EFFECTS OF α-LIPOIC ACID SUPPLEMENTATION ON ENDURANCE CAPACITY AND
ENERGY METABOLISM IN RATS
Tatsuhiro MATSUO and Kanako YAGI
Abstract
α -Lipoic acid (ALA) functions as a cofactor of mitochondrial enzymes, is closely related with mechanism of aerobic energy production. Therefore, ALA is expected to provide to improvement of endurance capacity and recov-ery of fatigue, but it is not well known that ALA has effect of energy metabolism. In this study, we investigated the promoting effects of ALA on energy metabolism in rats, used exercise time to exhaustion in loading swimming test and energy expenditure before and after exercise as indicators. Male Wistar rats were orally administrated 100mg/kg body weight of ALA for 4 weeks. The effects of ALA on exhaustion time were not observed, while resting metabolic rate was significantly increased by daily administration of ALA. Moreover, ALA increased total energy expenditure of the post-exercise, but the difference was not significant. These results suggest that ALA does not enhance endur-ance, but promotes activation of energy metabolism.
Key words:α-Lipoic acid; endurance capacity; energy metabolism; rat; swimming
Introduction
α-Lipoic acid (ALA) occurs endogenously in tissues and functions as a mitochondrial coenzyme for enzymatic reac-tions involved in the oxidative decarboxylation of α-keto acids (pyruvate dehydrogenase, α-ketoglutarate dehydroge-nase), is closely related with mechanism of aerobic energy production.(1)
It has been used as therapy for many diseases associated with impaired energy utilization. Dietary supple-mentation of ALA can act as a potent antioxidant and amelio-rate oxidative stress both in vitro and in vivo.(2−7)
Therefore, ALA is expected to promote energy production, provide to improvement of endurance capacity and recovery of fatigue. Hagen et al.(8) reported that ALA supplemented old rats
have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. These results sug-gested that ALA supplementation elevated energy production. However, research reports about the promoting effect of ALA on energy metabolism in vivo are limited, and it has not been known whether ALA supplementation influence energy me-tabolism during exercise. In this study examined the effects of ALA on endurance capacity and energy metabolism in rats. We used exercise time to exhaustion in loading swimming test and energy expenditure before and after exercise as indicators of endurance capacity and energy metabolism.
Materials and Methods
All procedures involving animals were approved by the Ex-perimental Animal Care Committee of the Kagawa University.
Animals and experimental design
Thirty-nine male Wistar rats (3 weeks old) were purchased from Japan SLC, Inc. (Shizuoka) and were acclimatized for a week under standard laboratory conditions (22 ± 1 ℃ , 50-60% humidity). The light/dark cycle was 12h with lights on from 8:00h to 20:00h. After the adaptation of a week, all rats were randomized by body weight to two groups. One group was a control (C, n=20) and the other was α-lipoic acid group (LA, n=19). Both groups were fed 5g of com-mercial rat chow (CE-2, Japan CLEA, Inc., Tokyo) twice a day (8:30-9:30h, 20:30-21:30h). All rats fed the diets isoenergetically during 4 weeks (from 4 to 8 weeks old) of experimental period. The LA group was administered orally α -lipoic acid (100mg/kg/day) dissolved in 0.2% of NaOH in physiological saline and the C group was administered orally 0.9% physiological saline at 10:30h.
Exercise time to exhaustion in loading swimming test
At the age of 6 weeks, all rats were forced to swim with loads equal to 6% of body weight attached to chest, and the
swimming time until fatigue was measured in all rats. A time point until fatigue was defined as the failure to rise to the sur-face of the water to breathe or not to move limbs below water by a modification described by Matsumoto et al.(9)
At 30, 60, 90, and 120 min after loading test, blood was collected from the tail vein to obtain plasma for analysis of plasma lactic acid concentrations. Plasma lactic acid concentration was deter-mined by enzyme method used lactate dehydrogenase by a modification described by Gutmann et al.(10)
and Wieland et
al.(11)
Energy metabolism measurement
Energy expenditure before and after exercise was mea-sured by the-breath-by-breath method, using a respiratory gas analyzer (LX-710 and G-102, Iizima Electronics Industry Corporation, Aichi, Japan) at the age of 7 weeks in all rats. The measurements of resting energy metabolism were carried out for 10 min. After all rats were forced to swim for 1h with loads equal to 4% of body weight attached to chest. Before measurements, all rats were housed quietly for 30 min in metabolimeter to adjust to measurement environment. After swimming, the measurements were carried out for 3.5h during the resting state. Respiratory quotient (RQ) and energy ex-penditure (EE) were calculated by using equations described by Weststrate.(12)
RQ = VCO2 / VO2
EE (kJ/min) = 4.184([4.686+1.096(RQ−0.707)] VO2)
Dissection of animals
At the end of experiment (8 weeks old), each group of rats was randomly assigned by body weight to two sub groups, the groups were killed before and after exercise. All rats swam for 30 min at 14:00 to adjust swimming exercise during ex-perimental period. We used an plastic pool (80×56×48 cm) filled to a depth of 40 cm with 30 ℃ water. The pre-exercise group was killed by decapitation 3.5h after administration (at 14:00h). The post-exercise group was forced to swim for 1h (from 14:00h to 15:00h) with loads equal to 4% of body weight attached to chest, and after the swimming load, rats were killed by decapitation immediately. Blood was collected to obtain serum, and liver, gastrocnemius and soleus muscles were removed immediately, frozen in liquid nitrogen, and kept at −80℃ until analysis.
Biochemical examination
Gastrocnemius muscle lactic acid content was determined
by enzyme method used lactate dehydrogenase by a modifi-cation described by Gutmann et al.(10)
and Wieland et al.(11)
Briefly, the muscle sample was added to 0.8N perchloric acid solution, then homogenized and centrifuged (14,000rpm, 1 min). The supernatant obtained was determined. Gastrocne-mius muscle lactate dehydrogenase activity was determined using the supernatant obtained via homogenized together with 0.1mol/L Tris-HCl buffer and centrifuged (14,000rpm, 1 min) by a commercial kit (lactate dehydrogenase C Ⅱ -test Wako, Wako pure chemical Industries, Ltd., Osaka). Gastrocnemius and Soleus muscles glycogen content were determined by a modification described by Lo et al.(13)
Serum and liver lipid peroxidation were determined by a modification described by Ohkawa et al.(14)
Statistical analysis
All values were expressed as mean±SD. Statistical analy-sis of differences between C and LA groups were performed with Student s t-test. Comparisons of the biochemical analy-sis in each group were performed by two-way ANOVA and Fisher s PLSD tests. Statistical significance was set at p value of <0.05. All analyses were performed with a commercially available statistical package (StatView J-5.0, SAS Institute Inc., Cary, NC).
Results
Exercise time to exhaustion in loading swimming test
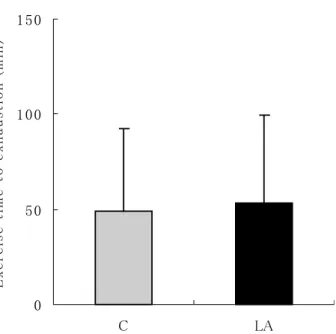
Exhaustion time in loading swimming test did not differ be-tween the C and LA groups, as shown in Fig.1. After 90 min swimming, plasma lactic acid concentration was significantly lower in the LA group than in the C group, however at 30, 90 and 120 min after swimming, plasma lactic acid concentra-tions did not differ between two groups, as shown in Fig.2.
Energy metabolism measurement
Resting metabolic rate (RMR) was significantly increased by ALA administration, and post-exercise energy expenditure during 3.5h was greater in the LA group than in the C group, however the difference was not significant (Table 1, Fig.3.). Pre- and post-exercise RQ did not differ between the C and LA groups, as shown in Fig.4.
Biochemical examination (Table 2)
Lactic acid content of gastrocnemius muscle in the pre-ex-ercise did not differ between the C and LA groups, but in the
post-exercise, it was significantly lower in the LA group than in the C group. Lactate dehydrogenase activity of gastrocne-mius muscle in the pre-exercise did not differ between the C and LA groups, but in the post-exercise, it was significantly higher in the LA group than in the C group. Glycogen con-tents of gastrocnemius and soleus muscles in the pre-exercise were significantly increased by ALA administration, but in the post-exercise, they did not differ between the C and LA groups. Serum and liver lipid peroxidation concentrations in both the pre- and post-exercise were significantly lower in the LA group than in the C group.
Fig. 1 Exercise time to exhaustion in loading swimming test. Values are expressed as the means and SD for 19-20 rats. C, control group ; LA, α-Lipoic acid group.
Fig. 2 Plasma lactic acid concentrations after loading swimming test. Values are expressed as the means and SD for 19-20 rats. *
Significantly difference from Control group (p<0.05, Student s t-tests). C, control group ; LA, α-Lipoic acid group.
Table 1 Resting metabolic rate (RMR), post-exercise en-ergy expenditure (PEP) and excess post-exercise energy expenditure (EPEE) by respiratory gas analysis. C LA Body weight g 132 ± 12 125 ± 8 RMR J/kg/min 724 ± 120 812 ± 99* PEE kJ/kg/3.5h 208 ± 21 221 ± 19 EPEE kJ/3.5h 7.39 ± 3.8 6.55 ± 3.2
Values are expressed as the means±SD for 19-20 rats.
*
Significantly difference from Control group (p<0.05, Student s t-tests). C, control group ; LA, α-Lipoic acid group.
Fig. 3 Changes in EE before and after exercise. Values are expressed as the means and SD for 19-20 rats.
*,* *Significantly difference from Control group (p
<0.05, p<0.01 , ANOVA with repeated measures and Fisher s PLSD tests). C, control group ; LA, α-Lipoic acid group.
Discussion
In this study, we demonstrated that ALA continuously-ad-ministered had no effect on improvement of endurance capac-ity of rats, but had effect on a significantly increasing resting metabolic rate. We also showed that energy expenditures after exercise increased but not significant by ALA continuously-administered. These results suggested ALA administration
increased energy metabolism in rats.
ALA functions as a cofactor of mitochondrial enzymes, is closely related with mechanism of aerobic energy production.(1) Because of essential biogenic factor for
pro-moting metabolism, it is not difficult to understand that ALA continuously-administered markedly enhanced energy ex-penditure in rats. Recent study reported that ALA suppresses AMP-activated protein kinase (AMPK) in the hypothalamus but activates it in skeletal muscle.(15)
AMPK functions as a fuel sensor in the cell and is activated when cellular energy is depleted.(16)
Lee et al.(15)
recently reported that ALA in-creased fatty acid oxidation in skeletal muscle by activating
AMPK. AMPK activation increases fatty acid oxidation by inhibiting acetyl-coenzyme A (acetyl-CoA) carboxylase (ACC) activity and by decreasing malonyl-CoA
concentra-tions.(17)
In this study, we were not observed that respiratory quotient increased by ALA-administrated. Therefore, it might be possible that ALA increases energy expenditure by the other mechanisms. In rodents, uncoupling protein (UCP)-1 in brown adipose tissue is the main regulator of basal energy expenditure, and the expression of this protein is increased by adrenergic stimulation.(18)
Lee et al.(19)
had shown that central administration of very small amounts of ALA increased ex-pression of UCP-1 mRNA in brown adipose tissue and energy expenditure in whole body.
We demonstrated that ALA induced significant increase of muscle glycogen accumulation before exercise and decrease of muscle lactic acid accumulation after exercise. ALA has been used as therapy for many diseases associated with im-paired energy utilization, such as typeⅡdiabetes and diabetic polyneuropathies.(20−22) This has been shown to protect against
oxidative stress-induced insulin resistance in vitro(8,23−24)
and to increase insulin-stimulated glucose uptake in skeletal muscle by improving insulin sensitivity.(25−26) In skeletal
muscle, glucose transport can be activated by two independent mechanisms, one is dependent and the other is insulin-independent.(27)
Recently, several studies have demonstrated that ALA can directly activate glucose transport by activation of AMPK.(1,28)
In this study, ALA enhanced skeletal muscle glycogen synthesis, expecting that glucose uptake in skeletal Fig. 4 Change in RQ before and after exercise. Values are
expressed as the means and SD for 19-20 rats. C, control group ; LA, α-Lipoic acid group.
Table 2 Biochemical analysis in each group of rats before and after exercise.
Pre-exercise Post-exercise ANOVA
C LA C LA Exercise(A) LA(B) A×B
Gastrocnemius lactic acid
(mg/g tissue weight) 62.0 ± 15.0 c 60.8 ± 10.6 c 124.3± 13.6 a 110.2 ± 14.2 b P<0.01 NS NS Gastrocnemius lactate dehydrogenase
(IU/g tissue weight) 756 ± 22 a 757 ± 33 a 688± 46 c 716 ± 11 b P<0.01 NS NS Gastrocnemius glycogen
(mg/g tissue weight) 3.986 ± 0.96 b 4.771 ± 0.87 a 2.011± 0.50 c 2.566 ± 0.76 c P<0.01 P<0.01 NS Soleus glycogen
(mg/g tissue weight) 0.781 ± 0.24 b 1.384 ± 0.33 a 0.055± 0.02 c 0.105 ± 0.02 c P<0.01 P<0.01 P<0.01 Serum lipid peroxidation
(nmol MDA equivalent/ml) 6.13 ± 0.29 c 5.00 ± 0.61 d 11.93± 1.40 a 10.28 ± 1.24 b P<0.01 P<0.01 NS Liver lipid peroxidation
mol MDA equivalent/g tissue weigh 249 ± 33 c 211 ± 24 d 363± 45 a 318 ± 41 b P<0.01 P<0.01 NS
Values are expressed as the mean±SD for 9-10 rats. Means with different superscripts within a row are significantly dif-ferent (p<0.05, two-way ANOVA and Fisher s PLSD tests).
muscle was promoted. In addition, this study implied that glycolytic system was inhibited because ALA was reported to enhance fatty acids oxidation.(15)
This could lead to accumula-tion of glycogen and reducaccumula-tion of lactic acid contents in skel-etal muscle. The importance of muscle glycogen on endurance performance has been demonstrated by the study reported that endurance capacity depended on accumulation of muscle gly-cogen.(29)
In contrast, it has been shown that lactic acid, me-tabolite formed by glycolytic system, decreased by endurance training, resulting that lactic acid utilization increased as the energy source.(30)
In this study, ALA reduced lactic acid con-tents in skeletal muscle after swimming. This finding suggests that ALA produces the same effects as endurance training, or ALA enhances the efficiency of training.
When much oxygen is taken into the body during exercise, level of oxygen in the body s tissues increase, and it followed that lipid peroxidative reactions are induced in blood, skeletal
muscle, myocardium and liver.(31)
There is strong evidence that exercise-induced oxidative stress decreases exercise per-formance.(32)
Some researchers indicated that ALA, a potent antioxidant, markedly reduced oxidative stress.(33−35)
In this study, serum and liver lipid peroxidation concentrations in both before and after exercise were significantly lower by ALA administration. Our results at least in part support previ-ous finding and ALA reduces oxidative damage, expecting to enhance exercise performance.
In conclusion, we demonstrated in this study that ALA did not contribute directly to enhance endurance capacity, but promoted accumulation of muscle glycogen and decreased accumulations of lactic acid and lipid peroxidation after ex-ercise. These results suggest that ALA supplementation leads to enhance the efficiency of training and reduce fatigue and biological oxidation damage by exercise. Thus, ALA may be useful as a supplement for athletes.
References
⑴ Henriksen, E. J., Jacob, S., Streeper, R.S., Fogt, D.L., Hokama, J.Y., and Tritschler, H.J., Stimulation by α -lipoic acid of glucose transport activity in skeletal muscle of lean and obese Zucker rats. Life Sciences., 61, 805-812 (1997)
⑵ Suzuki, Y. T., Tsuchia, M., and Packer, L., Thioctic acid and dihydrolipoic acid are novel antioxidants which in-teract with reactive oxygen species. Free Rad. Res.
Com-mun., 15, 255-263 (1991)
⑶ Scott, B. C., Aruoma, O. I., Evans, P. J., O Neill, C., Van Der Vleit, A., Cross, C. E., Tritschler, H. J., and Hal-liwell, B., Lipoic acid and dihydrolipoic acids as antioxi-dants. A critical evaluation. Free Rad. Res. Commu., 20, 119-133 (1994)
⑷ Han, D., Handelman, G., Marcocci, L., Sen, C. K., Roy, S., Kobuchi, H., Tritschler, H. J., Flohe, L., and Packer, L., Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors., 6, 321-338 (1997)
⑸ Xu, D. P., and Wells, W. W., alpha-Lipoic acid depen-dent regeneration of ascorbic acid from dehydroascorbic acid in rat liver mitochondria. J. Bioenerg. Biomembr., 28, 77-85 (1996)
⑹ Lykkesfeldt, J., Hagen, T. M., Vinarsky, V., and Ames, B. N., Age-associated decline in ascorbic acid concentra-tion, recycling, and biosynthesis in rat
hepatocytes-rever-sal with (R)-α-lipoic acid supplementation. FASEB J. 12, 1183-1189 (1998)
⑺ Kagen, V. E., Serbinova, E. A., Forte, T., Sata, G., and Packer, L., Recycling of vitamin E on human low den-sity lipoproteins. J. Lipid Res., 33, 385-397 (1992) ⑻ Hagen, T. M., Ingersoll, R. T., Lykkesfeldt, J., Liu, J.,
Wehr, C. M., Vinarsky, V., Bartholomew, J. C., and Ames, B. N., (R)-α-Lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxida-tive damage, and increased metabolic rate. FASEB J. 13, 411-418 (1999)
⑼ Matsumoto, K., Ishihara, K., Tanaka, K., Inoue, K., and Fushiki, T., An adjustable-current swimming pool for the evaluation of endurance capacity of mice. J. Applied Physiology., 81, 1843-1849 (1996)
⑽ Gutmann, I., Wahlefeld., L-(+)-lactate. Determination with lactate dehydrogenase and NAD. In Methods of Enzymatic Analysis , ed. Bergmeyer, H., Academic Press, New York, pp.1464-1468 (1974)
⑾ Wieland, O., Jagow-Westermann, B. V., L-(+)-Lactate. Determination with Yeast Lactate Dehydrogenase. In
Methods of Enzymatic Analysis , ed. Bergmeyer, H., Verlag, Chemie, Weinheim, and Academic Press, New York, pp.1483-1491 (1974)
⑿ Weststrate, J. A., Resting metabolic rate and diet-induced thermogenesis, a methodological reappraisal. J.
Clin. Nutr., 58, 592-601 (1993)
⒀ Lo, S., Russell, J. C., and Taylor, A. W., Determination of glycogen in small tissue samples. J. Applied.
Physiol-ogy., 28, 234-236 (1970)
⒁ Ohkawa, H., Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry., 95, 351-358 (1979)
⒂ Lee, W. J., Song, K. H., Koh, E. H., Won, J. C., Kim, H. S., Park, H. S., et al., Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle.
Bio-chemical and Biophysical Research Communications,
332, 885-891 (2005)
⒃ Hardie, D. G., Carling, D., The AMP-activated protein kinase-fuel gauge of the mammalian cell ? Eur. J.
Bio-chem.,246, 259-273 (1997)
⒄ Ruderman, N. B., Saha, A. K., Vavvas, D., and Witters, L. A., Malonyl-CoA, fuel sensing, and insulin resistance.
Am. J. Physiol., 276, E1-E18 (1999)
⒅ Dalgaard, L. T., and Pedersen, O., Uncoupling proteins: functional characteristics and role in the pathogenesis of obesity and TypeⅡ diabetes. Diabetologia., 44, 946-965 (2001)
⒆ Lee, W. J., Koh, E. H., Won, J. C., Kim, M-S., Park, J-Y., Lee, K-U., Obesity: The role of hypothalamic AMP-activated protein kinase in body weight regulation. The
International Journal of Biochemistry & Cell Biology.,
37, 2254-2259 (2005)
⒇ Jacob, S., Henriksen, E. J., Tritschler, H. J., Augustin, H. J., and Dietz, G. J., Improvement of insulin-stimulated glucose disposal in type 2 diabetes after repeated paren-teral administration of thioctic acid. Endocrinol.
Diabe-tes., 104, 284-288 (1996)
Sachse, G., and Willms, B., Efficacy of thioctic acid in the therapy of peripheral diabetic neuropathy. Horm.
Metab. Res. Suppl., 9, 105-107 (1980)
Ziegler, D., Hanefeld, M., Ruhnau, K. J., Meissner, H. P., Lobisch, M., Schutte, K., and Gries, F. A., Treatment of symptomatic diabetic peripheral neuropathy with the antioxidant α-lipoic acid. A 3-week multicentre random-ized controlled trial (ALADIN study). Diabetologia., 38, 1425-1433 (1995)
Maddux, B. A., See, W., Lawrence Jr, J. C., Goldfine, A. L., Goldfine, I. D., and Evans, J. L., Protection against oxidative stress-induced insulin resistance in rat L6 mus-cle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes., 50, 404-410 (2001)
Rudich, A., Tirosh, A., Potashnik, R., Khamaisi, M., Bashan, N., Lipoic acid protects against oxidative stress induced impairment in insulin stimulation of protein ki-nase B and glucose transport in 3T3-L1 adipocytes.
Dia-betologia., 42, 949-957 (1999)
Eason, R. C., Archer, H. E., Akhtar, S., and Bailey, C. J., Lipoic acid increased glucose uptake by skeletal muscle of obese-diabetic ob/ob mice. Diabetes Obese. Metab., 4, 29-35 (2002)
Madaoui, A. E., and Champlin, J., Prevention of hyper-tension, insulin resistance, and oxidative stress by alpha-lipoic acid. Hypertension., 39, 303-307 (2002)
Holloszy, J. O., and Hansen, P. A., Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Physiol. Biochem. Phar -macol., 128, 99-193 (1996)
Hayashi, T et al., Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes., 49, 527-531 (2000)
Bergstrom, J., et al., Diet, muscle glycogen and physical performance. Acta. Physiol. Scand., 71, 140-150 (1967) Shimomura, Y., et al., Exercise and metabolism in mus-cle cells: molecular aspects of energy metabolism during exercise and adaptation to exercise training. In Exer-cise, Nutrition, and Environmental Stress , eds. Nose, H., et al., Cooper Publishing Group, Traverse City, MI, pp.89-116 (2001)
Davies, K., et al., Free radicals and tissue damage pro-duced by exercise. Biochem. Biophys. Res. Commun., 107, 1198-1205 (1982)
Barclay, J. k., and Hansel, M., Free radicals may contrib-ute to oxidative skeletal muscle fatigue. Can. J. Physiol.
Pharmacol., 69, 279-284 (1991)
Borcea, V., Nourooz-Zadeh, J., Wolff, S. P., et al., Alpha-lipoic acid decreases oxidative stress even in diabetic pa-tients with poor glycemic control and albuminuria. Free.
Radic. Biol. Med., 26, 1495 (1999)
Packer, L., Kraemer, K., and Rimbach, G., Molecular aspects of lipoic acid in the prevention of diabetes com-plications. Nutrition., 17, 888-895 (2001)
Hagen, T. M., Liu, J., Lykkesfeldt, J., Wehr, C. M., In-geersoll, R. T., Vinarsky, V., Bartholomew, J. C., and Ames, B. N., Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc. Natl. Acad. Sci.,