Tech Bull Fac Agr Kagawa Univ, Vol 43, No 1, 51-56, 1991
An Improved Method for Protein Determination
in Persimmon Fruit
Toshiyuki
MATSUI
and HirotoshiK I ~ A G A W A
Different methods of protein determination were examined using persimmon cv Atago which contains high amount of tannin and is normally astringent Micro-KJELDHAL is accurate and stable against polyphenol compounds but takes time whereas LOWRY, dye reagent and micro-BIURET methods are affected by polyphenol compounds The protein content determined by the last three methods was lower when astringency was removed by heat treatment However, micro-KJELDHAL did not detect this difference in protein content between untreated and heat-treated fruits T o protect the protein from polyphenolic disturbance, trichloro acetic acid (TCA) was added to the fruit extract The protein value increased in the presence of TCA LOWRY and dye reagent methods sometimes resulted in negative readings and were thus not applicable to protein assay When the protein of persimmon fruits which contain large amount of tannin will be analyzed by micro-BIURET the protein value obtained when TCA is added (blank) should be subtracted from the sample reading (without TCA) Micro-BIURET method is rapid and the value obtained was comparable to that of micro-KJELDHAI
B&o)9
~-~%.$aEf4f3'+~;k,6'ZZ'
B E - > r9 Ylf3Bo)@Qo)?iZP$!?%@$j L k .<
9
n-.t-lb9'-~~&5 Ciil%TL-h>G %!, 7 7 1 ---1bt~%X~;k,6QT@Ed;3f;3h~7~.- E ,
a ' / !, - & - P . $ ~ 3 ~ & T ~ 2 L 7 z 9 Y l f 3 . $ P I i A B f J U & @ E Q T R % L f i G o ) b : 9 $1&<,$!, 711 -1~{t;f$@0&@Bf i f k . L i ~ L Q - h f r j , ?o-.t-1b9'- lL&r;GZfjblT, % @ E ~ ~ J u & @ B % % B B ' J ~ ~ Y/f9.$%o)BISEM,ijhQ-hh77',-. % '1 7 7 1 --1l.@%!fQi~rj9 Y j fP E % # l f 6 fc-@R, b !, 3 ~ I L @ @ (TCA) %%%M&$$JE~JuR Q 2 , 9 Y / f 3 B - $ P G i TCA o)@%TTf&kL
7:. '1 - & k & % 3 % % T I t @ & % 0 1 E % Z L f ~ . % T T , 9 Y ~ \ . ~ Z ~ ~ I Z I ~ % M T ~ Q ~ ~ - > ~ . 3 2 0 9 2.z
Y B . $ b r k + % % a ) 9 Y l f 3 B % 5 9 o - Y = V.Y i-$ktZk ->T%$fifQl%G~Li, TCA % f J U X 7 7 3 i j h Y z 9 Y / f 3 1 E ( 7 * 7 ~ 3 ) % W g o ) s a (TCA%$&T) ; ~ ~ ~ ; ~ L G I { D ; S ~ B ~ ~ ~ , E , ~ ~ ~ . : ? U - Y J . V . Y r j m , L ~ T L ;3hGf3rjhfz1iElCt, 5 ~ u - ? - I L Y ~ - I ~ % D ~ E R E ~ % ~ ~ L ~ ~ .
Introduction
The FOL.IN-DENIS(') method for analyzing total polyphenol is based on measurement of color produced when the hydroxy group is deoxidized while the LOWRY-FOL.IN(~) method is based on the color produced by the hydroxy groups of tyrosine and thyroxine Both methods use the phenol reagent, FOL.IN-CIOCAL:TEU The dye reagent assayc3) is based on the change in absorbance when Commassie Briliant Blue binds with protein This reagent
52 Tech Bull Fac Agr Kagawa Univ, Vol 43, No 1, 1991
is not known to react with tannin M~c~o-BIuRET(') method is very accurate since it is based on coloring of peptide bond but it can easily be affected by the presence of other substances M ~ C ~ O - K J E L D A H L ' ~ ) method is accurate but takes time This paper describes an improved method for protein determination in persimmon fruit based on deproteinization
Materials and Methods Materials
Persimmon fruit cultivars, Fuyu, Hiratanenashi and Atago were harvested from the field of Kagawa University located in Nagao, Kagawa Prefecture Samples were collected on the 15th of October, 1988, and 21st of July, 9th of August, 14th of September, 13th and 26th of October and 24th of November (only 'Atago'), 1989 Nine fruits were harvested a t random for every month
'Atago' (astringent type) harvested on the 5th of ~ e c e m b e r was used a s the test samples to determine the effect of tannin on protein measurement Astringency was removed by packing the fruits in plastic films and heating a t 40°C for 15 h'6)
Reagents
FOLIN-CIOCALTEU reagent and catechin were obtained from Nakarai Chemical Co, Ltd Dye-binding reagent was purchased from Bio-Rad Laboratories Co, Ltd Unless otherwise stated, guaranteed reagent (Wako Chemical Co , Ltd ) were used
Methods
Protezn extractzon : The fruit pulp was homogenized in a POTTER'S homogenizer with chilled water, twice the amount of sample (W/V) The homogenate was filtered through 4 layers of cotton cloth and the filtrate was centrifuged at 13 000 x g for 15 min The residue was rinsed with the same amount of water and centrifuged under the same condition The combined supernatant was used for protein assay
Pretreatment of the supernatant : Two m$ of the supernatant was diluted to 10 mP with water (A) ; two m l of the supernatant and 3 m$ of 10 % TCA was made up to 10 m$ with water (B) The solution was mixed vigorously in a tube mixer for 3 min and allowed to stand at room temperature for 30 min Both solutions were centrifuged at 3 000 rpm for 3 min and a color-producing reagent was added to the supernatant Protein was calculated using A-B
Protezn assays
(LOWRY) : Soluble protein content was determind by the method of LOWRY If a dialyzed solution was needed, a part of the total supernatant was dialyzed in 5 mM of sodium phosphate-citrate buffer (pH 7 4) for 12 h (@e-reagent assay) : The dye reagent was diluted five times with water and the standard assay procedure'') was performed
(MZCYO-BIURET) : Soluble protein content was determined by the method of GoA") with some modifications as described below Micro-BIURET reagent was prepared a s follows : 17 3 g of sodium citrate and 10 0 g of sodium carbonate was dissolved in about 60 m e of hot water and mixed with 1 73 g of copper sulfate in 10 m$ of water, and made up to a final volume of 100 m.@ with water To make alkaline, 0 2 m$ of 30 %NaOH was added to
Toshiyuki MATSUI and Hirotoshi KITAGAWA : Protein Determination in Persimmon Fruit 53
1 8 m l of solution (A) and (B) Then, 0 1 m l of micro-BIURET reagent was added and mixed vigorously in a tube mixer for 3 min After 10 min a t room temperature, the solutions were centrifuged a t 3 000 rpm for 5 min and was measured a t 330 nm against 3 % NaOH reacting with the same reagent Protein was calculated using A-B on the basis of a standard curve
(Mzcro-KIELDAHL) : The fruit pulp (less than 10 g) was mixed with 2 g of decomposition-catalyzer and 20 m l of sulfuric acid, and decomposed completely into ammonium compound The solution was filled up to 100 m l with water and part of it was distilled The resulting ammonium gas was collected a s ammonium sulfate in sulfuric acid The solution was titrated with 0 01 N NaOH and the amount of crude protein was calculated from the nitrogen value (6 25) On the other hand, a homogenized fruit pulp was placed in 200 m l Erlenmeyer flask and was added with 85 % ethanol, 10 times the amount of sample (W/V) and heated for 30 min in 85 "C water bath under a reflux condenser The residue was re-extracted with about twice the amount of solvent used in the first extraction until the extract tested negative for ninhydrin reaction The combined solution was concentrated and 20 m l of 10 % TCA was added to the solution and the precipitate was removed by centrifuga- tion The residue and the precipitate were analyzed by ~ ~ C ~ O - K ~ E L D A H L In the case of dialyzed sample, the homogenate was place in a Visking tube and dialyzed in running water for 12h
Results and Discussion
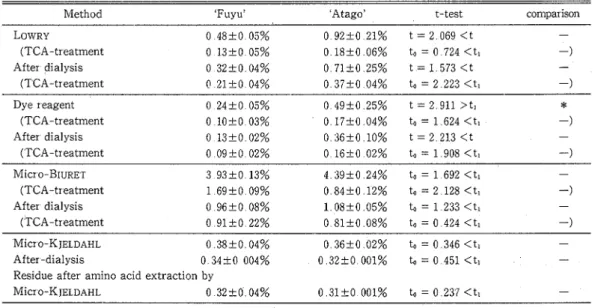
Cornpansons of conventzonal protezn assays : Table 1 shows the comparisons of protein values obtained from the different analyses, and their t-test@' There was significant difference between the protein content of 'Fuyu' and 'Atago' by dye reagent, a t 5 % level On the other hand, after deproteinization by the dye reagent there
Table 1 Comparisons of protein values obtained from different methods, and their t-test
Method 'Fuyu' 'Atago' t-test comparison
LOWRY 0 4 8 2 0 05% 0 92-10 21% t = 2 069<t - (TCA-treatment 0 13-10 05% 0 1 8 f 0 06% to = 0 724 <tl -) After dialysis 0 32+0 04% 0 71+0 25% t = 1 573 < t - (TCA-treatment 0 21-10 04% 0 3 7 1 0 0 4 % t o = 2 2 2 3 < t l -) Dye reagent (TCA-treatment After dialysis ( TCA-treatment Micro-BIURET (TCA-treatment After dialysis ( TCA-treatment
Micr 0-K JELDAHL O 38+0 04% O 3 6 + 0 0 2 % to=O346<tl - After -dialysis 0 34+0 004% 0 32+0 001% to = 0 451 <ti - Residue after amino acid extraction by
Micr o-K JELDAHL 0 32t-0 04% 0 3 1 1 0 001% to = 0 237 < t l - t (5, 0 05) = 2 571, tl (10, 0 05) = 2 228
*
: significant difference at 5 % level, - : no significant difference Values are means with SE (n = 6) t was calculated by the equation of COCHRAN'UJ54 Tech Bull Fac Agr Kagawa Univ, Vol 43, No 1, 1991
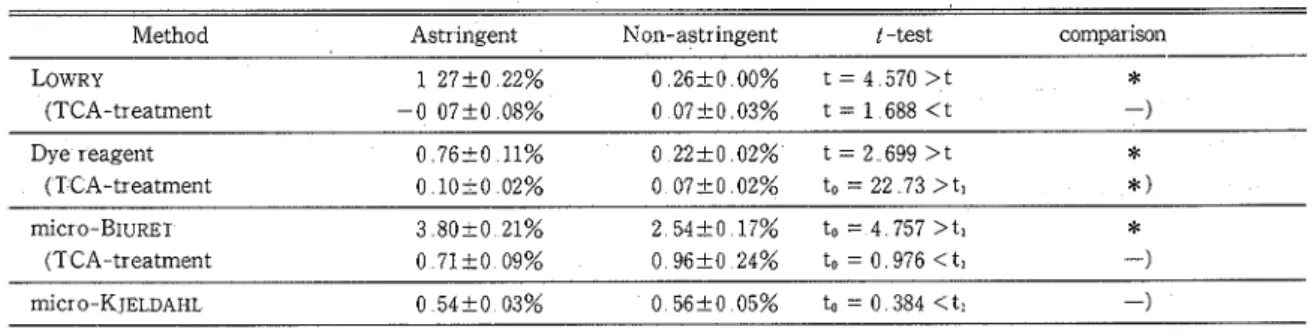
was no significant difference between the content in both cultivars a t 5 % level The protein content of 'Atago' in the middle of October 1988 was apt to be higher than that of 'Fuyu' by the methods of LOWRY, dye reagent and r n i c r o - B ~ u ~ E ~ According to ITOO('~), the soluble tannin content in astringent cultivar assayed by LOWENTHAL oxidation method(li) was higher than in the non-astringent It seemed that in the above mentioned methods, the difference in protein values of the two cultivars was due to the interference of tannins The effect of soluble and insoluble tannin on the protein values of 'Atago' was determinedy by comparing the protein values in fruit extracts where astringency was removed by heat treatment and that of extracts of non-treated fruits using the four methods (Table 2) The protein value of astringent 'Atago' was higher than that of non
'Table 2 Comparisons of proteinvalues in astringent non-astringent ' A t a g ~ ' ~
Method Astringent Non-astr ingent t -test comparison
Dye reagent (TCA-treatment rnicro-B~u~E~
('I CA-tr eatment
micr 0-K JELDAHL 0 54tO 03% O56+005% to=O384<ti -)
ti (8, 0 05) = 2 306, t (5, 0 05) = 2 571
*:
significant difference at 5 % level, - :no significant difference Values are means with SE (n = 5)aAtago is an astringent cultivar Astringency was removed by heat treatment (See methods)
-astringent The soluble tannin caused more interference in protein measurement than insoluble tannin The phenol reagent used in L.OWRY and FOLIN-DENIS methods may have deoxidized both protein and tannin, giving erroneous protein values Although the protein value obtained by K T E ~ D A H L method is affected by nitrogen compounds such as amino and nucleic acids, the nitrogen furnished by the amino acids appeared to be small as shown by non-significant difference between with or without extraction of amino acid (Table 1) On the other hand, the KJELDAHL method which is not affected by the presence of high levels of polyphenol compounds (tannins) gave comparable protein values for the astringent type 'Atago' and the non-astringent 'Fuyu' If protein is determined by using LOWRY, dye reagent and BIURET methods, the phenol compounds should be removed The conventional protein assays except KJELDAHL did not give accurate results Even dialysis did not remove these interfering compounds
Eflects of pretreatments on protezn assays
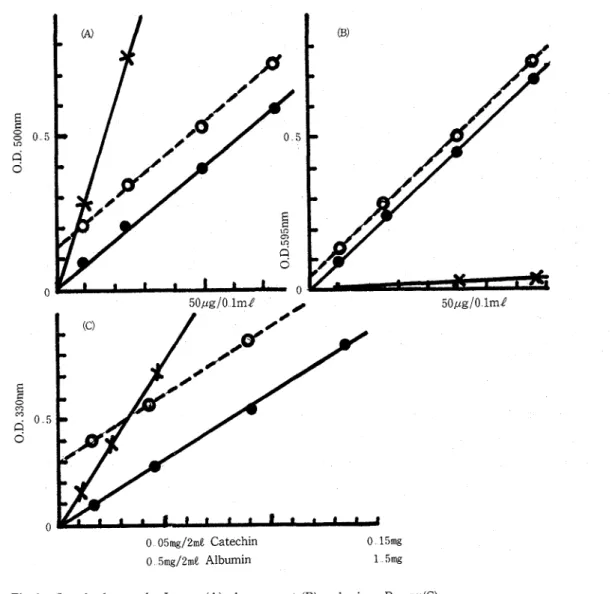
Fig 1 (A), (B) and (C) show the standard curves by LOWRY, dye reagent and micro-BIURET, respectively The standard curves of catechin and albumin, and the line obtained for 4 p g (A) or 100 p g (B) catechin plus 10, 25, 50, and 75 p g albumin were approximately straight In the micro-BIURET, the standard curves of catechin, albumin, and the line produced by 0 18, 0 45, 0 90 and 135 mg of albumin and 0 03 mg of catechin per 2 m l water were straight lines The sensitivity of LOWRY and dye reagent methods to albumin was almost the same while that of micro-BIURET was lower than the two methods On the other hand, the sensitivity of LOWRY to cathechin was about 1 9 and 120 times as high as that of micro-BIURET and dye reagent, respectively In spite of the lowest sensitivity of the dye reagent to authentic cathechin, its protein values seemed to be affected by
Toshiyuki MATSUI and Hirotoshi KITAGAWA : Protein Determination in Persimmon Fruit
0 05mg/2mQ Catechin 0 5mg/2mQ Albumin
Fig 1 Standard curve by LOWRY (A), dye reagent (B) and rnicro-B~uR~~(C)
(A) x-x , catechin (0 25 pglrne) : 0---0, catechin (4 pg/me)+albumin (0-75 pg/m$) :
-@, albumin (0-75 p g l m j ) (B) x-x , catechin (0-75 pg/me) : 0--0, cathechin (100 pg/ me) +albumin (0 - 75 p g l m l ) :
@-a,
albumin (0 - 75 pg) (C) : x-
x,
catechin (0-
0 05 mg/2 me) : 0---0, catechin (0 03 mg/mJ)+
albumin (0 - 1 3 mg/2 m l ) : @--0, albumin (0 - 1 3 mg/ ml) Values are means of duplicateanother polyphenol or unknown compounds in persimmon fruit (Tables 1 and 2) Seasonal changes zn p ~ o t a n contents of persznzmon fruzts
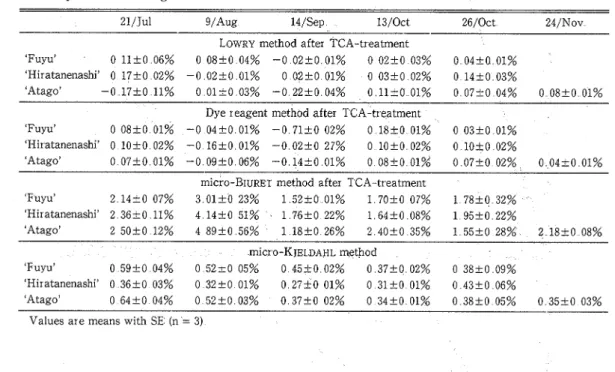
Table 3 shows the protein content during growth of persimmon fruit calculated as A-B (see methods) by L.OWRY dye reagent and micro-BIURET The protein values from July to September using LOWRY and dye reagent methods were sometimes negative The protein value by LOWRY method was negative in the presence of TCA whereas that by micro-BIUREI was positive(12) Thus, LOWRY and dye reagent methods which used TCA to eliminate tannin were not applicable for analysis of protein in persimmon fruit The protein value was not negative by ~ ~ C ~ O - B I U R E T after deproteinization in contrast to the values obtained by LOWRY and dye
56 Tech Bull Fac Agr Kagawa Univ, Vol 43, No 1, 1991
Table 3 Comparisons of protein content assayed by different methods after TCA treatment, during persimmon fruit growth
21/Jul g/Aug 14/Sep 1 3 / 0 c t 24/Nov
--- 26/0ct
LOWRY method after TCA-treatment
'Fuyu' 0 l l t 0 06% 0 08+0 04% -0 0 2 + 0 01% 0 0 2 5 0 03% 0 04+0 01% 'Hiratanenashi' 0 1 7 + 0 02% -0 02+0 01% 0 02+0 01% 0 03+0 02% 0 14+0 03%
'Atago' -0 1 7 f 0 11% 0 0 1 t 0 03% -0 22+0 04% 0 l l t O 01% 0 0 7 t 0 04% 0 08+0 01% Dye reagent method after TCA-treatment
'Fuyu' 0 0 8 t O 01% -0 0 4 f 0 01% -0 7 1 f 0 02% 0 1 8 4 0 01% 0 03+0 01% 'Hiratanenashi' 0 l o t 0 02% -0 1 6 f 0 01% -0 02+0 27% 0 l O f 0 02% 0 10+0 02%
'Atago' 0 07+0 01% -0 0 9 2 0 06% -0 1 4 f 0 01% 0 0 8 t 0 01% 0 0 7 t 0 02% 0 0 4 t 0 01% ~ ~ C ~ O - B I U R E T method after TCA-treatment
'Fuyu' 2 1 4 t 0 0 7 % 3 0 1 + 0 2 3 % 1 5 2 + 0 0 1 % 1 7 0 + 0 0 7 % 'Hiratanenashi' 2 36+0 11% 4 1 4 + 0 51% 1 76+0 22% 1 6 4 5 0 08% 'Atago' 2 5 0 + 0 1 2 % 4 8 9 + 0 5 6 % 1 1 8 4 0 2 6 % 2 4 0 + 0 3 5 % micxo-KJELDAHL method 'Fuyu' 0 59+0 04% 0 52+0 05% 0 45+0 02% 0 3 7 t 0 02% 'Hiratanenashi' 0 36+0 03% 0 3 2 4 0 01% 0 2 7 f 0 01% 0 3 1 4 0 01% 'Atago' 0 6 4 t 0 0 4 % 0 5 2 + 0 0 3 % O 3 7 + 0 0 2 % 0 3 4 + 0 0 1 %
Values are means with SE (n = 3)
reagent methods This means that micro-BIURET is stable against TCA Since the protein value with or without extraction of amino acid were not significantly different when analyzed by micro-KJELDAHL, the TCA pre-treatment procedure was omitted Micro-BIURET with pretreatment of extracts with TCA appeared to be a better colorimetric protein assay for persimmon fruit compared with LOWRY and dye reagent methods
References (1) FOLIN, 0 and DENIS, H : J Bzol Chem, 22,
305 (1915)
(2) LOWRY, 0 H , ROSEROUGH N J , FARR, A L and R E N D A L L , R J Bzol Chern, 193, 265 (1951)
(3) B R A D F O R D , M : Anal Bzochem, 72, 248 (1976)
(4) ANDO E , TERAYAMA H , NISHIZAWA K and YAMAKAWA T : Seikaggaku Kenkyu Ho (II), (Asakura Shoten, 'Tokyo), p 442 (1969) (5) KANDATSU M : Saishin Shokuhin Bunseki
Ho , (Dobun Shoin , Tokyo), p 62 (1964) (6) KITAGAWA H , MATSUI T and KAWADA H :
Engez Gakkaz Zasshz, 58 (suppl 2), 584 (1989) (7) Bio-Rad protein assay manual, (Bio-Rad Ltd,
California) p 8 (1986)
(8) SNEDECOR, G W and COCHRAN, W G : Statis- tical Methods, Sixth Edition (Iowa State Univ Press, Iowa), p 91 (1978)
(9) YOSHIDA M : Designs of Experiments for Ani- mal Husbandry, (Yoken Do, Tokyo), p 62 (1980)
(10) ITOO S : Persimmon, (AVI Publishing Inc, Conneticut), p 442 (1980)
(1U Jikken Nogei Kagaku, G e k a n , ( A s a k u r a Shoten, Tokyo), p 526(1969)
(12) PARVIN R , PANDE S V and VENKITASU
B R A M A N I A N ? A : Anal Bzochem, 12, 219 (1965)