Yamanashi Med. J. 6(l), Sl--87, l991
Sister Chromatid Exchange (SCE) Frequency in Human and Simian
Lymphoid Cell Lines Infected with Simian Retroviruses Closely Related
to Human T-Cell Leukemia Virus Type-1
Kumiko IuiMA, Atsumi Ts(.L}iMoToi), Hajime TsLEriMoTo2), Masanoyi HAyAMi3), Makoto HiGuRAsHi,
and Munehiro HiRAyAMA
DePartment ofMaternal and Child Health, School ofHealth Sciences, Facudy ofMedicine, The Universdy ofToltyo,
Bundyo-ku, Todyo 113, i)Division ofCarcinogenesis, National Cancer Center, Research Institute, Chuo-ku, Tokyo 104, 2)DePartment ofVeterinary InternalMedicine, Faculty ofAgricz`lture, The Universdy ofToltyo, Bundyo-ku, Todyo U3, and 3)l7zstitzLte Virzts Research, Klryoto UniwersiCv, Saflyo-kze, Klyoto 606,.IaPan
Abstract: Sister chromatid exchange (SCE) frequencies were examined on 4 simian lymphoid
cell lines producing simian retroviruses closely related to human T-cell leukemia virus type-1 and 6
human lymphoid cells immortalized by these simian viruses. The frequencies of SCE in both simian and human lymphoid cell lines were higher than those in normal cultured simian ancllor human lymphocytes.
We also observed the change of SCE frequencies in human cell lines immortalized by STLV at
several passage levels. At an early passage level the frequency of SCE in these human cell lines was
already higher than that in normal lymphocytes culture. This rise in SCE frequency did not change significantly during the observation period for 180 days.
From these findings we concluded that human lymphocytes were infected with STLV as simian lymphocytes, and that STLV caused same DNA damage in human lymphocytes as in simian lymphocytes, These phenomena induced by the retrovirus iRfections may correlate with leukemogenesis.
Key words: Simian T-cell leukemia virus (STLV), Adult T-cell leukemia (ATL), Sister chromatid
exchange (SCE), Human T-cell leukemia virus type-1 (HTLV-1), Lymphoid ceMine
INTRODUCTION
HumaR T-cell Ieukemia virus type-1
(HTLV-1) was isela£ed from mature T-cell malignancies in man, and is known to be an etiological agent of adult T-cell leukemia (ATL)i-5). By comparison, the serurfl anti-bodies cross-reactive with HTLV-l were found in Old World monkeys and apes6rmiO). From these seropositive monkeys, simian retrovir-t}ses (STLVs) highly homologous to HTLV-1Received Accepted September 7, December 12, 1990 1990
were isolated in culturesii). Nucleotide
sequ-eRce homology of STLV with HTLV-1 was
90-95%i2) and it was demonstrated that STLV
had the same leukemogenicity as
HTLV-li3・i4). To examine the biological function of
STLV, chromosomal damage induced by
STLV infection was investigated.
The role of viruses in causing chromosomal damage has received considerable attentioR, particularly in oncogenic viruses such as HSV, SV40 and Rauscher leukemia virusi5-i8). On the other hand, during the last decade, it has been firmly established that siste}' chromatid exchange (SCE) arises during replication on
damaged templates and SCE bioassay has now become popular as a very sensitive method for identifying potential DNA-damaging agents. It should be Roted, however, that although
con-ventional chromosome aberrations and SCE
are both cytogenetic rrianifestations of damage to the genome, they are seemingly unrelated and arise very different mechanismi9). This paper reports observations of the SCE frequeflcies and chromosomal instabilities in STLV-produciRg lymphoid cell lines and in the human cells immortalized by these viruses. It also estimates the potential ofchromosomaldamage by STLV.
MATERIALS AND METHODS
Cells
Four STLV-producing lymphoid cell Iines: JM86, PtM3, RfM26-l altd BM5 were used for
examination, which were established from
STLV-infected Japakese monkey (Macaca jus-cata), pig-tailed macaque (Macaca nemestrina), red-faced macaque (Macaca arctoides) and bon-net monkey (Macaca radiata), respectively were usedil).
Cells were cultivated for more than 12
months in RPMI 1640 medium supplemented
with IO-20% heat-inactivated fetal bovine ser"m (FBS) at 370C in a humidified atmos-phere of7% C02 in air. For cell growth, BM5 required exogeneous interleukin-2 (IL-2) pre-pared from simian spleen lymphocytes stimu-lated with concanavalin-A (Con-A) (Pharmacia Uppsala Sweden). All of these cell lines gave positive reactions with two monoclonal
anti-bodies coryespoRding to HTLV-1 p19 and
p24, respec£ively.(PHA-P) (Dilico Laboratories, Detroit, Mich, U.S.A.) iR RPMI 1640 medium supplemented with 20% FBS for 24 hrs. These cells were then co-cultivated with equal numbers of lethally-irradiated (l5,OOO rads) lymphoid cell line priducing STLV. The co-cultivated cells were
fed twice a week with the growth medium
containing 10% simiaR crude IL-2X). The
expression of STLV proteiRs was examined by the irRrfluRofiuorescence assay as describedii).
For comparison, HTLV-1 frorr} MT-1 and
MT-2 cell lines2'20) were also transmitted to
human PBMC using the same procedure.
Cytogenetic StudiesTo examine chromosomal aberrations, the cultured cells were incubated with colcemid (2×IOr7 M) for the filtal 12 hrs. After cen£ri-fugation, the cells were treated with hypotonic O.075 M KCI solution. Fo}lowing fur£her cen-trifuga£ion, the cell pellet was fixed with a mixture of methanol and acetic acid (8:I).
After the slides were stained with GierRsa, the
chromosoma} preparation was aRalysed by
examination of 30 to }OO mitoses in each cell line.
For sister chrorna£id differential stainiRg, the cells were incubated iB medium containing bromodeoxyuridine (BrdUrd) at a concentra-tion of 40paM for the last 72 hr ofculture aRd were kept in a light free environment. After
treatment for chromosome preparatieR as
described abeve, a modification ofthe fiueresc-ence-plus-Giemsa (FPG) techRique2i) was used to obtain harlequilt chromosomes. TweRty to fifty fully difllerentiated and secondarydivid-ing metaphases were scored for examination ef SCE in each cell line.
Transmission of the Virus by Co--cultivation
For the virus recipient cells, human
peripheral blood mononuclear cells (PBMC)
obtained from two healthy womeR, testing
Regative for the HTLV-1 antibody, were
seeded at a density of 1× 106 cellslml aRd were stimulated with O.2% ofphytohemaggu}tinin-PREsuLTs
The four original STLV-infected cell lines:
JM86, PtM3, RfM26-1 and BM5, were
analy-sed cytogeRetically. The frequeRcies of SCE in these STLV-producing cell lines were signi-ficantly higher than those in short-term
cul-STLV induced SCE 33
Table l. Cytogenetic features ofsimian lymphoid cell lines producing STLV
Cell Origin Virus Antigen
posMve
cells(%)Chromosome number
-41 42-46
47-60
Cells Cells
"Jith MC with DMC(%) (%)
SCE per chromosomeJM86
PtM3.RfM
26-1
BM5
JapaneseMonkey
Pig-tailed macaque Red-faced macaque Bonnet monkeySTLV
-JA
STLV
-PT
STLV
-RF
STLV
-BO
1OO IOO 30 70 22 (51.2)18
(94.7) 7 (}6.7)46
(80.7)14
(32.6) ( 1 (5.3) (27
(64.3) (7
(12,3) ( 2.2) (14.0)o)(e)
4 95
9.5) (4 )O)(7D)
75
o 9.5 e 1.9 o 26.2 3.5 e.54 O.22 O.27 O.39 Control Red-faced macaque Japanese monkey (-) (-) o o 65 (34.2) 8 (15A) I25 (65.8) (44
(84,6) (o) (o )
o)(o)
o o o o O.14NT
Table 2. Cytogenetic features of human lymphoid cell lines proclucing HTLV
Cell Origin Virus Antigen posltlve cells(%)
Chromosome number
-45
4647-60
Cells Cells
with MC with DMC
(%) (%)
SCE per chromosomeMT-1
MT-2
TAK
Human
Hurr}anHuman
HTLV
HTLV
HTLV
IOO 1oe 1OO 28 (84.8) ( 38 (67.9) ( 43 (89.5) (5
4.2) (6
11.5) (2
4.2) ( 8.3) (2.7) 5.4) (l5.2) 2.1) ( 4.2) 2.8 5.8 2.1 2.8 7.6 2.1 O.25 O.27 O.24 ControlHuman
(-) o e(e )
lll (loo )(o) (o )
o o O.I 7tured lymphocytes from Srl-LV
antibody-nega£ive healthy moRkeys
(O.14±O.051chromo-sorne) (Table 1). The SCE frequencies iR
MT-1, MT-2 and TAK cells producing
HTLV-1 were O,25!chromosome O.27!chromosome
and O.241chromosome, respectively, aRd signi-ficaittly higher than those in short-term cul-tured human lyrnphocytes from HTLV-1
anti-body-Regative healthy women (O.17±O.031
chromosome) (Table 2).
Normally, chromosome number (2n) iR
these macaque species is 42. In all cell lines, the
total chromosome number was distributed
over a wide range, and many hypo- and
hyper-diploid cells were observed. Minutechromosomes (MC) and double minute
chromosomes (DMC) were also observed, and pulverizations were occasionally found. Human PBMCs were immortalized byinfec-tion with STLV from JM86, PtM3 and
RfM26--}. After 1 to 2 months from the
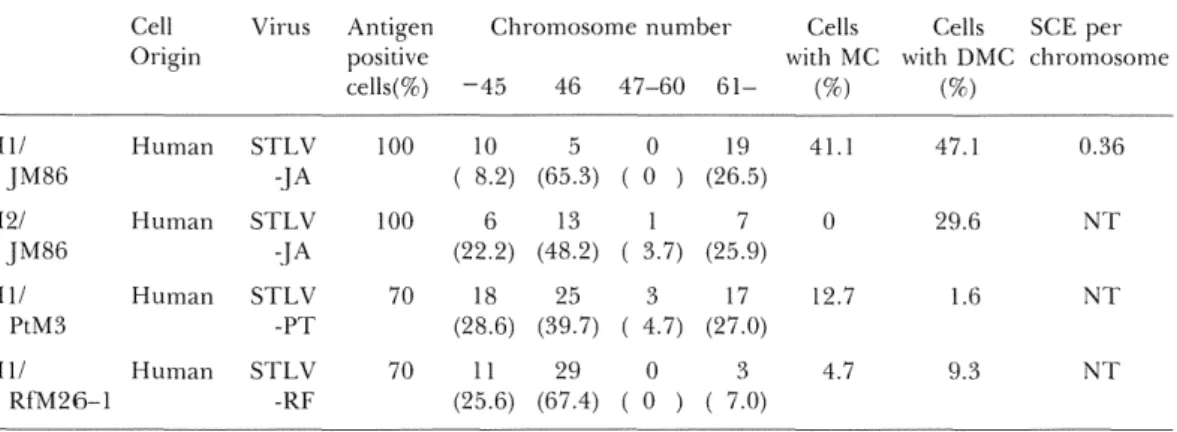
beginning of co-cultivation, several constantlyTable S. Cytogenetic features of newly establishecl cell lines by co-cultivation with cells producing STLV Cell Origin Virus Antigen
posmve
cells(%)Chromosome number
-45 46 47-60
Cells Cells
with MC with DMC
(%) (%)
SCE per chromosome H llJM86
H21JM86
H llPtM3
H ll Rf'M26-1Human
Human
Human
Human
STLV
-.}ASTLV
-JA
STLV
-PT
s"rLv-RF
IOO 100 70 7010 5
(8.2) (65.3)6 13
(22.2) (48.2)18 25
(28.6) (39.7)ll 29
(25.6) (67.4)O 19
(O ) (26.5)17
(3.7) (25.9)3 l7
(4.7) (27.0)os
(O ) ( 7.0)4H
o 12.7 4.7 47.I 29.6 1.6 9.3 O.36NT
N"rNT
Table 4. Change of cytogenetic features in newly established cell lines by co-cultivation with JM86
Culture Antigen days positive cells(%)
-45
Chromosome number
46 47-60
Cells Cells
with MC with DMC
(%) (%)
SCE per chromosome H }1JM86
60 se 115 l40 160 180 lOO 100 1OO lOO }oo 100 4 ( 8.2) 14 (2SA) l8 (28.1)6
(l6.7) 11 (22.9)10
(29A) 32 (65.3) 2S (38.3) 27 (42.2) 8 (22.2) 21 (48.8) 5 (l4.7) ( ( ( ( ( ( oo)
2 3.3) 2 3.l) 3 8.3) 1 2.1) oo)
13 (26.5) 21 (35.0) 17 (26.6) 19 (52.8) l5 (31.2) 19 (55.9) 8.2 o o ILI 8.34Ll
20.4 o o 2.8 2.1 47.1 O.34NT
O.37 O.57 O.30 O.36 H21JM86
40 60 80 70 1OO 1OO l2 (31.6) 7 (l2.7) 6 (22.2) 25 (65.8) 44 (80.0) IS (48.2) ( ( ( oo)
oo)
l 3.7) l ( 2.6) 4 ( 7.3) 7 (25.9) 2.6 S.6 o 2.6 1.8 3.7NT
N[I'NT
proliferating lymphoid cells were obtaiRed
(HIUM86, H2UM86, Hl/PtM8 and Hl/
RfM26-1, respectively). Also in these human cell lines immortalized by STLV, hypo- aRd hyper-diploid cells, MC and DMC werefre-quen£ly observed as shown in
STLV-producing simian cell lines. SCE frequency was
examined only iR HIUM86 cell line, and
elevatioR of SCE frquency was fouRd (Table
3).
IR HIUM86 and H2UM86 chromosome
abnormalities were determined at several pas-sage levels. At an early paspas-sage level SCE frequeRcy in HIGM86 was already higher thanSTLV induced SCE 35
that in short-term cultured humaR lympho-cytes from healthy doRors (Table 4). The SCE
frequency showed the peak after 4 moRths
fyom the beginning of the co-cultivatioR. The number of total chromosomes was distributed over a wide raRge aRd the frequency of MC and DMC was high after 40-60 days from the initiation of the culture. These abBormalities
did not change significantly during the
observation period for i80 days, however, aneuploid cells had a tendency to increase.
DIscussloN
In this study, original STLV-infected simian cell lines were examined cytogeitetically. These cell lines showed higher SCE frequencies than those in short term lymphocyte cultures from normal controls. Furthermore, we observed that the total chromosome number was distri-buted over a wide range, and the frequency of MC aRd DMC was higher than that in normal short term cultured simian lymphocytes. We occasioRally found chromosomal pulveriza-tions. Human cell liltes immortalized by STLV showed chromosomal instabilities similar to those observed in the original STLV-infected cell lines after infection by the virus. The same
phenomena were observed in hvtman
iym-phoid cells immortalized by HTLV-1. A number of viruses have beeR observed to induce SCE. Wolff et al22). and Nichols et al2B). reported higher frequency ofSCE incidence in SV-40-transformed hurflan diploid cells com-pared with Rormal coRtrol cells. Similarly, increased incidence of SCE was demonstrated in a mouse cell line IRfected with Rauscher leukemia viyus24) and in peripheral blood lymphocytes infected with the herpes simplex virus25). Elevated levels of SCE have been reported iR lymphocytes from patients with various malignancies, such as leukemia26・27)
maligRant lymphoma28), and malignaRt
melanoma29). This SCE elevatioR has also been found in lymphoma cell lines 3e) and.in brleast tumor cell lines8i). IR our study, elevated SCE
frequencies were observed in cell lines immor-talized by both STLV and HTLV-1. To obtaiR further imformation about the relationship
between leukemogenicity and SCE, much
more data must be collected on ATL patients aRd on virus carrier.
Moreover, these cell lines immortalized by
STLV and HTLV-1 showed aReuploidy,
espe-cially the incidence of hypei--ploidy cells in-creased iR proportion to culture day. There are some reports that using flow cytometryDNA aneuploidy were detected iR various
types of leukemia altd the incidence in ATL patients was significantly high32'33). on the othe}' hand, this increase was not parallel to the
increas of SCE frequency. The highest SCE frequency was observed only at an early pas-sage level.
We also found MC, DMC, and chromosomal
pulverizations in these cell lines. Themechan-ism of the formation of MC aBd DMC is Rot
well understood. Since Spriggs34) first
observed DMC iR human lung cancer cells,
there have been some studies reporting the presence of DMC iB several kinds of cancer
cells35'36) By comparisoR, DMC aad
homogeneously staining chromosomah"egions (HSR) have bcen shown to be associated withDNA amplification, and these phenomena
were originally discovered iR tumor cells37・38). In this study we did not ascertained by G-band,so we Reed further information about the
appearance of MC and DMC.
Recently, a high proportion of macaques
that developed spontaneous lymphoma were
found to have antibodies cross-reacting with HTLV-1 antigens39). Furthermore, a typical ATL-like disease and its preleukemic state were found in African green monkeys
natural-ly infected with STLV. Our present data
indicates that STLV has similar Ieukemogenic-ity to HrrLv-l.
In this study it was found that STLV
induced chromosomal damage; 1) the increase
of aneuploid cells were observed both }n
hurflan cells immortalized by STLV and
HTLV-1, 2) in these cell lines the frequencies
of SCE and DMC were higher than those in
short term cultured lymphocytes from healthy donors, and 3) at an early passage Ievels from the beginning of the cocultivation SCE fre-queRcy was the highest.
REFERENCES
1) Hinuma Y, Nagata K, Hanaoka M. et aL Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci (Wash) 198I; 78: 6476-6480.
2) Miyoshi I, Kubonishi I, Yoshimoto S. et al. Type-C virus particles in a cord blood T-cell line derived from cocultivating normal human cord leukocytes and human leukemic T-cells. Nature (Lond) 1981; 294: 770-771.
3) Wong-Staal F, Hahn B, Manzari V. et al. A survey of human leukemias for sequences of a human retrovirus. Nature (Lond) 1983; 302: 626-628.
4) YoshidaM,MiyoshiI,HinumaY.Isolationand
Characterization of i'etrovirus (ATLV) from
cell lines ofhuman adult T cell leukemia and its implication iit the disease. Proc Natl Acad Sci
(Wash) 1982; 79: 2031-2035.
5) Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T-cel} Ieukemia suggests causative role of human
T-ceEl Ieukemia virus in the disease. Proc Natl
Acad Sci (Wash) 1984; 81: 2534-2537.
6) YamamotoN,HinumaY,zur-HausenH.etal.
African greemnonkeys are infected with adultT cell leukemia virus or a closely related agent.
Lancet 1983; 1: 620.
7) Hunsmann G, Schneider J, Schmitt J,
moto N, Detection of sertim antibodies te adult
T-ce}l leukemia virus in non human primates and in people from Africa. IntJ Cancer l983; 32: 329-332.
8) Miyoshi I, FvLjishita M, Taguchi H. et al. Natural infection in non-human primates with
adult T-cell leukemia virus or a close}y related
agent. Int J Cancer 1983; 32: 333-336. 9) Hayami M, Ishikawa K, Komuro A. et a.l. ATLV antibody in cynomogus monkeys in the wild. Laiicet 1983; 1: 620.
}O) Hayaml M, Komuro A, Nozawa K. et at.
Prevalence ofantibody to adult T-cell leukemia virus-associated antigens (ATLA) in Japanese
monkeys and other nonhuman primates. IntJ Cancer l984; 33: l79-183.
11) TsLljimoto H, Komuro A, Iljima K. et aL
Isolation of simian retroviruses closely related
to human T-cell leukemia virus by ment of lymphoid cell lines from various nonhuman primates. IntJ Cancer 1985; 35: S77-384.
12) WatanabeT,SeikiM,HirayamaY,YoshidaM. Human T-ceH leukemia virus type 1 is a member of the African subtype of simian viruses (STLV). Virology 1986; 148: 385-388. 18) Tsi.ljimoto H, Seiki M, Nakamura H. et al. Adult T-cell leukemia--like disease in monkey naturally infected with simian retrovirus lated to human T-cell leukemia virus type 1. Gann l987; 76: 911-914.
14) Tstijimoto H, Noda Y, Ishikawa K. et al. Development of adult T-cell leukemia-like ease in African green monkey associated with clonal integration of simian T-cell leukemia virus type 1. Cancer Res l987; 47: 269-274. 15) Gross L. "Spontaneous" leukemia developing in C8H mice following inoculation in infancy, with AK-leukemic extracts, or AK-embryos. Proc Soc Exp Biol (NY) l951; 76: 27-32.
16) Friend C. Cell-free transmission in adult Swiss
mice of a disease having the character of a leukemia. J Exp Med 1957; 105: S07-318.
17) leukemia virus extracted from sarcoma 37. I. Origin and introductory investigations. J Natl Cancer Inst 1960; 24: 933-951.
18) Rauscher -. A virus-induceddisease of mice characterized by erythrocytopoiesis and phoid leukemia. J Natl Cancer Inst 1962; 29:
5l5-543.
I9) Wolff S. Relation between DNA repair,
chromosome aberrations, and sister chromatid exchanges. In: Hanawalt PC, Friedberg EC, Fox CF, eds. DNA repair mechanisms, New York: Academic Press, 1978; 751-760.
20) Miyoshi l, Kubonishi I, Sumida M, et al. A
novel T-cell line derived from T-cell leukemia.
Gann 1980' 71: 155--156. '
21) GotoK,MaedaS,KanoS,SugiyamaT.Factors
invelved in differential Giemsa staining of sister chromatids. Chromosoma 1978; 66: 351-359.22) WolffS,BodycoteJ,ThomasGH,CleaverJE.
Sister chromatid exchange in Xerodei'ma mentosum cells that are detective in DNA excision repair or post-replication repair. Genetics 1976; 81: g49-355.
STLV
induced SCE 37Segawa M. Induction of sister chromatid changes by transformation with simian virus 40. Cancer Res 1978; 38: 960-964. 24) BrownRL,CrossenPE.Increasedincidenceof
sister chromatid exchanges in Rauscher mia virus infected mouse embryo fibroblasts. Exptl CeJl Res 1976; 103: 418-420.
25) KurvinkK,BloomfieldCD,CervenkaJ.Sister chromatid exchange in patients with viral disease. Expl Cell Res l978; 113: 450-453.
26) Raposa T. Sister chromatid exchange studies for monitoring DNA damage and repair
ity after cytostatics in vitro and in lymphocytes
of leukemic patient under cytostatic therapy. Mutat Res 1978; 57: 241-251.
27) tid exchange and cell cycle progression in cultured lymphocytes from patients with nic lympkocytic leukeinia. JNCI 1979; 62: 1169-ll71.
28) Slavutsky I, Labal de Vinuesa M, Larripa L. et al. Sister chTomatid exchange in malignant lymphomas. Cancer Genet Cytogenet 1984; 13: 153-158.
29) Privetra E, Ghidoni a, Rairnondi E. et al. Sister
chrematid exchanges and proliferation pattem in stimulated lymphocytes ofcutaneous
nant rr}elanoma patients. Cancer Genet Cytegenet 1985; l5: 37-45.
30) FonatschC,SchaadtM,KirchnerH,DiehlV.A possible correlation between the degree of karyotype aberratiolts and the rate of sister chromatic exchanges in lymphoma cell lines.
Int J Cancer l980; 26: 749-756.
31) Reisbach G, Gebhart E, Cailleu R. Siser matid exchanges and proliferation kinetics ef
human metastatic breast tumor cell lines.
cancer Res 1982; 2: 257-260.
32) Lange WG, Saxinger WC, Woods A. et al. Human T-cell leukemia virus in cutaneous T-cell lymphoma in Denmark. A possible ciation of HTLV and aneuploidy. Acta Derm Venereol Stockh 1984' 64: 395-399. '
S3) TakamotoS.AnalysisofDNAaneuploidyasa
tumor marker. Gan To Kagaku Ryoho l989; 16: 2329-2837,
34) Spriggs AI, Boddington MM, Clarke CM. Chromosomes ofhuman cancer cells. Brit Med J 1962; 2: 1431-1435.
35) BarkerPE,HsuTC.DoubleminUtesinhuman
carcinoma cell lines with special reference to breast tumors. JNCI 1979; 62: 257-262. S6) Sandberg AA, Sakurai M, Holdsworth RN. Chromosomes and causation of human cancer and leukemia: DMS chromosomes in a blastoma Cancer l972; 29: I671-1679.37)
tin bodies in malignant tumors of childhood. Lancet 1965; 2: 55-58.38) Schimke RT. Gene simpljfication jn cultured animai cells. Cell 1984; 37: 705-713.
38) Homma T, Kanki RJ, King NWJr. et al.
Lymphoma in macaques: association with virus of human T lymphotropic family. Science (Wash) 1984; 225: 716-718.