Tech. Bull. Fac. Agr. Kagawa Univ., Vol. 36, No. 2, 117-125, 1985
SEASONAL CHANGES OF THE ACTIVITIES OF SUCROSE
SYNTHETASE AND SUCROSE PHOSPHATE
SYNTHETASE IN SUGARCANE.
Toshiyuki
MATSUI
In this paper the author reports on the seasonal changes of sucrose accumulation and the activities of sucrose syn- thetase and sucrose phosphate synthetase in stalks and leaves of the two varieties of sugarcane, Chikusha (Saccharurn sinense Roxb.) and N: Co 310 (S, oficinarum L ) The main purpose of this study is to decide the fitting season for harvest on the basis of sucrose synthetase and sucrose phosphate synthetase. Sucrose synthetase showed the highest activities at the rapidly growing period in the stalk of both varieties. It showed high activities in May, and June and August in N: Co and almost constant activities during the experimental period in Chikusha in the case of leaves. Sucrose phosphate synthetase in leaves of Chikusha showed the highest activities in May, whereas that of N: Co showed the highest activity in November The activity of sucrose phosphate synthetase in leaves was over 50 times as high as that in stalks of both varieties in June. The phenomenon agreed with the fact sucrose synthesis seemed to occur in leaves. Chikusha matured faster than N: Co.
Therefore, the sugar contents and the total weight of Chikusha gave the conclusion that the best harvesting season was in October, Furthermore, Chikusha gave lower activity of sucrose synthetase (sucrose decomposition) in the stalks in October than that in November, and almost constant activity of sucrose phosphate in September to Novem- ber. These data also supported that Chikusha should be harvested in late-October instead of mid-December. Concerning the optimum pH and temperature of both enzymes, there was no difference between N: Co and Chikusha.
*$$$%
T i t , "t I- 9 3 C D 2 &@,B E
(Saccharurn sinense Roxb.)t:
N : Co 310 (S oficinarum L.) 0 % k t 9 @ 3 T , t: s l % % k t: .;/%&&%% t: 3 % 9v@&%%%D~~I1t:tz3L~TI%TB0
ZO3aF.%DS%?$€l!& It, t: 3 % e & % t: s % 92@e&%%D%BIZ4~5L\T@%?d%@@fgJ8SkWTB
Z 2 t Z & B t: 3%&a@
% i t ,i5a"alt:
Bf
T%B&.E2fgJtZBk%B%.% L k 0 R D U e , N : CO Tit 5, 6, 8 B tZ /PIETCt%&fgJ~+i3 l3--2D%B%% L?:, B%D%D t: 3U
9 y@&R%%lt 5 tZEkk%BB% L , N : CO TItllEl T & 7 R o%
69 t: sl
9 ~%&&%%%!!ikIt, 68
l Z i S P P P I t :
Bf
D%BD5043I;A_t:T& 7 k LD%%lt, t: s U & R f i s % ~ @r
B t:c\i@%tz--%LR0/PIEIt
N : Cod:
4 9
<%%Lt:, % . = T , BED.;/ s I % f t & S f fi>%B%E@fgJ itloBt:L\i%%%@f:o S % i z ,B%ltllBd;QlOBDftzBLtBt:3I8&%%
(vEIUD%R) %Bfi$I&<,v @ e & % % % ~ t a r a k x / ~ - - ~ ~ & ~ t : ,
r ; h 5 a ~ " - a f i h 6 i t , + ~ ~ ~ 1 2 ~ + @ d : 1 ) ~ 1 0 ~ ~ ' @ i Z E @ T ~ 3 T & b 0 i 5 % % D E S pH, I E i z A L T , N : Co t$'~EtzbIt?$fi~->?:~CARDINI et al.l)z) have revealed that the synthesis and metabolism of sucrose occur by the following reaction. UDP*-glucose+fructose~ucrose+UDP [Sucrose synthetase (E. C. 2.4.1 1311
M S
UDP-glucose + fructose-6-phosphate
.
sucrose-6-phosphate + UDP [Sucrose6-phosphate synthetase (E. C. 2 4.1.14)] (2)4'5' "118 Tech.. Bull. Fac. Agr. Kagawa Univ,, Vol. 36, No. 2, 1985
Sucrose-6-phosphate+H,O~sucrose+phosphate [Sucrose-6-phosphatase (E. C. 3 1.3.2411
.
(3)2)7)8)The reactions (2) and (3) take part in the principal pathway of sucrose synthesis whereas the reaction (1) catalyses the cleavege of sucrose in higher plants. In sugarcanes (sucrose accumulating plant), the reaction (3) is very fast and non-reversible and so sucrose is accumulated. On the other hand, the sucrose breakdown also occurs by invertase9). However, there are the lack of information between invertases and sucrose synthetaseiO).
Here, the autho~ reports on the changes of sucrose synthetase and sucrose phosphate synthetase located in leaves and stalks during the vegetative period, and the difference of sugar accumulation between N: Co and Chikusha.
Materials and Methods
1. Materials. Sugarcane varieties N: Co and Chikusha were harvested in the field of Kurokawa "Wasanbon" sugar factory located in Hiketa, Kagawa prefecture on 25th of each month from May to November, 1980 Two sugarcanes of each variety were selected at random for every month.
2. Reagent. Sephadex and bovine serum albumin were obtained from Pharmacia Fine Chemicals. Lactate dehy- drogenase and pyruvate kinase were purchased from Sigma Chemical Company. Unless otherwise stated, guaran- teed grade reagents (Wako Chemical, Ltd.) were used.
3 Enzyme extraction. Each stalk and each leaf were ground in a cooled motor and pestle with twice volume of 0.1 M Tris-HC1 buffer (pH 7.5) containing 0.3 M mannitol, 10 mM MgCI,, 20 mM EDTA, and 20 mM cysteine4). The resulting homogenate was filtered through 2 layers of cotton cloth and the filtrate was centrifuged at 10,000 rpm for J O min. The supernatant was filled up to 10 ml with 0 1 M Tris-HC1 buffer (pH 7.5) and used as the crude enzyme.
4. Enzyme assay. In order to remove the disturbing substances during the assay, 2
ml
of the enzyme solution was applied to a Sephadex G-25 fine column (1.5x
18 cm), which had been equilibrated with 0.01 M Tris-HCI buffer (pH 7 0). The column was eluted with the same buffer at a flow rate of 10-12 ml per hr. Each 2 ml fraction was collected and was monitored by quantitative absorption at 500 nm for protein and reducing sugar described below. The fractions combined from tubes 3 to 5 were used for enzyme assays and the comparison of their properties. In the case of standard assay method for sucrose synthetase, the formation of sucrose was assayed spectrophoto- metrically by measuring the decrease in NADH at 340 nm, pH 7 5, and 37°C Following 3 min. preliminary incuba- tion of the sucrose synthetase in an assay mixture containing (in micromole) Tris-HC1, pH 7 5 (loo), MgCI, (5), KC1 (25), UDP-glucose (disodium salt 0.8), fructose (6), NADH (disodium salt 0.35), phosphoenolpyruvate (trisodium salt hydrate I), 0.1 unit of pyruvate kinase, and 0.5 units of lactate dehydrogenase in a total volume of 2 0 ml. The ~eaction was initiated by the addition of 0.2 ml crude enzyme. One unit of sucrose synthetase catalyzes the formation of 1 micromole of sucrose per min under these specified conditions. Distilled water was used as the blank experi- ment instead of pyruvate kinase and the other conditions were the same described aboveIn the case of the sucrose phosphate synthetase, the fructose-6-phosphate (disodium salt 6 micromole) was sub- stituted for fructose (10 micromole). Soluble protein was determined according to the method described by Lowry et a].") using bovine serum albumin as a standard.
5. Determination of sucrose and reducing sugar. An aliquot of the crude extract was added with 0 2
ml
of a saturated solution of lead acetate, followed by filtration. Then powdered sodium oxalate was added to exclude the excess lead as the precipitate. The final filtrate was used as the sample of sugar analysis. The reducing sugar in the assay was estimated by the method of Somogyiz2). To 5 0 ml of the deproteinized solution in a 10 ml flask was added 0.27 ml of concentrated hydrochloric acid, the mixture was heated at 60°C for 10 min and adjusted to 10 ml with water after neutralization by 0.5 N sodium hydroxidez3). The amount of sucrose was given as the diierence between the amount of total reducing sugar and that of reducing sugar multiplied by 0.9513).Toshiyuki M ~ r s v r : Sucrose Synthetase and Sucrose Phosphate Synthetase in Sugarcane 119
Result and Discussion
1 . Properties of sucrose synthetase and sucrose phosphate synthetase in apical leaves from sugarcane. The optimum pH values of sucrose and sucrose phosphate synthetases were 7.5 and 8.0, respectively, and the optimum temperature values were similar at 40°C Both enzymes showed the same characterization in two varieties.
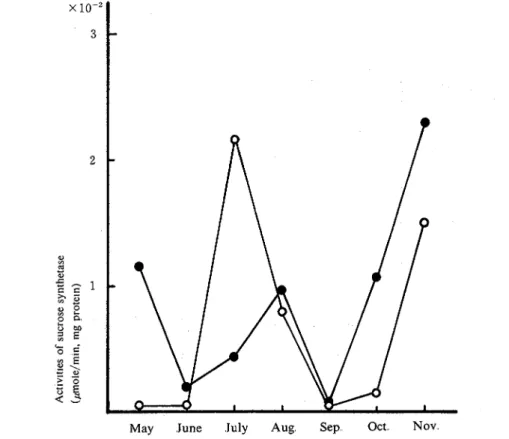
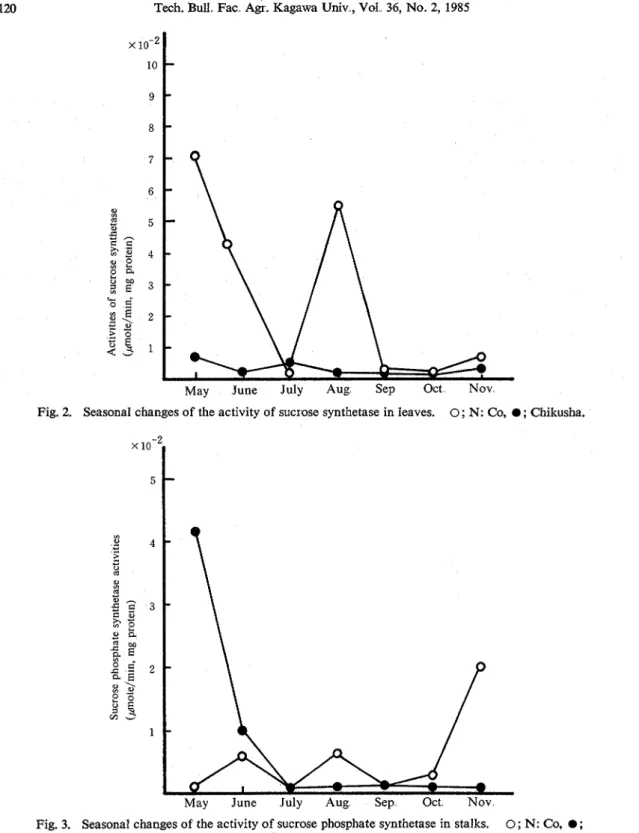
2. Seasonal changes of sucrose synthetase and sucrosephosphate synthetase from stalks and leaves. The activities of the sucrose synthetase from the stalks and leaves on average are shown in Figs, 1 and 2, respectively. In the case of sucrose synthetase in stalks N: Co showed higher activity than Chikusha only in July, whereas Chikusha showed higher activity throughout the experimental period in this study except July Sucrose synthetase was apt to give almost the same profile of activity in comparison to the acid invertase in both varieties14) whereas theacid invertase activity showed about 5 times as high as sucrose synthetase in the stalks As shown in Fig. 2, N: Co showed higher activity of sucrose synthetase in the leaf in May and June, and it increased suddenly in August, whereas Chikusha showed almost constant activity and the tendancy was similar to neutral invertase.
Figs 3 and 4 show the activities of sucrose phosphate synthetase from stalks and leaves o n average, respectively. Fig 5 shows the changes of the activities of sucrose phosphate synthetase from all parts of leaves. N: Co gave higher activity in stalks at the mature period, whereas Chikusha gave higher activity at the immature period. Sucrose phosphate synthetase in the stalks of Chikusha showed the highest activity in May, and it showed about 4-fold higher activity than that of N : Co. Sucrose phosphate synthetase in leaves of N: Co showed the activities about 5 times as high as that of Chikusha in June.
After photosynthesis thecarbohydrate moves from leaves to stalks and roots in the form of sucrose15)16). Sucrose
May .June .July Aug. Sep. Oct. NOV.
Tech. Bull. Fac.. Agr. Kagawa Univ., Vol.. 36, No. 2, 1985
Fig. 2. Seasonal changes of the activity of sucrose synthetase in leaves. 0; N: Co, 0 ; Chikusha.
Fig. 3. Seasonal changes of the activity of sucrose phosphate synthetase in stalks. 0; N: Co, 0 ;
Chikusha.
phosphate synthetase showed the highest activity in leaves in June Sucrose synthesis seemed to occur in leaves. This idea was supported by the presence of sucrose phosphate synthetase activity (sucrose synthesis activity) in leaves.
Fig. 4,
Fig. 5.
Seasonal changes of the activity of' sucrose phosphate synthetase in leaves. ; Chikusha. 5 M 2 E 7 L
-
0 E25
5 2z
$ 3
4 3 2 1 1 2 3 4 5 6 7Base Internode number TOP
Seasonal changes of the activity of sucrose phosphate synthetase in leaves of N: Chikusha (b). @; Aug
.
x ; Sep.. ; Oct,
0 ; Nov.0 ; N: Co,
122 Tech. Bull. Fac. Agr. Kagawa Univ., Vol. 36, No. 2, 1985
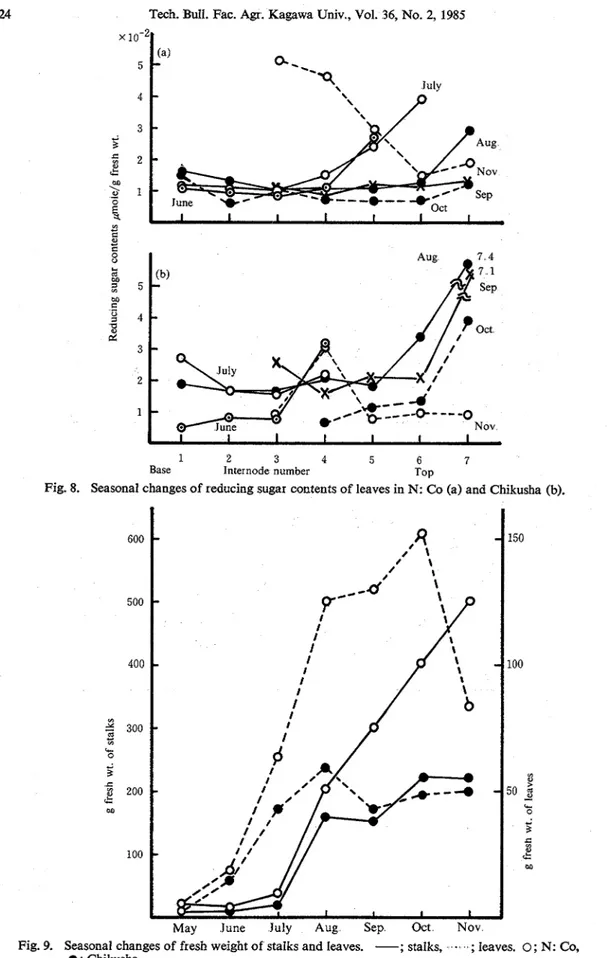
As shown in Fig. 5, the activity of sucrose phosphate synthetased in Chikusha was high at the second and/or third leaves from top in June and July, and it suddenly decreased in August. In Chikusha [Fig. 5(b)] its activity in leaves is apt to correlate with those in stalks, but in N: Co (a) it is not.
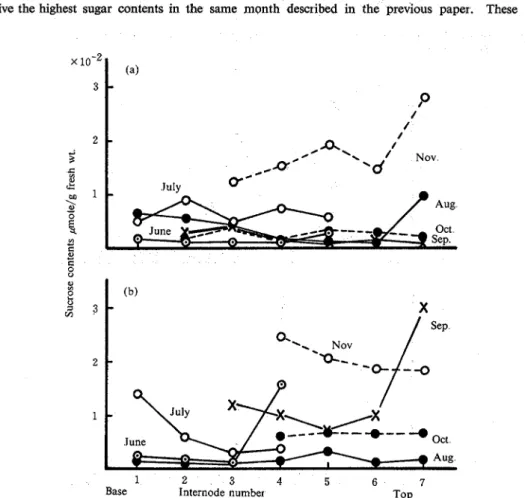
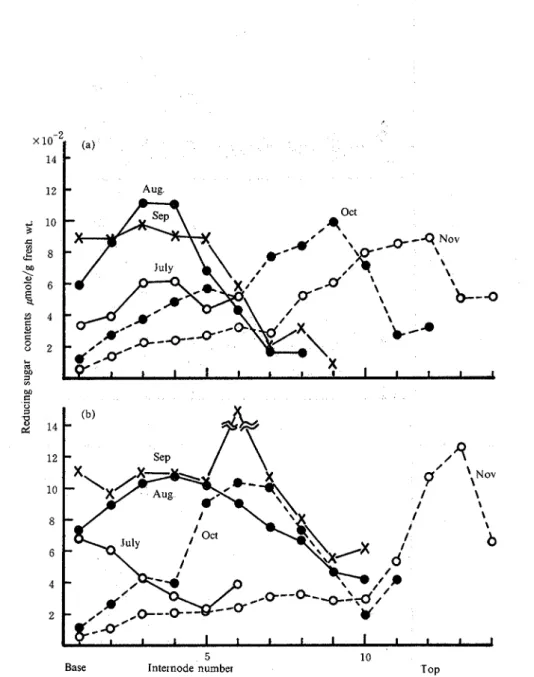
3. Seasonal changes of sucrose and reducing sugar contents in stalks and leaves. The accumulation of sucrose in leaves is shown in Fig. 6 In the previous paper14), the sugar accumulation increased from the base to the top of stalks, whereas it was apt to be higher at the top of leaves in the mature season. The leaves in both varieties began to die from the base in September. Figs 7 and 8 show the seasonal changes of reducing sugar contents of stalks and leaves, respectively. The highest accumulation of reducing sugars was transferred from the base to the top according to the growing stalks in both varieties. Though sucrose moved also from the base to the top, its high content remained in the base parts in both varieties As shown in Fig 8, Chikusha and N: Co are apt to give the highest contents of reducing sugars in the top of leaves from August to October Fig 9 shows the changes in total weight. In the previous paper, the total weight of sugarcane was shown to correspound to that of sucrose. The results in this paper were similar to those of the previous paper. N: Co was apt to show higher weight of leaves than Chikusha. The acid and neutral invertases from Chikusha showed lower activity in the stalks in October than the other variety The sugar contents and the total weight of Chikusha gave the conclusion that the best harvesting season was in October14) Therefore, it is advisable to harvest sugarcane Chikusha already in October instead of mid-December as has been usual. On the other hand, Chikusha gave lower activity of sucrose synthetase (sucrose decomposition) in the stalks in October than that in November and almost constant activity of sucrose phosphate synthetase in the harvest season. It also showed the highest weight of stalks in the same month and should give the highest sugar contents in the same month described in the previous paper. These data also
1 2 3 4 5 6 7
Base Internode number TOP
Toshiyuki MAISUI: Sucrose Synthetase and Sucrose Phosphate Synthetase in Sugarcane 123
5 10
Base Internode number TOP
Fig. 7. Seasonal changes of reducing sugar contents of stalks in N: Co (a) and Chikusha (b).
supported that Chikusha should be harvested in lateOctober instead of' mid-December.
The author wishes to thank Prof Shozaburo KIIAOKA of University of Osaka Prefecture, Prof. Sin'itir6
KAWAMURA of Kagawaken Meizen Jounior College and Dr. Kazutaka MIYATAKE of University of Osaka Prefecture, for their suggestions. He also thanks MIS Hidemi TANAKA (her single name ENOUCHI) of Kai3 Co., Ltd. for technical assistance.
Tech. Bull. Fac. Agr. Kagawa Univ., Vol. 36, No. 2, 1985
Fig. 9. Seasonal changes of fresh weight of' stalks and leaves. -; stalks, ,
."
, ; leaves. 0; N: Co,0 ; Chikusha.. x 10-2 5 4 3
$
"
2 -2
M2
-
1 -8
3 (a)-
a.
-
-
J 1 I t I 1 1 8 , d 8 2 5 - M3
4 - 3 d 3 2 1 (b)-
-
-
I I 1 I I I I 1 2 3 4 5 6 7Base Internode number TOP
Toshiyuki MAISUI: Sucrose Synthetase and Sucrose Phosphate Synthetase in Sugarcane 125
References 1) CARDINI, C. E., LELOIR, L.. F., and CHIRIBOGA, 10)
J. : J. Biol. Chem.., 214, 149 (1955)..
2) LELOIR, L., and CARDINI, C. E..: J. Biol. Cherni,., 11) 214, 157 (1955)..
3) NOMURA, T.., and AKAZAWA, T.: Arch. Bio-
chem. Bioph,ys., 156, 644 (1973). 1 2)
4) HAWKER, J. S.: Biochem. J., 105, 943 (1967).. 1 3) 5) MENDICINO, J.: J. Biol. Chem., 235,3347 (1960).
6) FEKEIE, M.. A. R..: Eur. J. Biochem.. 19, 73 14) (1971),,
7) HATCH, M. D.: Biochem. J., 93, 521 (1964). 15) 8) HAWKER, J. S.., and HATCH, M. D.: Biochem. J. 16)
99, 102 (1966)..
9) GLAZIOU, K. T. : Plant Ph,ysiol,., 35, 895 (1 960)..
MA~SUMO~O, H : Protein, Nucleic Acid and Enzyme., 24, 278 (1979).
LOWRY, 0. H., ROSEBROUGH, N. J., FARR, A. L
,
and RENDALL, R. J.: J. Biol. Chem., 193, 265 (1951).SOMOGYI, M.: J. Biol. Chem
,
195, 19 (1952). MATSUI, T., and YAMADA, K.: Eiyo To Syokuryo, 28, 371 (1975)MATSUI, T.: Nippon Shokuhin Kogyo Gakkaishi, in contribution.
SHIROYA, M : Plant Cell Physiol ,18,633 (1977)
HOUSIEY, T L., PEIERSON, D. M., and SCHRADER, L. E. : Plant Physiol., 59, 217 (1977).