-tion) is a promising technique thanks to the development of pre-cise methods for synthesizing functionalized magnetic nanopar-ticles (MNPs) [1]. MNPs with functionalized surfaces, which have high specificity to a tumor tissue, are used as heating ele-ments for hyperthermia. The process involved in the magnetic hyperthermia is based on the energy dissipation when a ferro-magnetic material is placed on an external alternating ferro-magnetic field. The energy dissipation of MNPs consists of the following two effects: the Néel relaxation and the Brownian relaxation. For efficient magnetic hyperthermia, the MNPs are required to have low toxicity and a high saturation magnetization in order to minimize the doses needed for temperature increase.
In this context, iron-platinum (FePt) nanoparticle is a promising candidate because presents a high Curie tempera-ture, high saturation magnetization, and high chemical stability
Digital Object Identifier 10.1109/TMAG.2006.872198
[2]. Recently, a colloid-chemical synthesis technique of FePt MNPs has been developed and the FePt MNPs are regarded as one of the most likely candidates for ultrahigh-density nano-sized magnetic recording materials [2][3]. The col-loid-chemically synthesized FePt MNPs have chemically disordered face-centered cubic (fcc) structure in which Fe and Pt atoms are randomly arranged and are superparamagnetic. Post-synthesis thermal annealing at a temperature over 580 C transforms the crystalline structure from fcc to chemically ordered phase. -phase FePt MNPs have a large mag-netocrystalline anisotropy ( J/m ) [4], and thus exhibit large coercivity at room temperature, even when their size is as small as several nanometers. For bio applications such as heating elements for hyperthermia, however, a superpara-magnetic fcc-phase FePt MNP is also an attractive material. For this purpose, it is important to know the targeted value of MNP size, because the mean size control attaining desirable average composition and uniformity of FePt MNPs is still a big challenge. Chen et al. have synthesized FePt MNPs of average diameter with a size of up to 9 nm by precisely con-trolling reaction conditions, such as the kind of solvent used, the amount of capping agents, temperature, temperature rising rate, and reaction time [5]. However, in general, it is difficult to synthesize FePt MNPs of average diameter that are larger than 5 nm with uniform size and composition distributions. Here, we theoretically considered an applicability of FePt MNPs to magnetic hyperthermia comparing with iron oxide and perme-ndur MNPs, and clarified the requirements for the synthesis of FePt MNPs when one applies FePt MNPs as heating elements for hyperthermia.
II. THEORY
The energy dissipation of MNPs in an alternating magnetic field is described as [6]
(1) where is the permeability of free space, T m/A; is the equilibrium susceptibility; and are the amplitude and 0018-9464/$20.00 © 2006 IEEE
the frequency of alternating magnetic field; and is the effective relaxation time given by
(2) where and are the Néel relaxation and the Brownian re-laxation time, respectively. and are written as
(3) (4) where is the average relaxation time in response to a thermal fluctuation; is the viscosity of medium; is the hydrody-namic volume of MNP; is the Boltzmann constant,
J/K; is the temperature. Here, and is the volume of MNP. The MNP volume and the hydrody-namic volume including the ligand layer are written as
(5) (6) where is the diameter of MNP; is the ligand layer thickness. The equilibrium susceptibility is assumed to be the chord susceptibility corresponding to the Langevin equation, and ex-pressed as
(7)
where ;
and is the volume fraction of MNPs. Here, and are the domain and saturation magnetization, respectively. The ini-tial susceptibility is given by . The tem-perature rise is calculated as where and are the effective density and the effective specific heat
calcu-lated as and ,
where subscripts 1 and 2 represent the MNPs and the medium, respectively.
III. HEATINGRATE OFAQUEOUSDISPERSIONS OFMNPS Based on the above-mentioned theory, we calculated the rate of temperature rise for aqueous dispersion of monodispersed equiatomic fcc-phase FePt MNPs varying the diameter of MNP in adiabatic condition. For comparison, we also estimated the rates of temperature rise for magnetite (Fe O ), maghemite ( -Fe O ), permendur (FeCo) and -phase FePt MNPs. In Table I, physical properties of each magnetic material are shown. The magnetocrystalline anisotropy con-stant of fcc-phase FePt MNPs is estimated by using the
relation , where denotes the blocking
temperature [7]. The temperature-dependent magnetization measurements of 4-nm fcc-phase FePt particles indicated that superparamagnetic behavior was blocked at 20 to 30 K [2].
Hence, we estimated as J/m for fcc-phase
TABLE I
PHYSICALPROPERTIES ANDMOSTOPERATIVESIZES
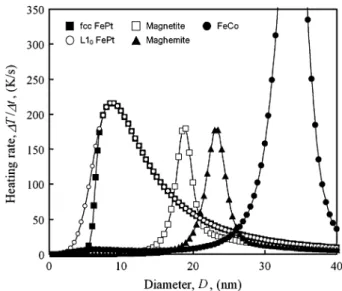
Fig. 1. Comparative heating rates as a function of particle diameter for var-ious MNPs. Filled squares and open circles represent fcc- andL1 -phase FePt, respectively. Open squares and filled triangles are magnetite and maghemite, respectively. Filled circles represent FeCo MNPs.H = 50 mT and f = 300 kHz.
FePt MNPs by setting K. The domain magnetization of FePt [8] and FeCo [9] MNPs are estimated by using bulk values of . The surface dead layer effect [10] and polydispersity of MNPs are not considered in our calculations. In practice, the magnetic anisotropy may vary considerably due to the shape contributions of MNPs. For simplicity, however, the shape effect is not taken into account in the present model. It has been pointed out that hysteresis losses are important especially for magnetic single domain particles with high magnetocrystalline anisotropy [11]. However, the hysteresis losses are not considered, because we assume MNPs are super-paramagnetic in this study.
Fig. 1 shows comparative heating rates for aqueous monodis-persions of the various MNPs listed in Table I, assuming
s and . Amplitude and frequency of applied mag-netic field were fixed at 50 mT and 300 kHz. The carrier liquid is pure water in all cases. Surface ligand layer thickness is as-sumed to be nm. On these conditions, fcc- and -phase FePt MNPs yield the largest heating rates in the size range of nm. Most operative sizes of each MNPs, , which give a maximum heating rate, are 9 nm for fcc- and -phase
Fig. 2. (a) Dependence of heating rates onH . By fixing f = 300 kHz, H is varied as 25 (bottom curve), 50 (middle) and 75 (top) mT. (b) Dependence of heating rates onf . By fixing H = 50 mT, f is varied as 100 (bottom curve), 300 (middle) and 500 (top) kHz. Filled and open squares represent the cases of fcc FePt and magnetite MNPs, respectively.
FePt MNPs, 19 nm for magnetite, 23.5 nm for maghemite, and 34 nm for FeCo MNPs (Table I). The FeCo MNPs have the highest heating rates as shown in Fig. 1. The maghemite MNPs also have large heating rates as well as magnetite MNPs. How-ever, the size ranges of FeCo and maghemite, where the heating is possible, are much larger than the typical size ranges of stan-dard ferrofluids ( – nm). In general, the stability of magnetic colloid becomes impaired when nm due to the spontaneous magnetization. On the other hand, it is diffi-cult to obtain independently isolated -phase FePt MNPs at this moment. Considering above-mentioned problems and other things such as chemical stability, we will focus on fcc FePt and magnetite MNPs hereafter.
Fig. 2(a) shows the dependence of heating rates on amplitude of applied magnetic field fixing kHz. Note that is varied as 25, 50, and 75 mT, and only the cases of fcc FePt and magnetite MNPs are plotted. The heating rates simply increase with increasing . In the case of fcc FePt MNPs, the increase in the heating rates is more prominent than that in the case of magnetite MNPs when increases. No change in is observed for both MNPs. Fig. 2(b) shows the dependence of heating rates on fixing mT. Note that is varied as 100, 300, and 500 kHz. The heating rates increase with in-creasing . Unlike in the case of Fig. 2(a), the heating rates of
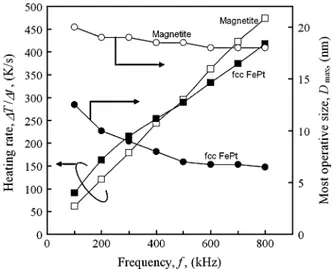
Fig. 3. Maximum heating rates (MHR) andD plotted as a function off . Filled and open squares represent MHR of fcc FePt and magnetite, respectively. Filled and open circles areD of fcc FePt and magnetite, respectively.
magnetite MNPs increase with increasing much faster than those of fcc FePt MNPs. The gradual decrease in with in-creasing is also observed for both MNPs.
In Fig. 3, the dependences of the maximum heating rates (MHR) and on ( MHz) for fcc FePt and magnetite MNPs are shown. The MHR of both MNPs linearly increase with increasing . At low frequency range ( kHz), the MHR of fcc FePt MNPs are larger than those of magnetite MNPs as shown in Fig. 3. On the contrary, the MHR of mag-netite MNPs are larger than those of fcc FePt MNPs when kHz. On the other hand, of fcc FePt MNPs decreases with increasing , while that of magnetite MNPs does not change much. Thus, in the low frequency region ( kHz), fcc FePt MNPs have an advantage that they can heat tumors to higher temperatures than magnetite MNPs. At the high frequency range ( kHz), of fcc FePt MNPs is quite smaller than that of magnetite MNPs. The saturation value of of fcc FePt MNPs is 6 nm. This result indicates that the accumulation of MNPs in the tumor could be enhanced, because the specific surface area of FePt MNPs increases when the size of MNPs decreases, and thus, the speci-ficity to a tumor tissue increases. Hence, the fcc FePt MNPs might have an advantage that the higher volume fraction of MNPs in the tumor tissue than that of magnetite MNPs can be attained. Consequently, the superiority of fcc FePt MNPs over the magnetite MNPs in hyperthermia is found to be prominent when is high and is low.
IV. BIOHEATTRANSFERMODEL
Several attempts to estimate the spatial temperature distribu-tion in localized magnetic hyperthermia using MNPs have been reported. For example, Andrä et al. calculated the spatial tem-perature distribution in breast carcinomas containing magnetic particles under external alternating magnetic field by modified heat conduction equations [17]. They compared the calculated results with in vitro measurements with muscle tissue, and found a good agreement between the calculation and the experimental
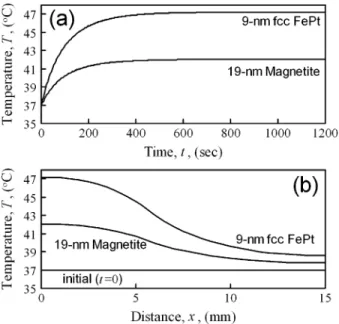
Fig. 4. (a) Time evolution of temperature at the center of the tumor (H = 50 mT, f = 300 kHz, and = 2 2 10 ). (b) Temperature distributions after applying external alternating magnetic field for 600 s. The interface between the tumor and normal tissue is located atx = 5 mm.
results. To estimate the temperature rise behavior in vivo, we solved the Pennes bioheat transfer equation [18] given by
(8) where is the thermal conductivity of tissue, 0.502 W/(m K) [19], [20]; is the density of blood, 1000 kg/m [21]–[24]; is the specific heat of blood, 4180 J/(kg K) [21]–[24]; is the blood perfusion rate, s [21]; is the tempera-ture of arterial blood, 310 K; and is the metabolic heat generation, 540 W/m . The effective density and the effec-tive specific heat of the MNP-loaded tumor are given as the volume average as mentioned above, setting
kg/m [19], [22]–[24], and J/(kg K) [19], [22]–[24]. By assuming a spherical tumor of 1 cm in diameter existing in the body core, we solved the one-dimensional form of (8) with the Neuman boundary conditions.
Fig. 4(a) shows the time evolution of temperature at the center of the tumor in the cases of 9-nm fcc FePt and 19-nm
magnetite MNPs ( mT and kHz). The
en-ergy dissipation is W/m for fcc FePt MNPs
and W/m for magnetite MNPs. In general,
cancer cells have a higher chance of dying when the temperature is above 42.5 C, and the rate of death drastically increases with increasing temperature [25]. Hence, fcc FePt MNPs work well while magnetite MNPs do not in these conditions. This result indicates that one can significantly reduce the doses of MNPs producing the same effect. In practice, the MNPs in tumor tissue are considered to be immobilized in cell plasma or sticking on cell membranes. Therefore, the Brown relaxation pathway is thought to be nearly irrelevant. To consider this effect, we in-creased the medium viscosity from the viscosity of water, and checked the changes in for both MNPs. In the case of mag-netite MNPs, is independent of , because the Néel relaxation
dominates the relaxation process originally. On the other hand, decreases with increasing in the case of fcc FePt MNPs. However, becomes to be independent of when increases more than 10 times the water viscosity. In such case, the value of is 70% of original value. Even in this situation, the energy dissipation of fcc FePt MNPs is larger than that of magnetite MNPs.
Fig. 4(b) shows the steady-state temperature distribu-tions after applying external alternating magnetic field of mT and kHz for 600 s. The distance from the center of the tumor is represented as . The interface between the tumor and normal tissue is located at mm. As seen in Fig. 4(b), the range where the temperature is higher than 42.5 C is mm. This indicates that the magnetic hy-perthermia is highly selective for cancer treatments. Although the biocompatibility and cytotoxicity of FePt MNPs are still unclear at this moment, they are known to strongly depend on the kinds of surface capping molecules [26]. Hence, we do not elaborate on the biocompatibility and toxicity of FePt MNPs in the present paper.
V. CONCLUSION
Theoretical assessment of chemically disordered fcc-phase FePt MNPs as heating elements for magnetic hyperthermia is carried out by combining the heat generation model and the bioheat transfer equation. In consequence, fcc FePt MNPs are found to have a superior heating capability compared with other MNPs such as magnetite. Thus, significant reduction of the doses of MNPs is expected. However, one needs to synthesize larger FePt MNPs than 6 nm for their use in hyperthermia.
ACKNOWLEDGMENT
The authors would like to thank Dr. H. Asatani (Solid/Powder Processing Laboratory, MCRC, Inc.) for his helpful discussion.
REFERENCES
[1] T. Neuberger, B. Schöpf, H. Hofmann, M. Hofmann, and B. von Rechenberga, “Superparamagnetic nanoparticles for biomedical appli-cations: Possibilities and limitations of a new drug delivery system,”
J. Magn. Magn. Mater., vol. 293, p. 483, 2005.
[2] S. Sun, C. B. Murray, D. Weller, L. Folks, and A. Moser, “Monodis-perse FePt nanoparticles and ferromagnetic FePt nanocrystal superlat-tices,” Science, vol. 287, p. 1989, 2000.
[3] S. Saita and S. Maenosono, “FePt nanoparticles with a narrow compo-sition distribution synthesized via pyrolysis of iron(III) ethoxide and platinum(II) acetylacetonate,” Chem. Mater., vol. 17, p. 3705, 2005. [4] K. Inomata, T. Sawa, and S. Hashimoto, “Effect of large Boron
addi-tions to magnetically hard Fe-Pt alloys,” J. Appl. Phys., vol. 64, p. 2537, 1988.
[5] M. Chen, J. P. Liu, and S. Sun, “One-step synthesis of FePt nanoparti-cles with tunable size,” J. Amer. Chem. Soc., vol. 126, p. 8394, 2004. [6] R. E. Rosensweig, “Heating magnetic fluid with alternating magnetic
field,” J. Magn. Magn. Mater., vol. 252, p. 370, 2002.
[7] V. Franco and A. Conde, “Influence of anisotropy on the grain size distribution derived from superparamagnetic magnetization curves,” J.
Magn. Magn. Mater., vol. 277, p. 181, 2004.
[8] T. Klemmer, D. Hoydick, H. Okumura, B. Zhang, and W. A. Soffa, “Magnetic hardning and coercivity mechanisms in L1(0) ordered FePd ferromagnets,” Scr. Metall. Mater., vol. 33, p. 1793, 1995.
[9] C. Desvaux, C. Amiens, P. Fejes, P. Renaud, M. Respaud, P. Lecante, E. Snoeck, and B. Chaudret, “Multimillimeter-large superlattices of air-stable iron—cobalt nanoparticles,” Nature Mater., vol. 4, p. 750, 2005.
[10] X. W. Wu, C. Liu, L. Li, P. Jones, R. W. Chantrell, and D. Weller, “Nonmagnetic shell in surfactant-coated FePt nanoparticles,” J. Appl.
Phys., vol. 95, p. 6810, 2004.
[11] R. Hergt, W. Andrä, C. G. d’Ambly, I. Hilger, W. A. Kaiser, U. Richter, and H.-G. Schmidt, “Physical limits of hyperthermia using magnetite fine particles,” IEEE Trans. Magn., vol. 34, no. 5, pp. 3745–3754 , Sep. 1998.
[12] R. C. O’Handley, Modern Magnetic Materials. New York: Wiley, 2000.
[13] J. K. Vassiliou, V. Mehrotra, M. W. Russell, E. P. Giannelis, R. D. McMichael, R. D. Shull, and R. F. Ziolo, “Magnetic and optical-prop-erties of gamma-Fe O nanocrystals,” J. Appl. Phys., vol. 73, p. 5109, 1993.
[14] R. V. Major and C. M. Orrock, “High saturation ternary cobalt-iron based alloys,” IEEE Trans. Magn., vol. 24, no. 2, pp. 1856–1858 , Mar. 1988.
[15] R. Kuentzler, “Low-temperature specific-heat of ordered and disor-dered FeCo,” Phys. Stat. Sol. B, vol. 58, p. 519, 1973.
[16] G. Hausch, “High-temperature specific-heat of FeNi and FePt invar-alloys,” J. Magn. Magn. Mater., vol. 92, p. 87, 1990.
[17] W. Andrä, C. G. d’Ambly, R. Hergt, I. Hilger, and W. A. Kaiser, “Tem-perature distribution as function of time around a small spherical heat source of local magnetic hyperthermia,” J. Magn. Magn. Mater., vol. 194, p. 197, 1999.
[18] H. H. Pennes, “Analysis of tissue and arterial blood temperatures in the resting human forearm,” J. Appl. Physiol., vol. 1, p. 93, 1948. [19] F. Duck, Physical Properties of Tissue: A Comprehensive Reference
Book. New York: Academic, 1990, pp. 167–223.
[20] H. F. Bowman, , Shitzer and Eberhart, Eds., “Estimation of tissue blood flow,” in Heat Transfer in Medicine and Biology. New York: Plenum, 1985, pp. 193–230.
[21] S. Tungjitkusolmun, S. T. Staelin, D. Haemmerich, J. Z. Tsai, J. G. Webster, F. T. Lee, D. M. Mahvi, and V. R. Vorperian, “Three dimen-sional finite element analyses for radio-frequency hepatic tumor abla-tion,” IEEE Trans. Biomed. Eng., vol. 49, no. 1, pp. 3–9, Jan. 2002. [22] L. A. Geddes and L. E. Baker, “The specific resistance of biological
material—A compendium of data for the biomedical engineer and physiologist,” Med. Biol. Eng., vol. 5, p. 271, 1967.
[23] O. P. Gandhi, G. Lazzi, and C. M. Furse, “Electromagnetic absorp-tion in the human head and neck for mobile telephones at 835 and 1900 MHz,” IEEE Trans. Microw. Theory Tech., vol. 44, no. 10, pp. 1884–1897, Oct. 1996.
[24] D. Simunic, P. Wach, W. Renhart, and R. Stollberger, “Spatial dis-tribution of high-frequency electromagnetic energy in human head during MRI: Numerical results and measurements,” IEEE Trans.
Biomed. Eng., vol. 43, no. 1, p. 88, Jan. 1996.
[25] W. C. Dewey, D. E. Thrall, and E. L. Gillette, “Hyperthermia and radi-ation—Selective thermal effect on chronically hypoxic tumor-cells in vivo,” Int. J. Radiat. Oncol. Biol. Phys., vol. 2, p. 99, 1977.
[26] A. Hoshino, K. Fujioka, T. Oku, M. Suga, Y. F. Sasaki, T. Ohta, M. Yasuhara, K. Suzuki, and K. Yamamoto, “Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their sur-face modification,” Nano Lett., vol. 4, p. 2163, 2004.
Manuscript received October 28, 2005; revised February 3, 2006. Corre-sponding author: S. Maenosono (e-mail: shinya@chemsys.t.u-tokyo.ac.jp).