Intracellular Signaling and Driving Mechanisms
Underlying Neuronal Growth Cone Guidance
by
Rurika Itofusa
A thesis submitted to
Graduate School of Life Sciences,
Tokyo University of Pharmacy and Life Sciences
in partial fulfillment of
the requirements for the degree of
Doctor of Philosophy, Life Sciences
Thesis Committee Chair:
Hiroyoshi Miyakawa
iii
This thesis is submitted to Graduate School of Life Sciences, Tokyo University of Pharmacy and Life Sciences, in order to fulfil the requirements for the degree of Doctor of Philosophy, Life Sciences. All the work has been performed at Laboratory for Neuronal Growth Mechanisms, RIKEN Brain Science Institute during the period from April 2005 to March 2015.
iv
Degree Recipient:
Rurika Itofusa
1999-2002: Bachelor of Life Sciences, School of Life Sciences, Tokyo University of Pharmacy and Life Sciences, Japan
2003-2004: Master of Life Sciences, Graduate School of Life Sciences, Tokyo University of Pharmacy and Life Sciences, Japan
2005-2014: Technical Staff, Laboratory for Neuronal Growth Mechanisms, RIKEN Brain Science Institute, Japan
Thesis Committee Chair:
Hiroyoshi Miyakawa, Ph.D.
Professor, Laboratory of Cellular Neurobiology, Graduate School of Life Sciences, Tokyo University of Pharmacy and Life Sciences, Japan
Thesis Committee Members:
Mitsuo Tagaya, Ph.D.
Professor, Laboratory of Molecular Cell Biology, Graduate School of Life Sciences, Tokyo University of Pharmacy and Life Sciences, Japan
Shigeru Yanagi, M.D., Ph.D.
Professor, Laboratory of Molecular Biochemistry, Graduate School of Life Sciences, Tokyo University of Pharmacy and Life Sciences, Japan
Masato Tanaka, M.D., Ph.D.
Professor, Laboratory of Immune Regulation, Graduate School of Life Sciences, Tokyo University of Pharmacy and Life Sciences, Japan
Hiroyuki Kamiguchi, M.D., Ph.D.
v
Acknowledgments
I would like to express my sincere gratitude to Dr. Hiroyuki Kamiguchi, Senior Team Leader of Laboratory for Neuronal Growth Mechanisms, RIKEN Brain Science Institute, for providing me this precious study opportunity in his laboratory. He gave me constant guidance, valuable advice and suggestions.
I would especially like to express my deep gratitude to Dr. Takuro Tojima, Staff Scientist of Laboratory for Neuronal Growth Mechanisms, RIKEN Brain Science Institute. I would like to thank him for allowing me to grow as a research scientist. His meticulous guidance was invaluable throughout all stages of this work. Without his enormous contribution, elaborated guidance, immense support, detailed advice and constant encouragement, this thesis would not have materialized. There are no words to express my appreciation.
I greatly appreciate the chair of my doctoral thesis committee, Professor Hiroyoshi Miyakawa, Laboratory of Cellular Neurobiology, Graduate School of Life Sciences, Tokyo University of Pharmacy and Life Sciences, for his extended discussion and valuable comments which have contributed to the improvement of the thesis. I also appreciate members of the committee, Professors Mitsuo Tagaya, Shigeru Yanagi and Masato Tanaka, Tokyo University of Pharmacy and Life Sciences, and Dr. Hiroyuki Kamiguchi for their critical reading of the thesis.
I am deeply grateful to Dr. Yoshihisa Kudo, Emeritus Professor of Tokyo University of Pharmacy and Life Sciences, who supervised my undergraduate and master’s theses. Even after his retirement, he continues to give me lots of valuable advice and warm encouragement. I am also grateful to Professor Masamitsu Iino, Graduate School of Medicine, The University of Tokyo and Associate Professor Mitsuhiro Morita, Graduate School of Science, Kobe University, who were my mentors when I was an undergraduate and graduate student.
I want to thank Drs. Atsushi Miyawaki, Hiroyuki Katayama, James H. Keen, Mark A. McNiven, Harvey T. McMahon and Roger Y. Tsien for providing plasmid constructs used in this study. I also thank RIKEN Brain Science Institute Research Resources Center for providing experimental materials and instruments.
I thank the members of Laboratory for Neuronal Growth Mechanisms, RIKEN Brain Science Institute for their support for the study.
Finally, I would like to thank my family. Without their constant support, I would never finish this thesis.
vi
Abstract
During embryonic nervous system development, individual neurons extend axons, long and thin processes that connect to distant cells, to establish precise neuronal networks. The growth cone, a highly motile amoeboid structure at the tip of the elongating axon, navigates the axon along the correct path by sensing concentration gradients of axon guidance cues presented in the extracellular local environment (Tessier-Lavigne and Goodman, 1996). Accumulating evidence indicate that intracellular second messengers, such as Ca2+ and cyclic AMP (cAMP), play crucial roles in the control of guidance cue-induced growth cone behaviors (Tojima et al., 2011). The guidance cue gradients evoke asymmetric increase in cytosolic Ca2+ concentration, with higher Ca2+ on the side of the growth cone facing the source of the cues, regardless of whether the cues are attractive or repulsive. Such asymmetric Ca2+ signals are necessary and sufficient to trigger both attractive and repulsive growth cone turning with respect to the cues. Importantly, the distinction between attractive and repulsive Ca2+ signals is the occurrence of Ca2+-induced Ca2+ release (CICR) from the endoplasmic reticulum (ER) Ca2+ store through ryanodine receptors (RyRs): Ca2+ influx through plasma membrane Ca2+ channels alone triggers growth cone repulsion, whereas the Ca2+ influx together with CICR triggers attraction (Ooashi et al., 2005). The occurrence of CICR can be controlled by cAMP signals: higher cAMP signals push the RyRs to the active state, allowing CICR, whereas lower cAMP signals inactivate the RyRs (Ooashi et al., 2005). In contrast to cAMP, however, less is known about the role of cyclic GMP (cGMP) in growth cone behaviors.
In the first part of this thesis (Chapter 1), I examine the role of cGMP and its upstream activator, nitric oxide (NO), in the regulation of CICR to control the directional polarity of growth cone guidance (Tojima et al., 2009). Using cultured embryonic chicken dorsal root ganglion neurons, I show that activation of the NO-cGMP pathway abolishes CICR and converts Ca2+-mediated growth cone attraction into repulsion. On the other hand, inhibition of the NO-cGMP pathway allows CICR and converts growth cone repulsion into attraction. Importantly, the NO-cGMP pathway counteracts the effect of cAMP on growth cone guidance. I also show that extracellular substrates affect the polarity of growth cone guidance via modulating cAMP and cGMP levels in an opposite manner. These results demonstrate a novel second messenger network that dictates bidirectional growth cone behaviors in response to the same guidance cues.
vii
subsequent vesicle-associated membrane-protein 2 (VAMP2)-mediated exocytosis on the side with Ca2+ elevation (Tojima et al., 2007). Furthermore, this asymmetric exocytosis is necessary for Ca2+-dependent growth cone attraction. However, it remains unknown what mechanisms drive growth cone repulsion downstream of Ca2+ signals.
In the second part of this thesis (Chapter 2), I examine the role of endocytosis in growth cone guidance (Tojima et al., 2010). Using total internal reflection fluorescence microscopy, I show that repulsive, but not attractive, Ca2+ signals induce an asymmetry in the frequency of clathrin-mediated endocytosis across the growth cone. I also show that pharmacologic or genetic inhibition of clathrin-mediated endocytosis abolishes Ca2+-dependent growth cone repulsion, but not attraction. My results, along with our previous report (Tojima et al., 2007), demonstrate that growth cone attraction and repulsion are driven by opposite membrane trafficking events: plasma membrane addition and removal, respectively (a-c in Figure).
In the last part of this thesis (Chapter 3), I further examine the antagonistic actions between exocytosis and endocytosis in determining the polarity of growth cone guidance (Tojima et al., 2014) (d in Figure). I show that growth cone turning direction depends on imbalance between VAMP2-mediated exocytosis and clathrin-mediated endocytosis on one side of the growth cone. I also identify the signaling pathways that regulate such localized imbalance between exocytosis and endocytosis downstream of Ca2+ signals. Repulsive Ca2+ signals facilitate clathrin-mediated endocytosis through the Ca2+/calmodulin-dependent protein phosphatase calcineurin and a 90-kD splice variant of phosphatidylinositol-4-phosphate 5-kinase type-1γ (PIPKI90). In contrast, attractive Ca2+ signals facilitate exocytosis but suppress endocytosis through Ca2+/calmodulin-dependent protein kinase II and cyclin-dependent kinase 5 that can inactivate PIPKI90. My results illustrating the antagonistic effects between exocytosis and endocytosis imply that endocytic and exocytic membrane vesicles carry functionally similar cargo molecules such as cytoskeletal and adhesion components.
viii
Figure. Localized imbalance between exocytosis and endocytosis steers the growth cone (a) In the absence of guidance cues, the growth cone migrates straight because the activities of
exocytosis (red line) and endocytosis (blue line) are kept symmetric across the growth cone width.
(b) The attractive cue (pink) evokes Ca2+ influx together with CICR and thereby activates exocytosis on the side of the growth cone facing the cue, while endocytosis remains symmetric. Such localized predominance of exocytosis over endocytosis causes attractive turning toward the cue. (c) The repulsive cue (blue) evokes Ca2+ influx without CICR and thereby causes endocytosis predominance on the side facing the cue, resulting in repulsive growth cone turning. (d) Application of CaMKII or Cdk5 inhibitor leads to balanced activation of both exocytosis and endocytosis on the side facing the attractive cue. Such asymmetries in exocytosis and endocytosis with the same polarities cause straight migration even if the cue is attractive.
References
Itofusa R, Kamiguchi H (2011) Polarizing membrane dynamics and adhesion for growth cone navigation. Molecular and Cellular Neuroscience 48:332-338.
Lowery LA, Van Vactor D (2009) The trip of the tip: understanding the growth cone machinery. Nature Reviews Molecular Cell Biology 10:332-343.
Myers JP, Santiago-Medina M, Gomez TM (2011) Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions. Developmental Neurobiology 71:901-923.
Ooashi N, Futatsugi A, Yoshihara F, Mikoshiba K, Kamiguchi H (2005) Cell adhesion molecules regulate Ca2+-mediated steering of growth cones via cyclic AMP and ryanodine receptor type 3. Journal of Cell Biology 170:1159-1167.
ix
Tojima T, Itofusa R, Kamiguchi H (2009) The nitric oxide-cGMP pathway controls the directional polarity of growth cone guidance via modulating cytosolic Ca2+ signals. Journal of Neuroscience 29:7886-7897.
Tojima T, Itofusa R, Kamiguchi H (2010) Asymmetric clathrin-mediated endocytosis drives repulsive growth cone guidance. Neuron 66:370-377.
Tojima T, Itofusa R, Kamiguchi H (2014) Steering neuronal growth cones by shifting the imbalance between exocytosis and endocytosis. Journal of Neuroscience 34:7165-7178.
Tojima T, Hines JH, Henley JR, Kamiguchi H (2011) Second messengers and membrane trafficking direct and organize growth cone steering. Nature Reviews Neuroscience 12:191-203.
x
Publications
Publications included as part of this thesis
I. *Tojima T, *Itofusa R, Kamiguchi H (2009) The nitric oxide-cGMP pathway controls the directional polarity of growth cone guidance via modulating cytosolic Ca2+ signals. Journal of Neuroscience 29:7886-7897. (*These authors contributed equally) [Chapter 1]
II. *Tojima T, *Itofusa R, Kamiguchi H (2010) Asymmetric clathrin-mediated endocytosis drives repulsive growth cone guidance. Neuron 66:370-377. (*These authors contributed equally)
[Chapter 2]
III. *Tojima T, *Itofusa R, Kamiguchi H (2014) Steering neuronal growth cones by shifting the imbalance between exocytosis and endocytosis. Journal of Neuroscience 34:7165-7178. (*These authors contributed equally) [Chapter 3]
IV. Itofusa R, Kamiguchi H (2011) Polarizing membrane dynamics and adhesion for growth cone navigation. Molecular and Cellular Neuroscience 48:332-338. [Part of General Discussion]
Publications not included in this thesis
V. Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Kamiguchi H (2007) Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nature Neuroscience 10:58-66.
xi
Table of Contents
page
Acknowledgments ---v
Abstract ---vi
Publications ---x
Table of Contents ---xi
List of Figures and Tables ---xii
General Introduction ---1
Chapter 1: The nitric oxide-cyclic GMP pathway controls the directional
polarity of growth cone guidance via modulating cytosolic
Ca
2+signals ---6
Chapter 2: Asymmetric clathrin-mediated endocytosis drives repulsive
growth cone guidance ---32
Chapter 3: Steering neuronal growth cones by shifting the imbalance
between exocytosis and endocytosis ---59
General Discussion ---98
References ---109
xii
List of Figures and Tables
page
Figure 1. CAMs regulate NO and cGMP levels in neurons ---20
Figure 2. The NO-cGMP pathway controls the direction of Ca2+-induced growth cone turning ----21
Figure 3. nNOS and RyRs are distributed over the entire region of the growth cone ---23
Figure 4. nNOS is involved in the regulation of Ca2+-induced growth cone turning ---25
Figure 5. The NO-cGMP pathway regulates RyR-mediated CICR in growth cones ---26
Figure 6. Counteractive effects of NO and cAMP on Ca2+-induced growth cone turning ---28
Figure 7. The NO-cGMP pathway controls the direction of NT-4-induced growth cone turning ---29
Figure 8. An extracellular gradient of NT-4 evokes asymmetric Ca2+ signals across the growth cone ---30
Figure 9. A model of bidirectional growth cone turning controlled by cyclic nucleotides and extracellular substrates ---31
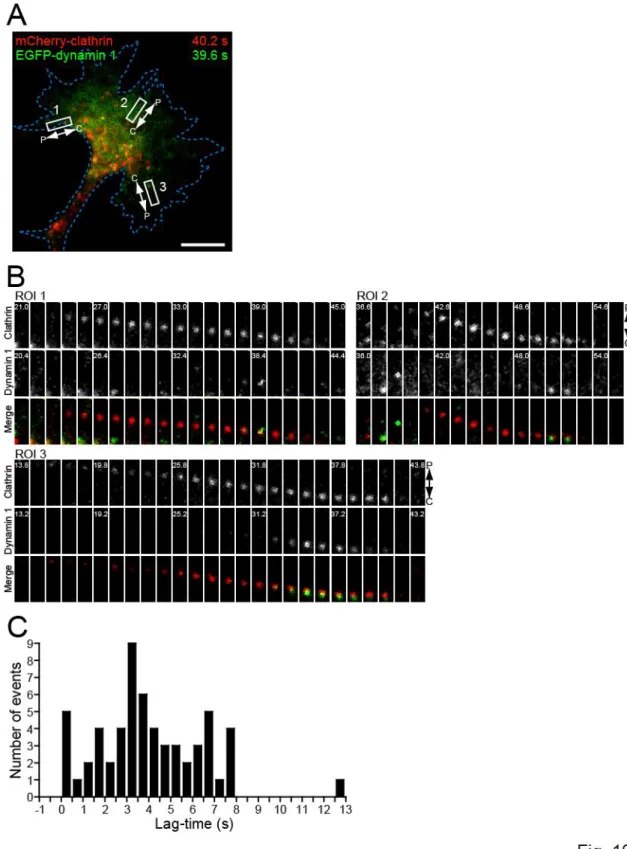
Figure 10. Visualization of clathrin- and dynamin 1-mediated endocytosis in growth cones ---45
Figure 11. Repulsive, but not attractive, Ca2+ signals induce asymmetric clathrin-mediated endocytosis across the growth cone ---47
Figure 12. Effects of endocytosis inhibitors on dynamics of clathrin and dynamin 1 puncta in growth cones ---49
Figure 13. Ca2+-induced repulsion but not attraction requires clathrin-mediated endocytosis ---51
Figure 14. Sema3A-induced repulsion requires clathrin-mediated endocytosis downstream of Ca2+ signals ---53
Figure 15. MAG-induced repulsion but not attraction requires clathrin-mediated endocytosis downstream of Ca2+ signals ---55
Figure 16. Asymmetric endocytosis/exocytosis across the growth cone is sufficient to trigger its turning ---57
Figure 17. Schematic of proposed signaling pathways for Ca2+-inducedgrowth cone turning ---75
Figure 18. Alignment of amino acid sequences of PIPKI---76
Figure 19. Embryonic DRG neurons express PIPKI---78
Figure 20. PIPKI90 mediates asymmetric endocytosis induced by repulsive Ca2+ signals ---80
Figure 21. PIPKI90 mediates Ca2+-induced growth cone repulsion ---82
Figure 22. Attractive Ca2+ signals suppress endocytosis via CaMKII and Cdk5 ---84
Figure 23. CICR is independent of CaMKII and Cdk5 ---86
Figure 24. Exocytosis-endocytosis imbalance underlies Ca2+-induced growth cone guidance ---88
Figure 25. The involvement of CaMKII, Cdk5 and membrane trafficking in MAG-induced growth cone turning ---90
Figure 26. Attractive MAG gradients suppress endocytosis via CaMKII and Cdk5---92
xiii
Figure 28. Exocytosis-endocytosis imbalance is necessary for growth cone turning induced by
asymmetric membrane perturbation ---95
Figure 29. Exocytosis-endocytosis imbalance underlies bidirectional growth cone steering ---96
Figure 30. Spatially regulated endocytosis and exocytosis in navigating growth cones ---105
Figure 31. Trafficking of the cell adhesion molecule L1 ---106
Figure 32. The distinction between attractive and repulsive Ca2+ signals ---107
Figure 33. Patterning growth cone adhesion by coordinated membrane trafficking ---108
Table 1. Combinations of culture substrates and pharmacological drugs to generate attractive or repulsive Ca2+ signals ---43
1
General Introduction
To create precise and complex neural circuits in the developing nervous systems, the growth cones, highly motile amoeboid structures at the tips of elongating axons, migrate along the correct path toward their appropriate target cells. On their routes to final targets, growth cones change their direction of migration by sensing extracellular gradients of axon guidance cues secreted by their intermediate and final targets (Tessier-Lavigne and Goodman, 1996; Chao et al., 2009; O'Donnell et al., 2009). A large number of axon guidance cues have been identified so far. Netrins, Semaphorins, Ephrins and Slits are classic guidance cue families discovered in the 1990s (Tessier-Lavigne and Goodman, 1996; Dickson, 2002; Huber et al., 2003). It is also known that morphogen families such as bone morphogenetic proteins (BMPs), Wnts and Hedgehog are reused to guide axons in later developmental stages (Yam and Charron, 2013). More recently, many extracellular molecules such as repulsive guidance molecule (RGM) (Monnier et al., 2002), Draxin (Islam et al., 2009), Engrailed-2 (Brunet et al., 2005), glial-cell-line-derived neurotrophic factor (GDNF) (Kramer et al., 2006), vascular endothelial growth factor (VEGF) (Erskine et al., 2011; Ruiz de Almodovar et al., 2011) and endocannabinoids (Berghuis et al., 2007) have been included in the list of guidance cues. It was originally thought that guidance cues can be categorized into two separate classes: attractive or repulsive cue. However, it is now evident that most guidance cues can act bidirectionally, i.e., the same guidance cue attracts or repels the growth cone depending on the circumstances such as types of axons, developmental stages and surrounding extracellular microenvironments. This is reasonable because individual growth cones must interpret guidance information differentially to choose their own routes to the final targets. More interestingly, after passing through an intermediate target, growth cones need to switch their responsiveness to the cue derived from the intermediate target from attraction into repulsion (Shewan et al., 2002). These findings indicate clearly that growth cones decide their turning direction to the cues through their intracellular signaling and driving machineries.
Second messenger signaling in growth cone guidance
2
elevations. Ca2+ imaging experiments showed that these guidance cue gradients evoke asymmetric Ca2+ signals, with higher amplitude Ca2+ elevations on the side of the growth cone facing the source of the cues, regardless of whether the cue is attractive or repulsive. Furthermore, even in the absence of extracellular cues, direct elevation of Ca2+ concentration by photolysis of caged Ca2+ compounds on one side of the growth cone triggers either growth cone attraction or repulsion, depending on the culture conditions (Zheng, 2000; Ooashi et al., 2005). These results indicate that asymmetric Ca2+ signals across the growth cone are required and sufficient for both growth cone attraction and repulsion.
Here, one important question is what constitutes the difference between attractive and repulsive Ca2+ signals. The majority of guidance cues primarily elicit opening of Ca2+ channels located on the plasma membrane, such as transient receptor potential (TRP) channels, voltage-dependent Ca2+ channels (VDCCs) and cyclic nucleotide-gated ion channels (CNGCs). This opening mediates “Ca2+ influx” into the cytosol from the extracellular space. In some conditions, the primary Ca2+ influx is further amplified by secondary “Ca2+ release” from the endoplasmic reticulum (ER) Ca2+ store through ryanodine receptors (RyRs) and IP3 receptors
(IP3Rs) (Berridge et al., 2003). Importantly, with the use of direct local Ca2+ manipulation, our
laboratory demonstrated that attractive Ca2+ signals consist of primary Ca2+ signals and secondary Ca2+ release, whereas repulsive Ca2+ signals contain primary Ca2+ signals only (Ooashi et al., 2005). This distinction is also applicable to growth cone guidance induced by physiological cues. For example, netrin-1-induced attraction is mediated by canonical TRP (TRPC) channels, L-type VDCCs and RyRs, whereas netrin-1-induced repulsion is mediated only by TRPC and L-type VDCCs (Hong et al., 2000; Wang and Poo, 2005). In addition, NGF- and BDNF-induced attraction are mediated by IP3Rs (Li et al., 2005; Akiyama et al., 2009), and Sema3A-induced
repulsion is mediated by CNGCs (Togashi et al., 2008).
Many previous studies showed that manipulation of cAMP signaling can switch the polarity of Ca2+-mediated growth cone guidance (Song and Poo, 1999). For example, growth cone attraction induced by netrin-1 (Ming et al., 1997), NGF (Ming et al., 1999), BDNF (Song et al., 1997) and MAG (Tojima et al., 2007) are converted into repulsion by suppressing cAMP signals. On the other hand, growth cone repulsion induced by MAG is converted into attraction by activating cAMP signals (Song et al., 1998). Such cAMP signals can modulate the activity of ER Ca2+ channels to switch the polarity of Ca2+-mediated growth cone guidance: higher cAMP signals push the channels to the active state, allowing secondary Ca2+ release, whereas lower cAMP signals inactivate the channels (Nishiyama et al., 2003; Ooashi et al., 2005; Akiyama et al., 2009). The cAMP levels in growth cones can be regulated by many factors, such as extracellular substrates (Hopker et al., 1999; Ooashi et al., 2005), neurotrophins (Gao et al., 2003), electrical activities (Ming et al., 2001) and developmental stages (Shewan et al., 2002). Therefore, it is most likely that growth cones choose their turning direction to the same cue depending on multiple environmental contexts that set cAMP levels.
3
signals counteract cAMP signals to establish axon-dendrite polarity in immature neurons (Shelly et al., 2010). In axonal growth cones, activation of cGMP signals antagonizes cAMP signals and thereby converts netrin-1-induced attraction into repulsion (Nishiyama et al., 2003). However, it remains obscure how cGMP signals affect cAMP and Ca2+ signals to control growth cone guidance and how cGMP levels in growth cones are set by environmental factors.
In Chapter 1 of this thesis, I examine the crosstalk between second messengers, Ca2+, cAMP and cGMP, in growth cone guidance (Tojima et al., 2009). In particular, I concentrate my effort on the role of cGMP and its upstream activator, nitric oxide (NO), in the regulation of Ca2+-induced Ca2+ release (CICR) through RyRs to switch the directional polarity of growth cone guidance.
Cytoskeletal dynamics in growth cone guidance
Next important question is what mechanisms act downstream of second messengers to drive growth cone guidance. Growth cone motility depends critically on dynamic remodeling of cytoskeletons, filamentous actin (F-actin) and microtubules (Lowery and Van Vactor, 2009; Dent et al., 2011). Earlier studies showed that both F-actin and microtubule dynamics are necessary for growth cone turning induced by guidance cues (Zheng et al., 1996; Buck and Zheng, 2002). Furthermore, even in the absence of any extracellular cues, local manipulation of cytoskeletal dynamics is sufficient to cause growth cone turning: the growth cone turns toward the side with stabilized cytoskeletons or away from the side with destabilized cytoskeletons (Buck and Zheng, 2002; Yuan et al., 2003; Koester et al., 2007; Marsick et al., 2010). Indeed, axon guidance cues induce cytoskeletal reorganization through multiple intracellular mechanisms including Rho-family GTPases such as RhoA, Rac and Cdc42 (O'Donnell et al., 2009; Hall and Lalli, 2010). For example, the semaphorin receptor plexin-B mediates repulsive guidance most likely by suppressing Rac and activating RhoA (Hu et al., 2001). BDNF-induced attraction depends on Cdc42, whereas lysophosphatidic acid-induced repulsion requires RhoA (Yuan et al., 2003).
Ca2+ signals can affect growth cone motility via regulating the activity of Rho-family GTPases. Ca2+/calmodulin-dependent protein kinase II (CaMKII)-mediated phosphorylation of Tiam1, a Rac1 guanine nucleotide exchange factor (GEF), enhances its GEF activity (Fleming et al., 1999). Ca2+ release from the ER evoked by attractive guidance cues leads to the activation of Rac and Cdc42 (Jin et al., 2005). CICR activates Cdc42 and inactivates RhoA, and CICR-mediated growth cone attraction requires Cdc42 but not RhoA activity (Jin et al., 2005). These results strongly suggest that, downstream of Ca2+ signals, Rho-family GTPases regulate cytoskeletal assembly and disassembly to drive bidirectional growth cone guidance.
Adhesion dynamics in growth cone guidance
4
turning away from the side with reduced 1-integrin function (Hines et al., 2010). These results strongly suggest that growth cone guidance depends on asymmetric changes in growth cone-matrix adhesiveness. Furthermore, accumulating evidence indicate that SFKs and FAK are involved in growth cone guidance in vitro (Li et al., 2004; Liu et al., 2004; Ren et al., 2004; Goh et al., 2008; Yam et al., 2009) and in vivo (Robles and Gomez, 2006; Woo et al., 2009). The attractive cues netrin-1 and BDNF increase SFK-dependent tyrosine phosphorylation at the tip of growth cone filopodia (Robles et al., 2005). Sonic hedgehog-induced growth cone attraction depends on asymmetric SFK-mediated tyrosine phosphorylation, and SFK inactivation on one side of the growth cone is sufficient to trigger its turning toward the side with higher SFK activity (Robles et al., 2005; Yam et al., 2009). These findings strongly support the notion that asymmetric changes in growth cone adhesiveness during turning are mediated by FAK-SFK phosphotyrosine signaling.
Ca2+ signals can regulate cell-matrix adhesion via the FAK-SFK pathway. In non-neuronal cells, CaMKII promotes adhesion turnover and cell motility through tyrosine dephosphorylation of FAK (Easley et al., 2008). In growth cones, activation of the Ca2+-calcineurin pathway leads to disappearance of phosphorylated FAK and cell detachment (Conklin et al., 2005). Moreover, asymmetric generation of filopodial Ca2+ transients activates the Ca2+-dependent protease calpain and thereby promotes repulsive growth cone turning (Robles et al., 2003).
Membrane trafficking in growth cone guidance
As described above, it is now evident that cytoskeletal and adhesion dynamics plays critical roles in the regulation of growth cone guidance. In addition to these well-established mechanisms, it is plausible that membrane trafficking system controls growth cone motility (Pfenninger, 2009). Axon growth requires an increase in the surface area of the neuronal cell. Earlier studies showed that exocytic fusion of membrane precursor vesicles occurs frequently in growth cones (Craig et al., 1995; Dai and Sheetz, 1995). These findings raised a possibility that localized facilitation of exocytic membrane addition causes growth cone turning. Recently, our laboratory showed that growth cone attraction is driven by asymmetric exocytosis across the growth cone (Tojima et al., 2007; Akiyama and Kamiguchi, 2010). Attractive Ca2+ signals facilitate microtubule-dependent centrifugal transport of intracellular vesicles toward the growth cone leading edge and subsequent vesicle-associated membrane-protein 2 (VAMP2)-mediated exocytosis on the side with Ca2+ elevation. Furthermore, blockade of VAMP2-mediated exocytosis abolishes growth cone attraction. Importantly, we found that repulsive Ca2+ signals do not facilitate asymmetric vesicle transport, and growth cone repulsion is not abolished by blocking exocytosis (Tojima et al., 2007). These results imply that growth cone repulsion is driven by unidentified machinery other than exocytic membrane trafficking.
5 repulsion.
In Chapter 2 of this thesis, I examine the driving machinery for growth cone repulsion. I show that repulsive Ca2+ signals facilitate asymmetric clathrin-mediated endocytosis, and growth cone repulsion but not attraction is abolished by inhibiting endocytosis (Tojima et al., 2010). In
Chapter 3 of this thesis, I further examine the antagonistic actions between exocytosis and
endocytosis in determining the polarity of growth cone guidance (Tojima et al., 2014). I also identify Ca2+-dependent signaling pathways that regulate localized imbalance of exocytosis and endocytosis in the growth cone. Taken together with previous reports from our laboratory (Ooashi et al., 2005; Tojima et al., 2007; Akiyama et al., 2009; Akiyama and Kamiguchi, 2010), my research creates a novel mechanistic concept for bidirectional growth cone guidance, in which polarized membrane trafficking acts, downstream of second messengers, as an instructive machinery to spatially localize steering apparatus such as cytoskeletal components and adhesion molecules (Itofusa and Kamiguchi, 2011; Tojima et al., 2011; Vitriol and Zheng, 2012) (for details, see also
6
Chapter 1
The nitric oxide-cyclic GMP pathway controls
the directional polarity of growth cone guidance
7
ABSTRACT
8
INTRODUCTION
Nitric oxide (NO), a gaseous messenger synthesized by NO synthases (NOS), is implicated in various processes of neural development, including neuronal migration (Haase and Bicker, 2003) and axon retraction (Trimm and Rehder, 2004; Stroissnigg et al., 2007). NO increases intracellular levels of cyclic GMP (cGMP) by activating soluble guanylyl cyclase (sGC) (Jaffrey and Snyder, 1995; Calabrese et al., 2007). Subsequently, cGMP activates protein kinase G (PKG) that phosphorylates many target proteins including ryanodine receptors (RyRs) (Suko et al., 1993). Dorsal root ganglion (DRG) neurons express neuronal NOS (nNOS) and PKG in early developmental stages followed by drastic downregulation of nNOS with age (Ward et al., 1994; Qian et al., 1996; Thippeswamy et al., 2005), implying a role of the nNOS-NO-cGMP signaling pathway in nascent DRG neurons. Indeed, it has been shown that genetic deletion of PKG causes guidance defects of DRG axons in the spinal cord (Schmidt et al., 2002).
The growth cone, a motile ending of an elongating axon, is guided along the correct path by various cues such as netrin-1, Semaphorin 3A (Sema3A) and neurotrophins (Tessier-Lavigne and Goodman, 1996; Song and Poo, 1999). An extracellular gradient of these guidance cues increases cytosolic Ca2+, with Ca2+ concentrations highest in the area of growth cone closest to the source of the cues (Henley and Poo, 2004; Henley et al., 2004; Nishiyama et al., 2008). These asymmetric Ca2+ elevations across the growth cone are sufficient to trigger turning to the side with higher Ca2+ (attraction) as well as to the side with lower Ca2+ (repulsion) (Zheng, 2000). It is well known that cyclic AMP (cAMP) modulates the asymmetric Ca2+ signals to determine the turning direction (Nishiyama et al., 2003; Henley et al., 2004) (Nishiyama et al., 2003; Henley et al., 2004). A previous study from our laboratory showed that high cAMP activates RyRs and thereby facilitates Ca2+-induced Ca2+ release (CICR) from the endoplasmic reticulum whereas low cAMP suppresses CICR through RyRs (Ooashi et al., 2005). It is also shown that asymmetric Ca2+ signals with and without CICR trigger growth cone attraction and repulsion, respectively. The cAMP levels in growth cones can be regulated by electrical activities (Ming et al., 2001) and environmental factors such as neurotrophins (Gao et al., 2003) and cell adhesion molecules (CAMs) (Hopker et al., 1999; Ooashi et al., 2005). While less is known about the role of cGMP in axon guidance, cGMP counteracts cAMP and converts growth cone attraction by netrin-1 to repulsion (Nishiyama et al., 2003). Nishiyama et al. (2003) also proposed that cGMP inactivates L-type voltage-dependent Ca2+ channels and RyRs, although the involvement of RyRs in cGMP-mediated regulation of axon guidance has not been tested experimentally. Furthermore, it is largely unknown how cGMP levels in growth cones can be controlled by environmental factors.
9
EXPERIMENTAL PROCEDURES Animals
Fertilized chicken eggs (Gallus gallus) were obtained from a local supplier and incubated at 38°C. Mice lacking nNOS (B6, 129S-Nos1tm1Plh) were obtained from the Jackson Laboratory and maintained at the RIKEN Brain Science Institute Animal Care Facility.
Cell culture
DRG neurons from embryonic day 9 chickens or postnatal day 0 mice were dissociated as described previously (Ooashi et al., 2005) and plated on a glass-based dish coated with laminin (approximately 10 g/ml; Invitrogen) or L1-Fc chimeric protein that consists of the ectodomain of the adhesion molecule L1 and the Fc region of human IgG (Kamiguchi and Yoshihara, 2001). The cultures were maintained in Leibovitz’s L-15 medium (Invitrogen) supplemented with N-2 (Invitrogen), 20 ng/ml nerve growth factor (NGF; Promega) and 750 g/ml bovine serum albumin (Invitrogen), in a humidified atmosphere of 100% air at 37°C.
Intracellular NO measurement
Intracellular NO was measured using the fluorescent NO indicator diaminofluorescein-FM (DAF-FM) (Kojima et al., 1999). Cultured DRG neurons were loaded with 3 or 5 M DAF-FM diacetate (Daiichi), a membrane-permeable analog of DAF-FM, for 30 min at 37°C, followed by a wash. After additional incubation for 30-60 min, fluorescence images of the neurons were acquired using a 100x objective (UPLSAPO, oil, NA 1.40; Olympus) on an inverted microscope (IX71; Olympus) equipped with a CCD camera (ORCA-ER; Hamamatsu Photonics). In some experiments, DAF-FM loading and post-incubation were performed in the presence of 50 M carboxyl-PTIO (PTIO; Dojindo) or 100 M NOC18 (Dojindo). PTIO was added 30 min before the onset of DAF-FM-loading to scavenge pre-existing NO in the cultures. For quantitative analyses, the background fluorescence was subtracted from the acquired fluorescence images, and the pixel intensities were then averaged within a growth cone or a cell body using AquaCosmos version 2.6 software (Hamamatsu Photonics).
Enzyme immunoassays
For cGMP measurements, chicken DRG neurons were cultured for 3 h on plastic culture plates (12-well, 22 mm) coated with either laminin or L1-Fc at an initial density of 1 x 106 cells/well. To block the degradation of cyclic nucleotides, the cultures were treated with the phosphodiesterase inhibitor IBMX (1 mM; Sigma) for 5 min. During this 5-min incubation with IBMX, some cultures were co-treated with either 50 M PTIO or 100 M NOC18. Intracellular cGMP concentrations were then measured using a cGMP enzyme immunoassay kit (GE Healthcare) according to the manufacture’s protocols (acetylation EIA procedure for intracellular cGMP measurement).
10
Some cultures were treated with PTIO or NOC18 for 5 min. Intracellular cAMP concentrations were measured using a cAMP enzyme immunoassay kit (GE Healthcare) according to the manufacture’s protocols (non-acetylation EIA procedure for intracellular cAMP measurement).
Growth cone turning assays
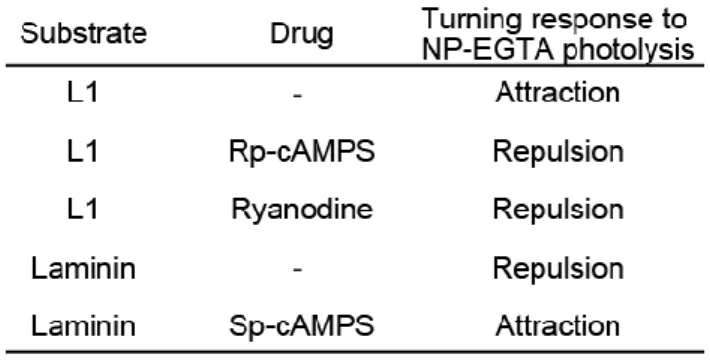
Growth cone turning was triggered by focal laser-induced photolysis (FLIP) of the caged Ca2+ compound o-nitrophenyl EGTA (NP-EGTA; Invitrogen) as described previously (Ooashi et al., 2005; Tojima et al., 2007). Spatial restriction of FLIP, as assessed by irradiating caged fluorescein maleimide (Dojindo), was approximately 1 µm in diameter in the x-y plane. Growth cone turning was triggered by a microscopic gradient of neurotrophin-4 (NT-4, 50 g/ml in pipette; PeproTech) as described previously (Song et al., 1998; Ming et al., 2001; Henley et al., 2004). In some experiments, 1 M of an acetoxymethyl (AM) ester derivative of BAPTA (BAPTA-AM; Invitrogen) was loaded into neurons at least 30 min before the turning assay experiments, as described previously (Ooashi et al., 2005). The following reagents were applied to some cultures at least 30 min before the turning assays: 100 nM L-NMMA (Tocris), 50 M PTIO, 100 M 8-bromo-cGMP (8-Br-cGMP; Calbiochem), 100 nM ODQ (Tocris), 100 M ryanodine (Latoxan), 10 nM KT5823 (Calbiochem), 100 M NOC18, 20 M Rp-cAMPS (Calbiochem), or 20 M Sp-cAMPS (Calbiochem). A higher concentration (40 M) of Sp-cAMPS was also used in Fig. 7.
Immunocytochemistry
To normalize the antigen distribution by growth cone thickness, a fixable analog of Alexa 594-conjugated dextran (MW = 10,000; Invitrogen) was pre-introduced into chicken DRG neurons by trituration loading (Nishimura et al., 2003a). The cultured neurons were fixed in fixation buffer (80 mM Na-PIPES, pH 6.9, 1 mM MgCl2, 1 mM EGTA, 1 mM GTP, 3% sucrose, 0.1%
glutaraldehyde, 4% formaldehyde) for 30 min at 37°C, permeabilized with 0.1% Triton X-100 for 60 min, and then incubated with mouse anti-nNOS monoclonal antibody (1:10, A-11; Santa Cruz Biotechnology) or rabbit anti-RyRs polyclonal antibody (1:5000, anti-C2) (Kuwajima et al., 1992) overnight at 4°C. Primary antibody binding was visualized with Alexa 488-conjugated goat anti-mouse or anti-rabbit IgG secondary antibody (1:200; Invitrogen). Ratiometric images were obtained by dividing Alexa 488 signals by Alexa 594 signals using AquaCosmos.
Imaging of FLIP-induced Ca2+ signals
FLIP-induced Ca2+ signals in a growth cone were visualized by simultaneous and ratiometric imaging of two fluorescent Ca2+ indicators, Oregon Green 488 BAPTA-1 (OGB-1; Invitrogen) and Fura Red (FR; Invitrogen), which rules out the artifactual fluorescence changes due to the growth cone movements and photo-bleaching of Ca2+ indicators (Gomez et al., 2001; Henley et al., 2004). As Ca2+ concentrations elevate, the fluorescence emission of OGB-1 increases and that of FR decreases. Cultured chicken DRG neurons were loaded simultaneously with 2 M OGB-1-AM, 2
11
observed under an inverted microscope (IX71) equipped with a 100x objective (UPLSAPO) and a CCD camera (ORCA-ER). OGB-1 and FR were simultaneously excited with a 75-W xenon lamp using an excitation filter (460-495BP; Olympus) and a dichroic mirror (72100bs; Chroma). The OGB-1 and FR emissions were split by a dichroic mirror (DM590LP; Hamamatsu Photonics) equipped in an emission splitter (W-view; Hamamatsu Photonics). The split OGB-1 and FR emissions were collected through a band pass filter (535AF45; Omega) and a long pass filter (BA610IF; Olympus), respectively. The images of OGB-1 and FR were simultaneously acquired every 22.1 ms at an exposure of 10.2 ms with CCD binning set at 8 x 8. For FLIP of NP-EGTA, five laser pulses (a pulse width of 5 ns) were shot onto a growth cone at 442-ms intervals, which corresponded to one laser pulse per 20 flames of Ca2+ imaging. The laser-shot timing was controlled by AquaCosmos and an electronic stimulator (Nihon Koden) such that a camera exposure was initiated 0.9 ms after the laser shot. This interval was sufficiently long to prevent laser-induced artifacts from affecting Ca2+ imaging (Ooashi et al., 2005).
For quantitative analyses, a region of interest (ROI; 2.6 m circular zone) was positioned within a growth cone such that the ROI was centered by the FLIP site. After background subtraction, fluorescence intensities (F) of OGB-1 and FR were averaged within the ROI. Relative fluorescence over the basal fluorescence (F/F0) was calculated individually for OGB-1 and FR
channels. Here, F0 is a mean of nine consecutive F values taken from 0 to 176.6 ms (before the
first laser shot). The F/F0 values for OGB-1 and FR channels were designated as ROGB-1 and RFR,
respectively. Changes in cytosolic Ca2+ levels were expressed as ROGB-1/RFR, where
ROGB-1/RFR= ROGB-1/RFR - 1. Positive and negative ROGB-1/RFR values indicate that Ca2+ levels
increase and decrease with respect to the basal Ca2+ level, respectively, where the basal Ca2+ level is the mean of nine flames taken from 0 to 176.6 ms (before the first laser shot). The effect of pharmacological agents on FLIP-induced Ca2+ elevations was evaluated by comparing the amplitude of ROGB-1/RFR spikes before and after 5-min treatment with the drugs in the same growth cone
(Fig. 5). The drug-induced changes in the amplitude of ROGB-1/RFR spikes were expressed as
Rafter/Rbefore, where Rbefore and Rafter indicate the mean of five peak ROGB-1/RFR values
induced by five laser pulses before and after the drug treatment, respectively.
Imaging of NT-4-induced Ca2+ signals
12
cone with 45-degree angle with respect to the original direction of axon elongation.
For quantitative analyses, two ROIs, near and far, were positioned on a growth cone as follows: a rectangle was positioned to cover the area of the growth cone perpendicular to its direction of growth, and this rectangle equally divided into three sections. The sections closer and further from the NT-4 source were then defined as the ‘near’ and the ‘far’ ROIs, respectively.
ROGB-1/RFR was used as a measure of cytosolic Ca2+ concentration.
Statistics
Data were expressed as the mean ± SEM. Statistical analyses were performed using Prism version 4.03 software (GraphPad). P values < 0.05 were judged statistically significant.
RESULTS
CAMs regulate NO and cGMP levels in DRG neurons
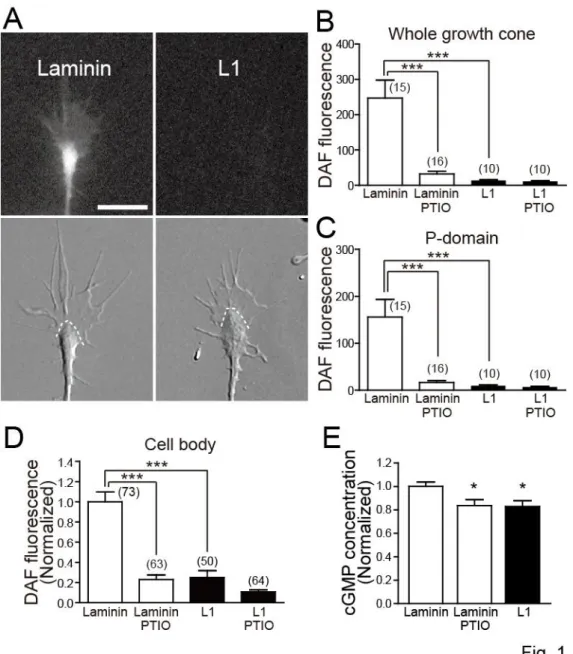
To test whether extracellular adhesive environments influence the activity of the NO-cGMP pathway, I cultured chicken DRG neurons on laminin or L1 substrate and measured intracellular levels of NO and cGMP (Fig. 1). Intracellular NO production was quantified using DAF-FM, a fluorescent NO indicator, that traps NO to yield a highly fluorescent triazole compound in a cell (Kojima et al., 1999). Because the reaction of NO with DAF-FM is irreversible, the intensity of DAF-FM fluorescence corresponds to cumulative NO production at the time of image acquisition. The intensity of DAF-FM fluorescence was higher in growth cones on laminin than those on L1, and the difference was negated by pre-treating neurons with PTIO, an NO scavenger (Fig. 1A-C). Similarly, neuronal cell bodies on laminin showed higher DAF-FM fluorescence than those on L1, and the difference was negated by PTIO (Fig. 1D). Treatment with NOC18, an NO donor, markedly increased DAF-FM fluorescence in neuronal cell bodies on L1: 1.00 ± 0.14 in untreated neurons (n = 54 cells) versus 1.98 ± 0.26 in NOC18-treated neurons (n = 53 cells), where the mean DAF-FM fluorescence intensity in untreated neurons was normalized to 1.00. The difference is statistically significant at the P < 0.01 level (unpaired t-test).
13
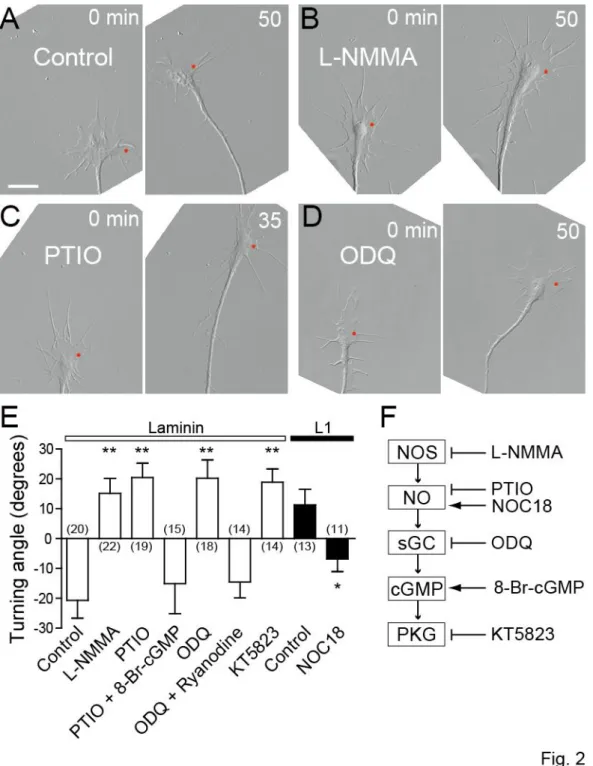
The NO-cGMP pathway controls Ca2+-induced growth cone turning
Next I examined whether the NO-cGMP pathway controls the directional polarity of Ca2+-mediated growth cone guidance (Fig. 2). Growth cone turning was triggered by producing spatially restricted Ca2+ elevations in the growth cone by FLIP of a caged Ca2+ compound, NP-EGTA compounded with Ca2+ in the cytosol (Zheng, 2000; Ooashi et al., 2005). This type of experimental manipulation has been used extensively to study causal relationships between Ca2+ and other signals in axon growth and guidance (Robles et al., 2003; Wen et al., 2004; Tojima et al., 2007). Consistent with a previous report (Ooashi et al., 2005), repetitive FLIP (3-s intervals) of NP-EGTA on one side of a chicken DRG growth cone induced bidirectional turning depending on culture substrates: turning away from the laser-shot side (referred to as repulsion) on laminin or turning toward the laser-shot side (referred to as attraction) on L1 (Fig. 2A, E). The Ca2+-induced repulsion on laminin was converted to attraction when NO signaling was blocked by bath application of either L-NMMA, an inhibitor of NOS, or PTIO (Fig. 2B, C, E). This conversion was rescued by simultaneous treatment with 8-Br-cGMP, an analog of cGMP (Fig. 2E, PTIO + 8-Br-cGMP), consistent with the idea that cGMP is a downstream effector of NO. The involvement of cGMP-associated signals in the regulation of growth cone turning was further tested using additional drugs: ODQ, an inhibitor of sGC, and KT5823, an inhibitor of PKG. Bath application of either of these two drugs converted repulsion to attraction on laminin (Fig. 2D, E). These results indicate that Ca2+-induced repulsion on laminin involves the activity of NOS-NO-sGC-cGMP-PKG pathway and that inactivation of this pathway converts the Ca2+-induced repulsion to attraction. Based on the previous finding that the turning direction of growth cones depends on the occurrence of CICR through RyRs (Hong et al., 2000; Ooashi et al., 2005), I tested whether CICR is involved in the cGMP-mediated regulation of growth cone turning. In the presence of a high dose (100 M) of ryanodine that traps RyRs in the closed state (Zucchi and Ronca-Testoni, 1997), ODQ could not convert repulsion to attraction (Fig.
2E, ODQ + ryanodine), suggesting that sGC-produced cGMP controls the turning direction via
modulating RyR-mediated CICR. I also tested whether increasing NO levels with NOC18 modulates growth cone turning responses. A previous report showed that treating Helisoma neurons with NOC7, another NO donor, inhibits neurite extension (Trimm and Rehder, 2004). In my experiments, however, bath application of NOC18 to chicken DRG neuronal cultures had no detectable effect on the rate of growth cone migration (data not shown). Repetitive FLIP of NP-EGTA on one side of a growth cone induced attractive turning on L1 (Fig. 2E). This attraction was converted to repulsion when NO levels were elevated by treatment with NOC18. Taken collectively, these results indicate that the NO-cGMP pathway plays a key role in switching the direction of Ca2+-induced growth cone turning probably by modulating the activity of RyRs.
14
= 21 wells each), where the mean cAMP concentration in untreated neurons was normalized to 1.00. Similarly, elevating NO levels with NOC18 had no effect on cAMP concentrations in neurons on L1: 1.00 ± 0.04 in untreated neurons versus 0.96 ± 0.04 in NOC18-treated neurons (n = 21 wells each). These results indicate that the NO-cGMP pathway switches the direction of growth cone turning independently of cAMP.
NO-mediated switching of growth cone turning direction depends on nNOS
There are three isoforms of NOS: nNOS, endothelial NOS (eNOS) and inducible NOS (iNOS). Previous reports showed that developing DRG neurons in vivo strongly express nNOS proteins (Qian et al., 1996; Thippeswamy et al., 2005). I examined the subcellular distribution of nNOS proteins in cultured DRG growth cones (Fig. 3). To normalize nNOS immunofluorescence by the growth cone thickness, the growth cone cytosol was co-labeled with Alexa 594-conjugated dextran. The spatial profile of the ratio of nNOS immunofluorescence to Alexa 594-dextran fluorescence represented that nNOS proteins were distributed over the entire region of growth cones on laminin and L1 (Fig. 3A). I also showed the homogenous distribution of RyR proteins in growth cones (Fig.
3B).
To test whether nNOS is responsible for producing NO that controls axon guidance, I analyzed the turning behaviors of DRG growth cones derived from an nNOS-gene knockout mouse line (Huang et al., 1993) (Fig. 4). On the laminin substrate, asymmetric Ca2+ signals generated by repetitive FLIP caused attractive turning of nNOS-deficient growth cones whereas wild-type growth cones exhibited repulsive turning (Fig. 4A, B, D). The attraction of nNOS-deficient growth cones was converted to repulsion by NOC18 treatment (Fig. 4C, D), suggesting that the lack of NO production is responsible for the altered turning behavior caused by nNOS deficiency. These data indicate that nNOS in neurons is involved in the regulation of growth cone turning induced by Ca2+ signals.
RyR-mediated CICR is regulated by the NO-cGMP pathway
15
pharmacological treatments. Consistent with a previous study (Ooashi et al., 2005), the mean amplitude of five Ca2+ elevations generated in growth cones on laminin was increased after 5-min treatment of these growth cones with Sp-cAMPS, a cAMP agonist (Fig. 5C). It was already shown that the Sp-cAMPS-induced increase in Ca2+-signal amplitude was abolished by simultaneous treatment with a high dose of ryanodine, indicating that Sp-cAMPS facilitates an additional increase in Ca2+ via RyRs, i.e., CICR (Ooashi et al., 2005). Similarly, FLIP-induced Ca2+ elevations on laminin were enhanced after treatment with either L-NMMA, ODQ or KT5823 (Fig. 5A, C). The enhancement of Ca2+-signal amplitude by these drugs was abolished by simultaneous treatment with a high dose of ryanodine (Fig. 5C, L-NMMA + ryanodine, ODQ + ryanodine, KT5823 + ryanodine), indicating that inhibition of the NO-cGMP-PKG pathway augments Ca2+ signals by facilitating RyR-mediated CICR. On the other hand, FLIP-induced Ca2+ elevations on L1 included CICR components that were blocked by inactivating RyRs (Fig. 5D). Elevating NO with NOC18 also suppressed the amplitude of FLIP-induced Ca2+ elevations on L1 (Fig. 5B, D). These results are consistent with the idea that the NO-cGMP-PKG pathway is a negative regulator of RyR-mediated CICR: high NO-cGMP activities prevent CICR, and low NO-cGMP activities allow the occurrence of CICR.
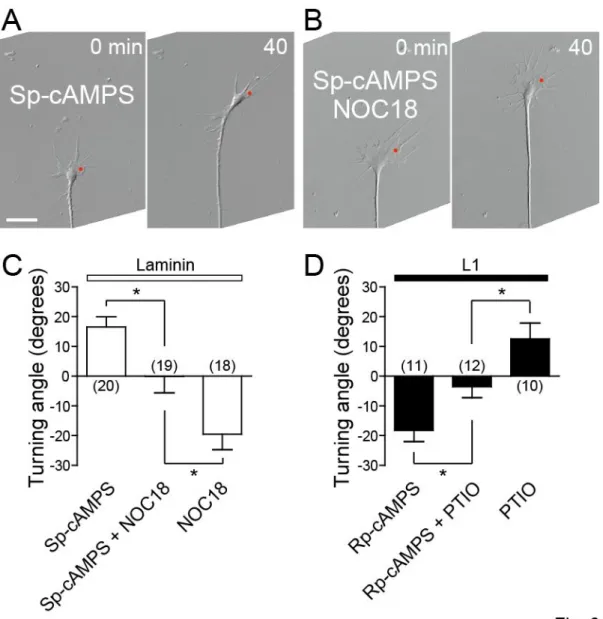
NO counteracts the effect of cAMP on growth cone turning
A previous report demonstrated that the polarity of growth cone turning induced by an extracellular gradient of netrin-1 is determined by relative activities of cAMP to cGMP, i.e., higher and lower ratio leads growth cone attraction and repulsion, respectively (Nishiyama et al., 2003). Based on this finding, I examined the counteraction between the NO-cGMP and cAMP pathways in determining the direction of growth cone turning induced by asymmetric Ca2+ signals (Fig. 6). When the NO-cGMP and cAMP pathways were simultaneously activated with Sp-cAMPS and NOC18 on laminin, repetitive FLIP of NP-EGTA on one side of a chicken DRG growth cone triggered no turning response (Fig. 6A-C), indicating the antagonizing activities of the two pathways. I also assessed growth cone turning when the two pathways were simultaneously inactivated. In the presence of both PTIO and Rp-cAMPS, a cAMP antagonist, the growth cone on laminin exhibited no turning response with respect to asymmetric Ca2+ signals (1.88 ± 2.92 degree, n = 12 growth cones), whereas the control growth cone treated with Rp-cAMPS alone exhibited repulsive turning (-12.69 ± 5.99 degree, n = 12 growth cones). The difference is statistically significant at the P < 0.05 level (unpaired t-test). This antagonism between PTIO and Rp-cAMPS was also confirmed in growth cones on L1 (Fig. 6D). Taken together, these results are consistent with the notion that relative, not absolute, activities of the NO-cGMP and cAMP pathways are the critical determinant of the directional polarity of growth cone guidance.
The NO-cGMP pathway is involved in NT-4-induced growth cone repulsion
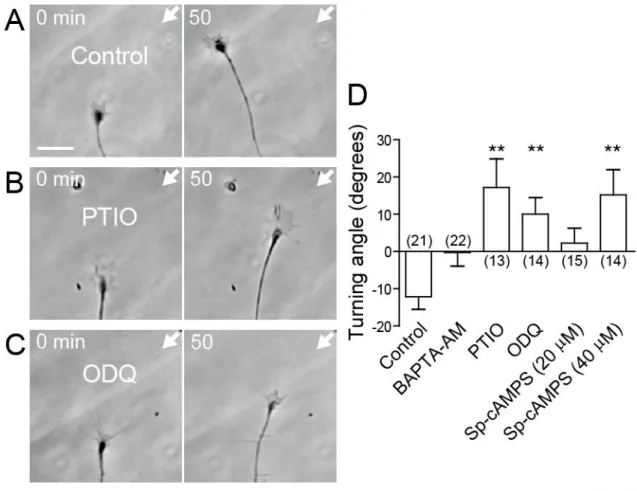
16
DRG growth cone induces growth cone collapse and axon growth inhibition (Paves and Saarma, 1997). The possibility that NT-4 acts as a guidance cue was tested by generating an extracellular gradient of NT-4 across a chicken DRG growth cone. I found that the NT-4 gradient repelled the growth cones on laminin (Fig. 7A, D). This NT-4-induced repulsion was abolished by pretreatment with a membrane-permeable Ca2+ chelator BAPTA-AM (Fig. 7D). Using simultaneous and ratiometric imaging of OGB-1 and FR, I also showed that the NT-4 gradient evoked asymmetric Ca2+ signals, with Ca2+ concentrations higher on the side of the growth cone facing the NT-4 source (Fig. 8). These results suggest that NT-4 repels the growth cones via asymmetric Ca2+ signals. I then examined whether the NO-cGMP pathway is involved in NT-4-induced repulsion. Bath application of PTIO or ODQ converted the NT-4-induced repulsion to attraction (Fig. 7B-D), indicating that the activity of NO-cGMP signaling is a critical determinant of whether NT-4 attracts or repels the growth cones. The effect of cAMP on NT-4-induced repulsion was also tested (Fig.
7D). Bath application of Sp-cAMPS (20 M) caused the growth cones to migrate practically straight in the NT-4 gradient. When I applied a higher concentration of Sp-cAMPS (40 M), the growth cones were attracted by the NT-4 gradient. These results are consistent with the notion that the directional polarity of NT-4-induced growth cone turning is controlled by the NO-cGMP and cAMP pathways in a counteractive manner.
DISCUSSION
In this chapter, I demonstrate that endogenous NO in DRG neurons is involved in repulsive axon guidance and that extracellular substrates influence the turning responses of the growth cone via modulating the NO-cGMP pathway. The counteraction of the NO-cGMP and cAMP pathways determines the occurrence of RyR-mediated CICR, thereby switching the direction of growth cone turning with respect to asymmetric Ca2+ elevations. My data also suggest that this regulatory mechanism plays a role in growth cone turning induced by an extracellular gradient of a physiological cue, NT-4.
17
the efficiency of RyR-mediated CICR via the two counteractive pathways. Such mechanisms may operate in vivo, for example, when a growth cone is migrating through an intermediate target that secretes a guidance cue (Chao et al., 2009). CAMs present in the intermediate target could modulate cyclic nucleotide and Ca2+ signaling in the growth cone, thereby switching its response to the guidance cue from attraction to repulsion.
Regulation of CICR by cyclic nucleotides
There are several possible signaling cascades that link the NO-cGMP pathway with RyRs. Welshhans and Rehder (2007) reported that, downstream of the NO-cGMP-PKG pathway, cyclic adenosine diphosphate ribose regulates RyR-mediated CICR to control filopodial dynamics of
Helisoma neuronal growth cones. The crosstalk between NO-cGMP and cAMP is also possible.
For example, cGMP activates cAMP-phosphodiesterases, causing a reduction of cAMP levels (Zaccolo and Movsesian, 2007). However, my enzyme immunoassay data suggested that cAMP concentrations in chicken DRG neurons are not regulated by the NO-cGMP pathway. The most likely explanation is that PKA and PKG modulate CICR by phosphorylating RyRs (Suko et al., 1993). Although the physiological relevance of PKG phosphorylation of RyRs remains unknown, PKA phosphorylation potentiates the ion-channel activity of RyRs (Wehrens et al., 2006; Xiao et al., 2006). I propose that RyRs are the site of counteraction of the cAMP-PKA and NO-cGMP-PKG pathways for controlling the occurrence of CICR.
Regulation of NO and cyclic nucleotide levels
In this chapter, I show that laminin-mediated adhesion stimulates the production of cGMP via NO. While intracellular signals responsible for laminin-induced NO elevations remain unclear, there is one report suggesting an extracellular mechanism (Rialas et al., 2000). It showed that, in PC12 cells, bath application of a synthetic peptide, LQVQLSIR, derived from the laminin-1- globular domain elevates NO levels within seconds of treatment. Because the LQVQLSIR sequence mediates laminin binding to syndecan, a cell surface proteoglycan, it is possible that laminin increases NO levels via binding to syndecan. In contrast to cGMP, DRG growth cones on laminin contain lower levels of cAMP than those on L1 (Ooashi et al., 2005). It was reported that laminin decreases cAMP levels in Xenopus retinal growth cones via YIGSR sequence in the laminin-1-1 chain (Hopker et al., 1999), suggesting that laminin controls the amount of cAMP and cGMP via distinct binding mechanisms on the neuronal surface. Furthermore, Hopker et al. (1999) has demonstrated that the YIGSR peptide applied to developing retina causes axons to be misdirected at the optic nerve head in vivo, stressing the importance of extracellular matrices in growth cone guidance.
Involvement of NO in growth cone guidance
18
suggested by Castellani et al. (2002), in which treatment of cortical neurons with 7-nitroindazole, a NOS inhibitor, affects Sema3A-induced axon guidance. In this chapter, using nNOS-deficient neurons and various pharmacological agents, I have clearly demonstrated that endogenous NO synthesized by nNOS controls axon guidance. The nNOS activity is regulated by direct-binding of calmodulin (Su et al., 1995) and phosphorylation by Ca2+/calmodulin-dependent protein kinase II (Komeima et al., 2000). Furthermore, nNOS can be proteolytically cleaved by calpain, a Ca2+-dependent protease (Hajimohammadreza et al., 1997). Therefore, the NO-cGMP pathway can be regulated downstream of Ca2+ signals, in addition to its role upstream of CICR. The Ca2+-mediated nNOS regulation raises an intriguing possibility that neuronal electrical activity influences the turning behaviors of growth cones by changing the amount of cGMP via NO. An analogous mechanism has been reported in which cAMP acts downstream of Ca2+ for the regulation of axon guidance by electrical activity (Ming et al., 2001).
To test for the involvement of nNOS in axon guidance in vivo, I examined axon trajectories in nNOS-knockout mice at various developmental stages (embryonic day 10-13 and postnatal day 0) by whole-mount and section immunohistochemistry (data not shown). As my in vitro experiments were performed using DRG neuronal cultures supplemented with NGF, the corresponding subpopulation of DRG axons in vivo was labeled by an antibody against TrkA or calcitonin gene-related peptide, markers for the NGF-dependent nociceptive neurons. However, I found no detectable guidance errors of the DRG central and peripheral projections in nNOS-knockout mice. While subtle axon guidance defects are often undetectable in mammals, my observations suggest the presence of complex regulatory mechanisms of cyclic nucleotide signaling by various CAMs and extracellular matrices in vivo. Furthermore, the loss of nNOS could be compensated by supplying NO from different sources, e.g., by the action of eNOS or iNOS.
Control of growth cone guidance by cyclic nucleotides
Previous in vitro studies using Xenopus spinal neurons delineated two groups of diffusible guidance cues in terms of the modulatory effects of cyclic nucleotides on growth cone turning (Song and Poo, 1999). The turning responses to group I cues, such as netrin-1 and brain-derived neurotrophic factor (BDNF), depend on the cytosolic level of cAMP, whereas those induced by group II cues, such as Sema3A and neurotrophin-3, depend on the cGMP level instead. For both groups, elevating and lowering the levels of cyclic nucleotides favors growth cone attraction and repulsion, respectively. However, these grouping criteria have been revised by more recent work. For example, the growth cone turning response to netrin-1 depends not only on cAMP but also on cGMP (Nishiyama et al., 2003). This report provides evidence that the ratio of cAMP to cGMP sets the polarity of netrin-1-mediated guidance with high ratios favoring attraction and low ratios causing repulsion. This notion is consistent with my conclusion that these two cyclic nucleotides control Ca2+-mediated axon guidance in a counteractive manner.
19
pathway, indicating the antagonizing effects by these two pathways (Dontchev and Letourneau, 2002; Chalasani et al., 2003). It has also been reported that, via activating the cAMP-PKA pathway, BDNF abolishes collapse of chicken retinal growth cones induced by an exogenously applied NO donor (Gallo et al., 2002). These findings are well in agreement with my model. In Xenopus neurons, however, cyclic nucleotides regulate Sema3A-induced growth cone behaviors in different ways. Asymmetric cGMP elevations across the growth cone that are not accompanied by PKG activation mediate Sema3A-induced repulsion by triggering Ca2+ influx through cyclic nucleotide-gated channels (Togashi et al., 2008). When cGMP has activated PKG in the growth cone, PKG causes membrane depolarization and thereby switches Sema3A-induced repulsion to attraction (Nishiyama et al., 2008). The differential roles of the cGMP-PKG pathway in Sema3A-mediated axon guidance could be explained by distinct expression profiles of ion channels that are regulated downstream of PKG. In Xenopus spinal neurons, PKG causes depolarization by opening saxitoxin-sensitive Na+ channels, which stimulates Ca2+ influx through voltage-dependent Ca2+ channels leading to growth cone attraction (Nishiyama et al., 2008). On the other hand, cGMP inactivates RyRs via PKG in chicken DRG neurons, allowing the generation of Ca2+ signals without CICR that trigger growth cone repulsion.
20
Figure 1. CAMs regulate NO and cGMP levels in neurons
A, Fluorescence (upper panels) and corresponding DIC (lower panels) images of chicken DRG
21
Figure 2. The NO-cGMP pathway controls the direction of Ca2+-induced growth cone turning
A-D, Time-lapse DIC images of chicken DRG growth cones on laminin in the absence (A, control)
22
24
Figure 3. nNOS and RyRs are distributed over the entire region of the growth cone
A, B, nNOS (A) or RyR (B) immunofluorescence in chicken DRG growth cones on laminin or L1.
Alexa 594-conjugated dextran that had been introduced into growth cones was used as a measure of cytoplasm thickness. The pseudo color images show the ratio of immunofluorescence (FnNOS or FRyRs) to Alexa 594 fluorescence (Fdextran). C, Negative-control immunocytochemistry in which the
25
Figure 4. nNOS is involved in the regulation of Ca2+-induced growth cone turning
A-C, Time-lapse DIC images of DRG growth cones derived from wild-type (WT; A) or
27
Figure 5. The NO-cGMP pathway regulates RyR-mediated CICR in growth cones
A, B, Chicken DRG growth cones were loaded with NP-EGTA and two Ca2+ indicators, OGB-1 and FR. Shown are a growth cone on laminin with L-NMMA treatment (A) and a growth cone on L1 with NOC18 treatment (B). FLIP-induced Ca2+ elevations in the growth cones were quantified by ratiometric Ca2+ imaging. Before (left panels) and after 5-min treatment with the indicated drugs (right panels), the Ca2+ signals were analyzed in the same growth cones under the same FLIP conditions. The black and white images show fluorescence intensities of FR. The red spots and the black circles in the FR images represent, respectively, the sites of laser irradiation and ROIs used to quantify Ca2+-signal amplitude. The ROI was defined as a 2.6 µm-diameter-zone centered by the FLIP site. The pseudo color time-lapse images show changes in the ratio of OGB-1 to FR fluorescence intensities (ROGB-1/RFR; where R = F/F0, for details, see EXPERIMENTAL
PROCEDURES). Digits in the pseudo color images represent milliseconds after single FLIP. Scale bars, 10 m. The graphs show time-course changes in ROGB-1/RFR values averaged within
the ROI. Note that five laser pulses (red arrowheads) triggered five Ca2+ elevations (ROGB-1/RFR
spikes). The dashed lines indicate the mean of the five peak ROGB-1/RFR values before the drug
treatment (Rbefore) or the mean of those after the drug treatment (Rafter). C, D, The effects of the
indicated drugs on FLIP-induced Ca2+ elevations were evaluated in growth cones on laminin (C) or L1 (D). ‘Control’ indicates that drug treatment was omitted. Rafter/Rbefore in the y-axis
28
Figure 6. Counteractive effects of NO and cAMP on Ca2+-induced growth cone turning
A, B, Time-lapse DIC images of chicken DRG growth cones on laminin in the presence of
29
Figure 7. The NO-cGMP pathway controls the direction of NT-4-induced growth cone turning
A-C, Time-lapse phase-contrast images of chicken DRG growth cones on laminin that were exposed
30
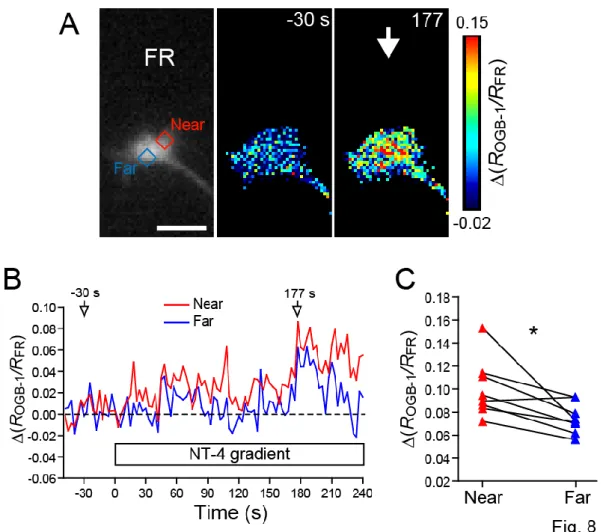
Figure 8. An extracellular gradient of NT-4 evokes asymmetric Ca2+ signals across the growth cone
A, Chicken DRG growth cone on laminin was loaded with two Ca2+ indicators, OGB-1 and FR. The left panel shows represents FR fluorescence. The red and blue rectangles indicate the near and far ROIs, respectively. The pseudo color images show changes in the ratio of OGB-1 to FR fluorescence intensities, ∆(ROGB-1/R FR). Digits represent seconds after the onset of NT-4
application. The white arrow indicates the direction of NT-4 gradient. Scale bar, 10 µm. B, Time-course changes in ∆(ROGB-1/R FR) in the near (red line) and far (blue line) ROIs drawn in (A).
The x-axis represents seconds after the onset of NT-4 application. C, NT-4 induced Ca2+ elevation was compared between the two ROIs in each growth cone (n = 8 growth cones). The y-axis indicates ∆(ROGB-1/R FR) at the time- point of peak ∆(ROGB-1/R FR)whole after the onset of NT-4
application, where ∆(ROGB-1/R FR) whole is averaged ∆(ROGB-1/R FR) over the whole growth cone.
∆(ROGB-1/R FR) in the near ROI was significantly greater than ∆(ROGB-1/R FR) in the far ROI. * P <
31
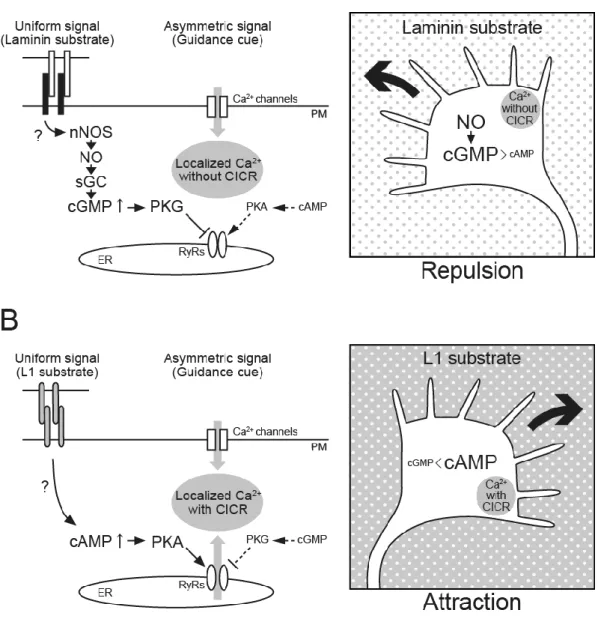
Figure 9. A model of bidirectional growth cone turning controlled by cyclic nucleotides and extracellular substrates
A, A laminin substrate, which is presented as a uniform signal, stimulates the NO-cGMP-PKG