Ishikawa Agricultural College
NII-Electronic Library Service
Ishikawa
4AgriculturalCollege
BuiLRIAR,IshikawaAgr.Coli.6:6T-66(]999)
Identification
of
strains
of
Mesothizobium
huakuii,
root
nodule
bacteria
of
Astragatus
sinicus,
by
the
polymerase
chain
reaction
Masao
TAcHIMoTO
I.aboratory
ofMicrobiological
Resources,
Research
Institute
of
Agrieultural
Resources
Ishikawa
Agricu]tural
College,
Nonoichi,
Ishikawa,
921-8836,
JAPAN
Strains
of
Mesof'hi.zohium
huakuii,
root nodulebactcria
ofAstf'agaius
sinicus.. wereidentified
from
DNA
po]ymorphism
amplified
by
the
polymerase
chain reaction(PCR)
using somcknown
()rrandomprim-ers.
Five
strains ofM.
huakuii
isolated
in
japan
and
China
were examined usingSPH
(a
randomprimer),
nit/HDK(a
part
ofthe
sequence of n(f'gene)
andERIC
(both
ends of an repetitiveintergenic
consensusDNA
sequence ofentericbacteria).
DNA
bands
amplified
withprirners
showeddistinctly
ditferent
band
patterns
between
groups
of strainsisolated
in
Japan
and
those
iso]uted
in
China.
Although
it
was sometimesdifficult
to
idcntify
strainsbe[onging
to
the
samegroup
w'ithon[y
one
primer,
strains
of
a
group
couldbe
identit'ied
by
cornparison ofLhc
resu]ts obtained withdifi'erent
PCR
pi'imers.
Strains
of
M.
huakuii
iso]ated
randomlyfrom
a ricefield
soil were examincdby
PCR,
and severaltypcs
of strainswere
found
to
survive
atthe
samesite.
This
identifieation
method usingPCR
wasalso
usefu1to
investigate
the
infection
rateof
inoculatcd
strains
in
a
pot
cultivation
experiment.
Key
words :Mesorhizobittm
huakuii,
Astragaius
siniciis,PCR,ERIC,
nifHDK
Introduction
The
taxonomy
of root nodulebacteria
ofChinese
milk vetch(Aslragafus
sinicLtsL.)
i'emained unclear unti]it
was classificd asRhizobittm
huakuii
by
Chen
et at.in
1991'i'.
It
was reclassifiedias
Mesorhizobiuni
huaketii
accordingto
its
physio]ogical
andphylogenetic
properties
to
distinguish
it
from
othcr rhi7obialbacteria.
Chinese
rnilk
vetch
is
aleguminous
plant,
bcing
cu[tivatedin
the
temperate
area
of
Far
East
Asia
especiallyin
China,
andit
has
notbcen
paid
attention
by
agricu]tural researchers ofthe
western world exceptin
a
few
casesS'.
The
role ofChincse
mi]k vetch asgrecn
manurein
riceficlds,
how-ever,has
become
lower
in
China
with
the
increase
of oil erops sueh as oi]seed rape as cashcropsi).
In
Japan
a]so,Chinese
milk vetch was ",idelygrown
as a wintercrop
on
rice
tlelds
asgreen
manure.but
the
cultivation areaof
Ch{nese
milk
vetchhas
rapidlydecrcased
afterWorld
War
II
withthe
spread
of
chemicalfertilizers.
However,
thc
ro]e ofChinese
milk vetchis
now
being
reassessed sincethe
soilfertility
of arableland
in
Japan
is
though{
to
be
continuously
lower
andthe
estab]ishment of a sustain-ab]e agriculture systemis
required,
The
root nodu]ebacteria
ofA. sinicttsare
considered
to
survive
as
indigenous
heterotrophic
inhabitants
of Japa-nese soils,because
Chinese
milk vetchhas
been
grown one
rice
t'ic]ds
sincethe
17
th
century.Therefore
the
nodulesofA.
sinicusare
usua]lyformed
by
indi.g.enous
root nod-ulcbacteria.
In
such
cases,
thc
et'fect ofthe
inoeulation
ofM.
huakuii
mustbe
evaluated
by
checking
if
the
root nod-u]es areformed
by
the
inoculant
strains
or other native ones.It
is
ratherdifficult
to
identify
the
strains
of root nodulebacteria
by
such conventional mcthodsas
intrinsic
antibiotic
resistance. serologica] reaction, enzymclinked
immunosorbent
assays
(ELISA}
or restrictionfragmcnt
lcngth
potymorphisms
<RFLP)
which requirecomplicated
procedures.
Recently,
the
polymerase
chain reaction(PCR)
using
random
primers
has
become
one ofthe
mostetTective methods
to
identify
bacterial
strains andit
has
been
usedfor
rhizobialbacteria,
However,
PCR
has
notbeen
appliedto
M.
htfakttii.
In
this
paper,
I
reportthe
utility and
detailed
conditions ofPCR
to
identify
strains
ofM.
huakt{ii.
This
method was also appliedto
cstimatethe
infection
rate
of
inocutated
strains onthe
root oi'A.
sinicetsin
a
pot
experimcnt.
Mcthods
andMaterials
Strains
qf'M.
huakuii
Thc
strains usedin
thc
experiment wcrethe
five
strains
Iisted
in
Table
1.
Three
of
them
wereisolated
in
Japan
and the othertwo
in
China.
The
strain103T
is
the
Ishikawa Agricultural College
IshikawaAgriculturalCollege62
Bullcttn
ot'RIAR,Ishikawa
Agricuitural
College
Nc).
6
C
T999}
Table
1,Strains
usediti
the
experinientsStra]ns
Ongin
i03T(NAU)
1
so05
(ACCC)
B39C)1
([A(t・)
912
cEAC)
Type
stainof it4.ht"tkttit
CNanjmg
A.gricultural
Universit.v.
China)
Stram
used inChina
tAgrieultural
Culture
Co]lectton.
Chtna)
IKoliLteb},
N{urooka,
Hirosh"na
Univ
.Jupan.
Isolatc
b},
Tokachi
Federation
ot'Agr.
Coop
.
Japan
Isolute
in this.i'F.iF.a-rs/!.!s.blls.eniL.As,r.
Coll,Japan
.
1'able
2,Primers
uscdfor
PCR
Ss,mbolSPH
1n]fHDKER]C
1RERIC
2
Base
seguenve5'-GACGACGACCGACGAC-3.'
5'-GGTTATCGAAATCAGCAGCCACAGCGC-3'
5'
ATGTAAGCTCC.T(}GGGA']'1'CAC-3'
5'-AAGTAAG']'GACI'GGGGTGAGCG-3'
Rcmark
Random
primer
Part
ei'mfgene
In{crgcmcconsensusrepelitn,c
scquence et'enteric
bacterui
Reference
6)
9)
4)
type
strain of iW.ht.takttii.
Prci)at'ation
of'
l)iYTA
,fbr
PCR
The
bacteria
to
be
tested
were culturedin
TY
liquid
mcdium(Bactotryptonc
<Difco)
5
g,
yeast
extractCDit'co)
3
g,
CaC12'6
H]O
1.3
g.
disti11eci
water1
L,
pH
7.0)
at26
℃
ft)r
aboult-・o
da>'s
untilthe
logarithmic
growth
stage,
The
culturedbacteria]
body
was
colleeted
b>,
centrifuga-tion
(1O.OOOXg,
1O
mm),
and
washed withph>,siological
saltne solution.
Tbe
eo]lectcd
bacterial
bodx,
wassus-pended
withan
adequate
amounr of s.terilized watcr and ciispensedinto
1.5
mL mlcrotubcs and centrifuged again.To
the
precipitat]t
was added200
pl.
ot'steri]izcd water,and
the
suspension was stocked ut20'C.
The
suspen-sion
was meltedjust
befoi'e
use, andtO
,uL
ofProteinase
K
solution(1
mg mL i) and50
yl.
ofBL
buffcr
solution(Tris
40
mM,Tween-20
1
9E・,
Nonidet
P-40
O.5%,
EDTA
・
2
Na
1
mM.pH
8.0)
were acldedand
incubated
at60
℃for
20
mmto
dissolvc
the
bacterial
body.
The
reaction was-stopped
by
heating
at95
℃t'or
IO
min andthe
supernatant wasco]lected
by
centrifugation and used as crudeDNA
so]ution.The
c)ptica]densjty
at260
nm ofthe
crude
DNA
solutton w'as measured andthe
concentration
of
DNA
was adjustedto
150
yg
uL
itbrPCR,
1]CR
The
reaction
so]ution containedlO
uL of10
×buffer
i
(Takara
Bio),
1.5rnM
rvls,C]!,
O.2mM
dNTP,
1pM
Primer<s),
2.S
unlts ot'Taq
DNA
po]ymerase
CTakara
Bi())
and5
pL
oi'
the
diluted
erudeDNA
solutionc750
pg
astemplate
DNA).
the
totah,olumc
ol'which was adjustedto
100
pL.
The
primers
uscd mPCR
were
as
listed
in
Table
2.
SPH
1
wasa
random singlcprimer
andthe
oth-ers were
directed
primers
that
are apart
of aknown
sequence(nifHDK)
or of a conscnsus sequence(ERIC).
The
reaction mixture wastaken
into
a1.5
ml. microtube.over[aid with
]OO
pL
ef mineral oil andset
in
a
thermal
c.vcler(Astec
PC-700).
The
te;nperature
condition
efPCR
with
SPH
1
and
nitl-IDK was asfollows
i
pre-run
at94
℃for
1
min,30
cycles ofdenaturjng
at94
℃for
1
min, annealing atSO
℃for
1
min,polymeriLation
at72'(.;
for
1,)'
min, andposl-run
at65
℃
for
2
min,The
tempera-ture
condition ofPCR
withERIC
priiners
was as{'ollows
;
pre-run
at95
℃foi'
5
min,30
c>,clesof
denature
at
94
℃for
min, annealingat
52
℃for
]
min,po]ymerization
aL65
℃
for
8
min,
and
post-run
at65"(:
for
16
min.The
PCR
products
werc separatedfrom
minera] oi]by
addition ot'100
uLof
chloroform
isoamyl
alcohol mixture(24
:1
vlv)
and
electi'ophorcscd
with2`7c,
agarose(.ttmplisize
aga-rese,Bio-Rad)
in
[XTBE
(pH
8.2)
under a1(}O
V
for
about45
tnin.As
DNA
size markers, aIOObase-pair
ladder
andKilo
base-pair
[adder
(Pharmacia)
wer'celec-trophoresed
atthe
sumetime.
The
agarose
s,e]
was
staincd with
ethidium
bremide
solution(2
,ug
L-i)
andDNA
fragments
wcredetected
underUV
light
of26e
nm.inoc'ulation
e.xl?eritnei'tt }i,iththe
strains of root JtoduiehacteJ'ia
Air-dried
soi]
(gray
low[and
soil, sand.vcluy
toam)
was mixed with river sancl at
the
i'atioof1
:1,
and2.5
kg
of
the
mixturepcr
Wagner
pot
of]15,OOO
a was usedi'or
the
pot
cultivation cxperimen{.Nodu]e
bacteria
shownin
Table
1
k-'ereculturcdtn
TY
liquid
medium
at26
℃for
about
two
days
and
the
suspension ofbacteria
(3'--9
×1OS
CFU
mL-i,2
inLpe;'
pot)
wasinoculated
to
the
seecllings ofA. sinic'us(cv.
Gifu-hinode-wasc).
which weresown
at
Ishikawa Agricultural College
NII-Electronic Library Service
Ishikawa
'AgriculturalCollege
63
TACHTMOTO
:Identificanon
ofstrains ofMeso
nhizobiumhuakuii
by
PCR
the
density
of nineper
pot,
Calcium
superphesphate andpotassium
chloride were supplied asbasal
fertilizers
at
the
rate ofO.5
g
per
pot
asP20s
andKiO,
respectively.A.
sinictts wasgrown
in
agreenhouse
at about20
℃for
2
months.The
cultivation
experiment was carried out withthree
rep]icates.The
growth
and
nitrogen content ofA. sinicus were measuredafter
harvesting,
and
the
root
nodules were counted and randomly sampled at
the
rateof
ten
nodulesper
treatment.
The
isolated
bacteria
from
colonies
fbrmed
onyeast
extraet mannitol agar medium(YM)
weresubjected
to
PCR
and examinedfbr
nodulei'c)rmution
by
inocugated
strains
or otherindigenous
strains ofM.
huakuii,
Results
andDiscussion
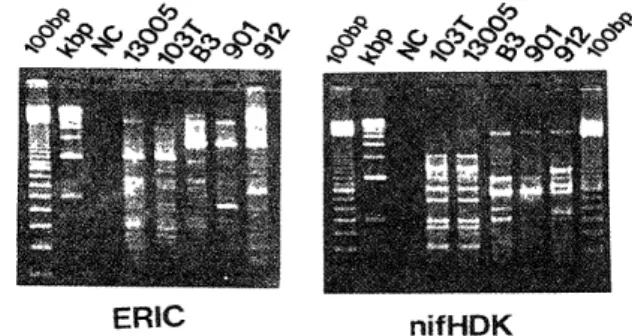
DNA
fragments
were amptified with anyprimers
usedin
this
experiment
as shownin
Fig.
1.
Electophore-sedDNA
band
pattems
were similarin
eachgroup
ofChinese
strains andJapanese
strains,
and
it
was easyto
determine
to
whichgroup
isolated
strains
be]onged,
Within
agroup,
identifieation
withon]y
one
primer
such
NcSSSi
LSPst/Sfi,g・cs'/di3'
siss'.6S.IStb{}iesi/di3f.cS5'
';tt.t'
t.t
ERiC
nifHDKFig.
1,
DNA
bancl
patterns
of testetistrains ofMesorhi.zohit{m
huakuii
by
PCR
.
Le
ft,
ERIC
primers
;
Right,
nitHDKprimer,
M
1OO,
1OO
base-pair
[adder
DNA
sizc marker ;Mk,
kilo
base-pair
iadder
DNA
size marker;
NC,
negative control,s8"gr
g
i
2
3
4
s
6
7
s
g
w
$8
Fig
2.DNA
band
patterns
oi' strainsiso[ated
t'rom
aficld
soilby
PCR
with nifHDKprimer.
M
100.
1OO
basc-pair
ladder
DNA
size rnarker;
Mk.
k][o
base-pair
ladder
DNA
stzemarker ;NC,
negative eonLrol,as nifHDK was sometimes
difficutt,
but
the
strainscou]d
be
identified
by
comparison ofthe
resu]ts obtained withdifferent
primers.
This
PCR
methodis
consideredto
be
usefulto
investigate
the
int'ection
rate ofinoculated
nod-ulebacteria
in
A.
sinicus.
The
nodulebacteria
isolated
from
nodules
which were randomly sampledfrotn
A.
sinicusgrown
in
one
ricefield
showed
variousDNA
po]ymorphisms
by
PCR,
This
suggestes
that
varioustypes
ofindigenous
strains ofM,
huakuii
inhabit
even
in
the
soi]
ofthe
same site.The
inoculation
experiment
was
carried
out withWagner
pots
oflf5,OO()
ato
confirmthe
effect of strains ofM.
h"akttii
as
inoculants
in
a condition where indige-nous nodulebacteria
survived.
As
shown
in
Table
3,
the
growth
and nitrogen contentwere
significantly
stimulatedby
the
inocu]ation
of13005,
B3
and
901
except
I03T,
The
investigation
ofisolates
from
nodules ofA.
sinicus
by
PCR
(Figs,
3
and4)
suggestedthat
ahigh
percentage
ofthe
noduleswere
infected
withthe
inoculants
excepL103T,
As
summarizedin
Tablc
4,
the
infection
rate with103T,
13005,
B3
and901
was
O%,
100%,
639e
and88%,
respectively.AIthough
103T
is
the
type
strain
ofM.
huakuii,
it
seemedto
]ack
aplasmid
containing nodgene
which
is
relatedto
the
nodulationof
leguminous
plantsi'.
The
ability of103T
to
form
nodulesin
A.
sinicus was notconfirmed
alsoin
this
experiment.Grewth
and
tota]
nitrogen
content
ofthe
plants
{noculated
withthe
strains
except
103T
were
significantlyhigher
than
the
eontrol withoutinocu]ation,
but
there
was no significant differ-encebetween
the
three
inoculants
ofl3005,
B3
and901.
There
was no significantdiderence
in
the
number ofbig
nodules(>3
mmin
diameter)
between
the
treatments,
but
the
total
number of nodules was significantly in-creasedby
the
inoculation
treatments
except103T.
It
is
estimatedthat
inoculation
treatments
causedthe
increase
of small nodu]esin
A,
sinicus.The
soil
used
in
this
experiment was estimatedto
have
ahistoryof
growingA.
sinicuh'.However,
asit
was usedin
an air-dried condjtion.the
density
ofindigenous
M,
huakuii
was ratherlow.
Iess
than
10]
CFU
g-i
andthis
might
have
causedthe
high
inoculation
effect
in
this
experiment,
It
maybe
necessaryto
confirmthe
effecL
of
inocu]ation
in
fresh
soil.
The
inoculation
of somestrains
of
M.
huakuii
was
quite
effectivefor
Chinese
milk vetchin
soils whichhad
nohistory
of
growing
Chinese
milkvetch
in
such countries asU.
S.
A.
and
Nepal]'").
How-ever,in
China
whereChinese
milk vetchhad
been
grown
for
along
period.
the
effect
ofinoculation
was sometimes not so clear2) andit
has
notbeen
cont'irmed whether nod-ulesof
Chinese
milk vetch werereally
formed
by
Ishikawa Agricultural College
エshlkawa Agrloultural College6
〆1
尋
BulleUn
eiRI
へR
、
1
,
shikawa へgricul
[uralCollcge
No
6
(」999
),
、。。,
ぱ 轟
3
鯲
轟
辱
B3
ぱ
轟
9
翻
轟
Flt43
DN
へb
、
mdpaUem
、ol I、{>laLc
丶m 匸hc
pQl
expe 睦1men しobLEuncdby
PCR
w貝h
1111HDKpllmolM10
(1
.
IOO
buse
−
p
.
田la
〔1
⊂1c
【DN
八 丶1/e ma 【kc1
、
Mk
ktlo
ba
〜c−
1
}、np:laddel
DNA
丶1zc IllalkolNC
ne撃 IUI、e しbo1
し〕[じachphLtc
indteate〜the
UcallrLcnL
arld dno 、、 丶md [cule Lhご】neculatcd 、[lam、〔130
〔〕5
B3
、
901
103
t
’
)尋
鱒
3
τ
ぱ
13
◎
05
吾
B3
♂ 轟
go
唱
FI
/24DN
へband
pattern
丶ol 匸s〔、lutc
〜in thep
〔,L expcnlncntobttunedb
}PCR
LklthER
【C
p
【1111c正、Ml
〔)0
100
base
−
1
>alrladdelDNA
、1∠c inarke [Mk
、
klk
〕ba
丶
e−
pUII
I
.
LddcrDN
へ丶tze MIL[ke
[、
NC
、
ncg、
虹nvc し〔}nU 〔}l
The
Ishikawa Agricultural College
NII-Electronic Library Service
IshikawaAgriculturalCollege
65
TAcH[MoTo
/Identification
ofstrainsofn4esorhi.7obi"m
huakuii
by
PCR
Table
3.
Inoculation
test
by
pot
cultivation
-. ・-
=.・StrainsShoot
growth
N
content mgper
potRoot
nodules
per
plantInt'ecnonrate
D.W.
(g)
perpot
big<>S
mm) totalinfccted1tolal(`",)Control103T1300S901B3
3.3ttO,18a'
3.82
±O.13a
5,29
±O,7eb
6.59
±O.30b
620
±・
O.77
b
107
±1a
153
±8
ab244
±30be
300
±.
17
c276
±27c
2.96.01.23.54.5
8a
18a1OO
b146c103
bc
O/8
(
O)
10tlO(100>
5f8
(
63)
7f8
(
88)
*
Same
]etters
indieate
no significantdifference
by
Duncan's
rnultip]e rangetest
atp=O.OS
(n=3).
Tabie
4.
Infection
rates ofinoculated
strainsin
thepot
expcrimentPnmer
Inoculated
strain nitHDKERIC
+
±+
±Infection
rateinfected1totat{%.)
I03T13005B3901
o*103s 7o53
2ooo
o1075 oel2
8ooo
0f8<
O)
1OAO
(1OO)
518
(
63)
718
(
88)
+,
same astheinoculated
strain±, almost
thc
samebut
part]y
different
from
theino
¢uluted strain--,
different
from
theinoculated strain' number of
iso]ates
C=
nodules)The
PCR
method
with adequateprimers
is
thought
to
be
usefu]to
identify
the
strains
ofM.
huakuii,
but
there
were some cases
in
whichaccurate
identification
wasdif-ficult
because
oflow
reproducibi1ityof
DNA
band
pattern.
It
was necessaryto
perform
PCR
amplificationwith
stan-dard
strains underthe
same conditions.To
increase
the
reliability
of
the
PCR
method, we must considerthe
cul-ture
conditions,
refining
procedure
ofDNA
templates,
temperature
setof
PCR
and
so
on.
As
it
is
known
that
M.
huakuii
easilylose
plasmids
during
culturc]']3),the
change
of
plasmid
compositiondur-ing
the
culture orin
the
soilmay
be
one
of
the
factors
causing
the
variation ofDNA
band
putterns.
Acknowledgements
The
authoris
gratefu1
to
Prof.
Murooka,
Hiroshima
University
(currently
Osaka
University)
for
providjng
the
strains
of103T
andB3
ofM,
huakuii,
andthe
Renge
Society
of
Japan
fbr
supplying
the
strain of13005.
Literature
Cited
1)
Chan,
C.
L.,
T,
A.
Lumpkin
andC.
Characterization
ofBrad.vrhizobiumS.sP'Root
1988.
(Astragatus
sinicus
L,)
using
serological agglutination,intrinsic
antibiotic resistance,
plasmid
visualization, andfie]d
peifbrrnance.
Plant
and
Soil
109
:85-91.2
)
Chan,
H.
K.,
F.
D.
Li
andY.
Z.
Cao
1992.
istics,
distribution,
ecology and utilizationof
galus
sinicus-rhizobia symbiosis.in
"The nitrogenfixation
and
its
researchin
China,"
Springer-Verlag,
Shanghai
Scientific
andTechnical
Publishers,
hai,
439-455.
3
)
Chen,
W.
X.,
G.
S.
Li,
Y.
L.
Qi,
E.
T.
Wang,
H.
L,
Yuan
andJ.
L.
Li
1991.
Rhizobium
httakuii
sp. nos,.iselated
from
the
rootnodules
ol'
Astiugalus
sinicus.Int.
J.
Syst
Bacteriol,
41(2)
:275-280.
4)
de
Bruijin,
F.
J.
1992.
Use
of repetitive(repetitive
extragenic
palindromic
and enterobacterial repetitiveintergeneric
consensus)
sequences andthe
erase chain
reaction
to
fingerprint
the
genomes
ofRhi.7.ohium
melilotiisolates
and
other soilbacLeria.
App.
Environ.
Microbiol.
58(7)
/2180-2]87,
5
)
Dooley,
J,
J.,
Hanison
S.
P.,
L.
R,
Mytton,
M.
Dye,
A,
Gresswell,
L.
Skot
andJ,
B.
Beeching
1993.
Phylo.oenetic
grouping
andidentification
ofhittm
isolates
onthe
basis
of
random
amp]ified
morphic
DNA
profiles,
Can.
J.
Microbiol.
39
:
Ishikawa Agricultural College
エshikawa Agrioultural College66
Bullctin
DfRIAR
,
且shlkaw こ虹A
呂11iculturalCollege
No
.
6
〔1999
)6
)Harrison
.
S.
P
.
,
L
.
R
.
My
宅toll
,
L,
Skot,
M
,
Dye
,
and
A
,
Gressw・
eHl992
.
Characterizalion
ollR
’z’ぞo わ此〃ア1is
〔}lates
by
amphfica τioll
ofDNA polymorphtsms
using 1
』
alldomprimcrs
、
Can
.
J
.
Microbiol
、
38
:iOO9
_
1015
.
7
)
Jarvi
〜,
B
.
D
.
W
,
,
P
.
Van
Bcrckum
,
W
.
X
.
Chen,
S,
M .
N
〔}ur andM
.
P
.
Fernadez
l
997,
Transfer
of
1
〜hizo−
∠フ
1
雄 ηloti
.
ノ〜hio
,,
θbit
・
tηi.
ht
‘akt・
tii、
尺Jzi
こ0わit
’
t〃1(」
iCE
.
厂1
,
RhiN
こob ’‘〃 ηiηedile ”rafteL〃 η
,
andRhi
こθhii
〃11ti
αn.
shanense:oMey θ 厂
hi
:oわie
”ngen.
nov
.
ll
〕t
.
J.
Sys
しBacterio1
.
47
(
3
)
:895−898.
8
)
Lumpkin
.
T
.
A
.
1993
.
In
しroduction ofChinese
Inilkvetch
to
coulltries otherthan
EasL
Asia
andits
1
’
eSeal−
Ch.
〃t’
‘
Renge
∠enShO
(
AII
ab〔}UtChinese
mi 】
k
vetch )∴
T
,
Yasue
(
Ed
.
),
N
〔,sansongyoson
Kyokai
,
Tokyo
,
130
−
S43
(in
japanese
).
9
)Richardson
,
A
,
E
.
.
L
.
A .
Vieca1
’
s、
J.
M
.
Watso
冂 and
A
.
H
,
Gibson
1995
.
Differentiation
〔)f
尺んizob
〜L,〃7strains using tlle
polymcrase
chain reaction with 1’
a11−
dom
anddirected
primers
.
Soil
Bio1
.
Blochcm
.
27
:
515−524.
10
,Tan
,
Z
,
Y
.
,
X
.
1
.
)