細胞性粘菌キイロタマホコリカビの核分離
全文
(2) Journal of Hokkaido University of Education (Section II B) Vol. 30, No. 1 September, 1979. ^^igaW^^ffi^ (^2^B) ^30^ ^1-?- Bgffi 54 -^ 9 J!. Isolation of Nuclei from the Cellular Slime Mold Dictyostelium discoideum. Fusayuki KANDA Biological Laboratory, Kushiro College, Hokkaido University of Education, Kushiro 085. ftBB^T : MW?^ ^ u ^^3 ij ^7 fc-^^Ef. W?H±WWW^W^ Summary. Nuclei of the cellular slime mold Dictyostelium discoideum were isolated and purified by a method which uses a low concentration of a non-ionic detergent, Triton X-100. The critical. concentration of the detergent which could bring about the breakage of almost all of the cells was 0.075% (v/v). Magnesium ions used in the preparation of nuclei did not significantly affect the stability of nuclei when the concentration of the ions was increased to 10 mM or decreased to 1 mM. The yields of crude and purified nuclei isolated by this method were 91.3% and 69%, respectively.. Introduction Dictyostelium discoideum has many unique advantages as a model eukaryotic system for studies on the relationship between gene expression and morphological changes during development.. For the isolation of nuclei of D. discoidenm amoeba cells, Cocucci and Sussman (1970) have used Cemulsol NPT 12 as a detergent to break the plasma membrane. This method was developed by some workers and has been commonly used for the isolation of nuclei in the investi-. gation of nuclear DNA(Firtel, 1972; Firtel & Banner, 1972) and RNA of this organism ( Firtel & Lodish, 1973; Kessin, 1973; Soil & Sussman, 1973; Jacobson et al., 1974). However, the nature of Cemulsol NPT 12 is not clear as yet and the concentration of the detergent used in the experiments was much higher than that of other ones used for the preparation of nuclear materials of mammalian cells (Perry & Kelley, 1968). In recent years, several. (17).
(3) Fusayuki KANDA. workers have begun to use Triton X-100 for the isolation of nuclei of D. discoideum (Coukell & Walker, 1973; Charlesworth & Parish, 1975; Pederson, 1977), but details on the isolation of nuclei have not been reported.. In this paper, a method with low concentration of Triton X-100 for the isolation of nuclei from D. discoideum is described.. Materials and Methods Cells : Amoeba cells of Dictyostelium discoideum, strain NC-4 (haploid), were grown on nutrient agar plates with Escherichia coli (Banner, 1947). In the late log phase of growth, the cells were harvested, washed several times by centrifugation at 2,500 rev./min for 2—3 min with SM. buffer (1.45% KHzP04 and 0.96% NazHP04- 12H20, pH 6.4) as described previously (Kanda et al., 1974; Kanda, 1977a). Cell fractionation: Although a part of the method is described in the Results section, this section is confined to a description of the standard isolation technique. All the procedures for the preparation of nuclei and cytoplasmic were conducted at 0 — 4 °C. The cells of D. discoideum were suspended in 5% sucrose containing various concentrations of Triton X-100 and 3 mM magnesium acetate at a concentration of 2 x 108 cells/ml. The cells were then disrupted in a loose-fitting Potter-type homogenizer. After standing for 10 min, an equal volume of 22% sucrose containing 3 mM magnesium acetate was added to the cell lysate.. It was then centrifuged at 3,000 rev./min for 10 min in an RPR 18 rotor of a Hitachi centrifuge. After centrifugation the pellet was washed further twice by centrifugation with 13.5% sucrose containing Triton X-100 and 3 mM magnesium acetate. The finally obtained pellet was named crude nuclear fraction. The supernatant fraction was centrifuged at 10,000 rev./min for 10 min. in a ft: 1 rotor of a Kubota centrifuge. The supernatant fluid obtained in this centrifugation was named soluble cytoplasmic fraction and the sedimented material named mitochondrial fraction. Purification of nuclei: The purification of nuclei was conducted by a modification of the method of Cocucci and Sussman (1970). The crude nuclear pellet was resuspended in 4 ml of 0.25 M sucrose containing 3 mM magnesium acetate, followed by the addition of 8 ml of 2.3 M sucrose containing 3 mM magnesium acetate. The suspension containing crude nuclei was layerd over. 15 ml of 1.95 M sucrose solution containing 3 mM magnesium acetate and then centrifuged for 45 min at 17,000 rev./min and 4°C in an SW 25.1 rotor of a Spinco L ultracentrifuge. The nuclei collected through 1.95 M sucrose were termed purified nuclei.. Estimation ofDNA: The amount of DNA was estimated according to the method advanced by Burton (1956) as described previously (Kanda, 1977b).. (18).
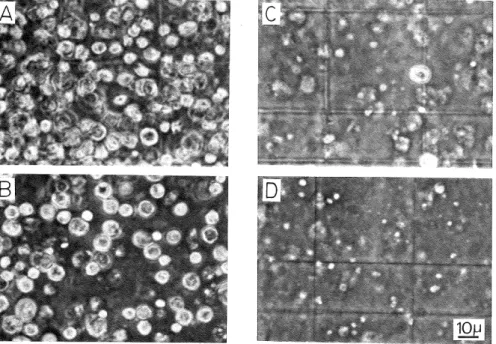
(4) Isolation of Nuclei from the Cellular Slime Mold Dictyostelium discoideiim. Results and Discussion. Cell lysis with Triton X-100: In order to determine the critical concentration of Triton X-100 which could bring about the breakage of almost all of the cells, the amoeba cells of D. discoideum were suspended in a solution containing various concentrations of Triton X-100 and homogenized. in a Potter-type homogenizer with several strokes (see Materials and Methods). The cell disruption was microscopically monitored.. When 0.1% Triton X-100 was adapted for the cell breakage, cells lysed rapidly (Fig. 1) and a large number of nuclei which appeared intact under phase-contrast microscopy were obtained. A microscopic observation of the nuclear preparation revealed that the nuclear sample contained. less than 0.1% unbroken cells with no significant amount of cytoplasmic materials (Fig. 2). Figure 3 shows that the critical concentration of Triton X-100 which could bring about the breakage of more than 98% of the cells was 0.075% (v/v). On the other hand, 1-2% of the total cells remained unbroken after the homogenization though the concentration of the detergent was increased to 0.1% (v/v). However, about half of the remaining unbroken cells were lysed when the suspension of homogenized cells stood for 10 min. The rest portion (0.5—1%) of the un-. broken cells could be further disrupted during the washing of the crude nuclei by centrifugation. Fig. 1. Disruption of the plasma membrane of D. discoide'um with 5% sucrose containing various concentrations of Triton X-100. A, 0% ; B, 0.05% ; C, 0.075% ; D, 0.1%.. (19).
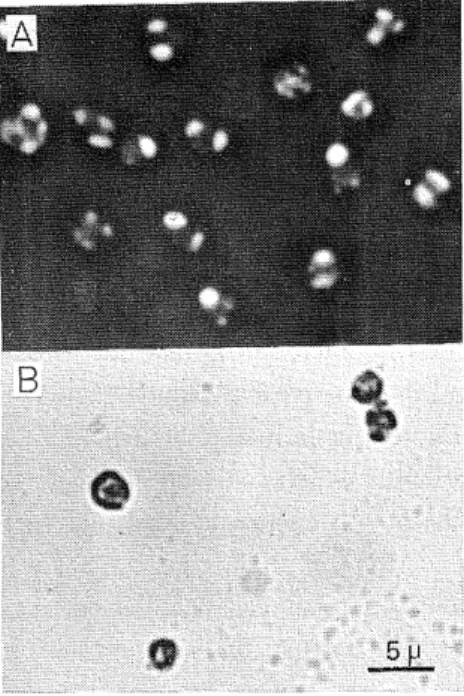
(5) Fusayuki KANDA. 100h. Fig. 2. Nuclei under phase-contrast microscopy. Crude nuclei were prepared as described in Materials and Methods and further washed twice by. centrifugation with 13.5%. sucrose containing 0.1% Triton X-100 and 3 mM magnesium acetate. A, viewed. with negative contrast lens; B, viewed with positive contrast lens.. 0 Fig. 3.. 0.05 0.1 0.15 ~02. Concentration of Triton X-100(°/o). Effect of Triton X-100 on the disruption of cells. The amoeba cells of D. discoideum were suspended in 5% sucrose containing 3 mIVt magnesium acetate and various concentrations of Triton X-100. The cells were then disrupted in a Pottertype homogenizer. Cell lysis was monitored under microscopy and the number of undegraded cells was counted.. (20).
(6) Isolation of Nuclei from the Cellular Slime Mold Dictyosteliiim discoideum. with a solution containing 0.1% Triton X-100. Thus, D. discoideum amoebae were routinely homogenized in a solution containing 0.08%. Triton X-100 at a cell density of 2 x 108 cells/ml. Initially, Brij 58 of a non-ionic detergent was used to disrupt the plasma membrane of this organism, but exerted almost no effect. Although the concentration of this agent was increased. to 1%, only 16% of the initial cells were broken. Effect ofmagnesium ions on the stability of nuclei: The stability of nuclei was represented by the amount of DNA which remained in the crude nuclear fraction plus mitochondrial fraction against the initial amount of DNA of the whole cells. The cells were divided into two parts; one being suspended in 0.08% Triton X-100 solution containing ImM magnesium acetate and the other in 0.08% Triton X-100 solution containing 10 mM magnesium acetate at a cell density of 2 x 108 cells/ml. The cell suspensions were then homogenized and fractionated as described in. Materials and Methods. As shown in Table 1, there was no significant difference between the two different concentrations of Mg2+. However, at 1 mM Mg2+, a small amount of DNA released to cytoplasmic. fraction. This suggests that a small number of nuclei were broken during the course of the preparation of nuclei at low concentration of Mg +.. Table 1. Effect of magnesium ions on the stability of nuclei. Concentration of Mg2+ Fraction Amount of DNA ODsoo x 103 % crude nuclei + mitochondria 274.5 98.7. ImM. soluble cytoplasm 3.5 1.3 crude nuclei + mitochondria 281.0 100.0. 10 mM. soluble cytoplasm 0.0 0.0 Amoeba cells of D. discoideum which proceeded to the early aggregation stage of morphogenesis were harvested and washed by centrifugation with an ice-cold TKM buffer. The cells were suspended in 5% sucrose containing 0.08% Triton X-100 and 1 mM or 10 mM magnesium acetate at a concentration of 2 x 108 cells/ml. The cells were then disrupted in a loose-fitting Potter-type homogenizer. After standing for 10 min, an equal volume of 22% sucrose containing 1 mM or 10 mM magnesium acetate was added to the cell lysate. It was then centrifuged at 3,000 rev./min for 10 min in an RPR rotor of a Hitachi centrifuge. The crude nuclear fraction was obtained by the washing of the pellet with 13.5% sucrose containing 0.1% Triton X-100 and 1 mM or 10 mM magnesium acetate. The soluble cytoplasmic and mitochondrial fraction were prepared as described in Materials and Methods. The amount of DNA was estimated according to the method advanced by. Burton (1956).. (21).
(7) Fusayuki KANDA. Yields of nuclei in various steps during preparation: Table 2 shows the yields of nuclei at several steps in the purification process of nuclei. . The yield of nuclei was determined by the amount of DNA in the nuclear fractions in each step. The yield of purified nuclei was 69% at the final step. If only the crude nuclei are required, the yield of nuclei could be increased to 91.3%.. Table 2. Yields of nuclei in various steps during the preparation of nuclei. Amount ofDNA. Fraction. ODeoo X 10s. 207 189 143. Total cells Crude nuclei Purified nuclei. OA 70. 100.0 91.3 69.1. The cells were divided into three equal portions. Two-thirds of the cells were suspended in 5% sucrose containing 0.08% Triton X-100 and 3 mM magnesium acetate at a concentration of 2 x 108 cells/ml. The cells were then homogenized and an equal volume of 22% sucrose containing 3 mM magnesium acetate was added to the cell lysate. The purified nuclei were obtained from a half-portion of the crude nuclei. The crude and purified nuclei were prepared as described in Materials and Methods. The amounts of DNA in the fractions of whole cells, crude nuclei and purified nuclei were estimated according to the method of Burton.. Triton X-100 of non-ionic detergent has been usually employed as a useful tool for the isolation of mammalian nuclei (Perry & Kelley, 1968). The present study shows that in the case of D. discoideum Triton X-100 is able to be used in the same manner as mammalian cells for the isolation of nuclei.. Acknowledgement The author thanks Dr. M. Iwabuchi of Hokkaido University for his encouragement during the course of this study.. (22).
(8) Isolation of Nuclei from the Cellular Slime Mold Dictyostelium discoideum. References. Bonner, J.T. 1947. Evidence for the formation of cell aggregates by chemotaxis in the development of the slime mold D. discoidewn. ]. Exp. Zool. 106 : 1—26. Burton, K. 1956. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 62: 315—323. Charlesworth, M.C. and Parish, R.W. 1975. The isolation of nuclei and basic nucleoproteins from the cellular slime mold Dictyostelwm discoidenm. Eur. J. Biochem. 54: 307—316. Cocucci, S.M. and Sussman, M. 1970. RNA in cytoplasmic and nuclear fractions of cellular slime mold amoebas.. J. Cell Biol. 45: 399-407. Coukell, M.B. and Walker, 1.0. 1973. The basic nuclear proteins of the cellular slime mold Dictyostelium discoideum. Cell Differentiation 2: 87—95. Firtel, R.A. 1972. Changes in the expression of single-copy DNA during development of the cellular slime mold Dictyostelium discoideum. ]. Mol. Biol. 66: 363—377. Firtel, R.A. and Banner, J. 1972. Characterization of the genome of the cellular slime mold Dictyostelium discoideum. ]. Mol. Biol. 66: 339-361. Firtel, R.A. and Lodish, H.F. 1973. A small nuclear precursor of messenger RNA in the cellular slime mold Dictyostelium discoideum. J. Mol. Biol. 79: 295—314. Jacobson, A., Firtel, R.A. and Lodish, H.F. 1974. Synthesis of messenger and ribosomal RNA precursors in. isolated nuclei of the cellular slime mold Dictyostelium discoideum. J. Mol. Biol. 82: 213—230. Kanda, F. 1977a. Nuclear ribonucleoprotein particles in the cellular slime mold Dictyostelium discoideum. J. Biochem. 82: 59-66.. Kanda, F. 1977b. Study on nuclear ribonucleoprotein particles containing pulse-labeled RNA in the cellular slime mold Dictyostelium discoidenm. Dr. thesis, Hokkaido Univ., Japan. Kanda, F., Ochiai, H. and Iwabuchi, M. 1974. Molecularweight determinations and stoichiometric measure-. ments of 40-S and 60-S ribosomal proteins of the cellular slime mold Dictyostelium discoideum. Eur. J. Biochem. 44: 469-479.. Kessin, R.H. 1973. RNA metabolism during vegetative growth and morphogenesi s of the cellular slime mold Dictyostelium discoideum. Dev. Biol. 31: 242—251. Pederson, T. 1977. Isolation and characterization of chromatin from the cellular slime mold, Dictyostelium. discoideum. Biochemistry 16: 2771—2777. Perry, R. and Kelley, D.E. 1968. Messenger RNA-protein complexes and newly synthesized ribosomal subunits: Analysis of free particles and components of polyribosomes. J. Mol. Biol. 35: 37—59. Soil, D.R. and Sussman, M. 1973. Transcription in isolated nuclei of the slime mold Dictyostelium discoideum.. Biochim. Biophys. Acta 319: 312-322.. (23).
(9)
図


関連したドキュメント
Laplacian on circle packing fractals invariant with respect to certain Kleinian groups (i.e., discrete groups of M¨ obius transformations on the Riemann sphere C b = C ∪ {∞}),
In [1, 2, 17], following the same strategy of [12], the authors showed a direct Carleman estimate for the backward adjoint system of the population model (1.1) and deduced its
Eskandani, “Stability of a mixed additive and cubic functional equation in quasi- Banach spaces,” Journal of Mathematical Analysis and Applications, vol.. Eshaghi Gordji, “Stability
Finally, we give an example to show how the generalized zeta function can be applied to graphs to distinguish non-isomorphic graphs with the same Ihara-Selberg zeta
Given the invariant measure µ of an ergodic and reversible continuous-time Markov process (X t ) t≥0 with carré du champ operator Γ (see below for the definition), we pro-
She reviews the status of a number of interrelated problems on diameters of graphs, including: (i) degree/diameter problem, (ii) order/degree problem, (iii) given n, D, D 0 ,
[56] , Block generalized locally Toeplitz sequences: topological construction, spectral distribution results, and star-algebra structure, in Structured Matrices in Numerical
We will later see that non-crossing and non-nesting set partitions can be seen as the type A instances of more general constructions:.. ▸ non-crossing partitions NC ( W ) , attached