[Mem. Fac Agr Kagawa Univ. No 16 (1963).]
STUDIES ON
THE
PENTOSE ISOMERASES
OF
LACTIC
ACID
BACTERIA
Kei YAMANAKA
Contents
INTRODUCTION 2
Acknowledgments 4
PART I STUDIES ON T H E PENTOSE ISOMERASES 4
I Methods 4 1 Microorganism 4 2 Culture 5 3 Chemicals 5 4 Enzymatic Assay 5 5 Determination 6
I1 Detection and Distribution of Pentose Isomerase Activities 6
1 Detection of Ketopentoses 6
2 Identification of Ketopentoses 8
3 Effect of Optical Isomers 10
4 Summary 11
I11 Relation between Carbon Source and Pentose Isomerase Production 11
1 Introduction 11
2 Acid Production from Pentoses 13
3 Effect of Sugar as Carbon Source on Isomerase Production 13
4 Discussions and Summary 13
I V Effect of Metals on Isomerase Production 17
1 Introduction 17
2 Methods 17
3 Effects of Metallic Ions on D-Xylose Isomerase Production 19 4 Effects of Metallic Ions on L-Arabinose Isomerase Production 20
5 Summary 20
V Purification and Proper ties of Pentose Isomer ases 2 1 1 Preparation of Isomerases
2 Chromatography on DEAE-Cellulose
3 Properties 22
4 Summary 25
VI Separation of D-Xylose and L-Arabinose Isomerases 26
1 Introduction 26
2 Preparation of Enzymes 26
3 Heat Treatment with Manganese Ions 26
4 Fractionation by Acetone at Various pH's 26
5 Separation by Chromatography on DEAE-Cellulose Column 2 7
6 Summary 29
PART I1 STUDIES ON T H E GLUCOSE ISOMERASE 29
I Production of D-Glucose Isomerase from Hetrolactic Acid Bacteria 29
1 Introduction 29
2 Methods 30
3 Effects of Cultural Conditions and Carbon Sources 30
5 Summary
11. Purification and Proper ties of D-Glucose Isomer ase from Lactobaczllur breuir 1 Preparation and Purification of D-Glucose Isomer ase
2 Column Chromatography on DEAE-Sephadex G-50 3 Properties
4 Summary
I11 Some Remarks on the Identity of D-Xylose Isomerase and D-Glucose Isomer ase
I Introduction
2 Fractionations and Chromatographies 3 Effect of Thermal Inactivation 4 Inhibition by the Other's Substrate 5 Discussions and Summary IV Reaction Mechanism
1 Introduction
2 Effect of Arsenate on Enzyme Activity 3 Inhibition by Xylitol
4 MICHAELIS Constants for Substrates and Manganese Ions 5 Discussions 6 Summary V Conclusions Literatures Resum6 in Japanese INTRODUCTION
Pentose isomerase is the enzyme which catalyzes the interconversion of an aldopentose and the corresponding ketose, and it was found mainly in bacterial cells. COHEN first demonstrated the formation of D-ribulose from D-arabinose by extracts of a mutant strain of E~cherichia coli grown on D-arabinose, and named a s D-arabinose isomerase('). Similarly, L-arabinose isomerase was also found in the cell-free extracts of Lactobacillus pentosus by
LAMP EN(^'),
and by BURMA et al.(7'19) and in Aerobacter aprogenes by SIMPSON and woo^(^'*^^). These activities were found only in the celIs grown on L-arabinose.D-Xylose isomerase which isomerizes D-xylose to D-xylulose has been demon-, strated in the extracts of the cells grown on D-xylose of Pseudomom hydrophila by HOCHSTER and W A T S O N ( ~ ' ~ ~ ~ ) , of L. pentosu~ by MITSUHASHI and
LAMP EN(^^),
and of Pas- teurella pe,stis by SLEIN(~'). Other pentose isomerases were also reported in bacteria: L-xylose and L-lyxose isomerases from Aerobacter a e r ~ ~ e n e s ( ~ - ~ ) .It is well known that there are two types on the glucose fermentation by lactic acid bacteria: the homo-type of them (homofermenter) ferments glucose to two moles of lactic acid, and the other (heterofermenter) yields each one mole of lactate, ethyl alcohol and carbon dioxide from glucose. On their pentose fermentations, however, any differences can not be found between the products by both types of bacteria. D-Xylose and L-arabinose are converted quantitatively into acetic and lactic acids and reports on isotopic analyses of the decomposition products of
1-c14
labelled ~ - a r a b i n o s e ( ~ ~ ) , ~ - x y l o s e ( ' ~ ' ~ ~ ) or ~ - r i b o s e ( ~ ) by L. pentoaceticus or L. pentosusStudies on the Pentose Isomerases of Lactic Acid Bacteria 3
pentose phosphate before cleavage of the pentose chain was occurred(15), and the isomerization of pentose would take place a t the early stage of these fermentations. On homofermenter, the arabinose and xylose isomerases have been demonstrated from L. plantarum (L. pentosus) and further studies on the mechanism of pentose fer- mentation by this bacterium were reported by HORECKER et al@, 19,20). They propos- ed that the first step in the fermentation of L-arabinose by L. plantarum is isomeriza- tion to L-ribulose. Though the activities of these enzymes from heterofermenters have yet not been investigated. Consequently, we had few available papers on the purification, properties and reaction mechanism of pentose isomerase from bacteria a t that time when I began the study on the pentose isomerase.
Our major research efforts during the past several years and a t the present time are concerned primarily with the pentose isomerases of lactic acid bacteria, especially on heterofermenters, In this Memoir, it was summerized the essential data obtained for over seven years not only on the pentose isomerases but also on the D-glucose isomerase which was found in 1961 by myself. Our original reports have been published mainly in English under the titles of "Studies on the pentose isomer ases from lactic acid bacteria" and of "Sugar isomer ases" in Agricultural Biological Chemistry (formerly Bulletin of the Agr icultur a1 Chemical Society of Japan) and some of them under the title of "Studies on the utilization of sugar isomerases" in Japanese as follows :
K. YAMANAKA; Tech Bull Fac Agr , Kagawa Unzv, 9 , 146 (1958)
Xylulose, Formation enzymatique par bacille lactique (in French)
K YAMANAKA; Bull Agr Chem Soc , 22, 299 (1958)
Part 1 Formation of ketopentoses by lactic acid bacteria (Formerly, Studies on the pyruvate and carbohydrate metabolisms by lactic acid bacteria Part IX)
K YAMANAKA; Zbzd, 24, 305 (1960)
Part 2 Some properties of pentose isomerases by hetero-type of lactic acid bac- ter ia
K YAMANAKA; Zbzd ,24, 310 (1960)
Part 3 Relation between carbon source and pentose isomerase production K YAMANAKA ; Agr Bzol Chem , 25, 272 (1961)
Part 4 Effects and requirement of metal
K YAMANAKA; T e ~ h Bull Fac Agr , Kagama Unzv , 13, 185 (1962)
Part 5 Formation of D-xylose isomerase by Lactobacillur fermentum (in Japanese)
K. YAMANAKA and T HIGASIHARA; Agr Bzol Chem ,26, 162 (1962) Part 6 Effects of metals on isomerase production
K YAMANAKA ; Zbzd ,26, 167 (1962)
Part 7 Chromatography of pentose isomerases
K YAMANAKA; Zbzd ,26 176 (1962)
Part 8 Separation of D-xylose and L-ar abinose isomer ases
K YAMANAKA; J Fermentatzon Arsoczatzon, Japan, 20, 305 (1962) Purification of isomer ases (in Japanese)
K YAMANAKA; J Starch Sweetener Techno1 Rer Soc Japan, No 26, 44 (1962)
On the sugar isomerases, especially on the glucose isomerase from lactic acid bacteria (in Japanese)
K YAMANAKA; Agr Bzol Chem ,27, 265 (1963)
Sugar isomerases Part 1 Production of D-glucose isomerase from Heterolactic Acld Bactei ia
Part 2 Purification and proper ties of D-glucose isomerase from Lactobaczllus breuzs.
K YAMANAKA; Nzflon N6gei Kagaku Kazrhz (J Agr Chem Soc Japan). 37, 231 (1963)
Studies on the utilization of sugar isomerases Part 1 Basal conditions for D-
glucose isomerase reaction (in Japanese ) K Y A M A N P K A ; Zbzd, 37, 237 (1963)
Part 2 Reaction of glucose isomerase on starch hydrolyzates and their hydrols. (in Japanese)
Acknowledgments
The author wishes to express his gratitude to Dr. Hideo KATAGIRI, Emeritus Professor of Ky8to University, Prof. Kakuo KITAHARA, director of the Institute of Applied Microbiology, T8kyo University, Prof. KGichi OGATA, Ky6to University, Prof. Tadao HATA, director of the Research Institute for Food Science, KyGto University, Prof. Ytihei MORITA, the same Institute. He also wishes to acknowledge his indebtness to Dr. Yoshiro KUROIWA, the Research Institute of Kirin Brewers Co
,
Dr. Taiji KUROKAMI, the former Dean of this Faculty, Former Prof. Kenji KATAKURA, and Prof. Kazutami IMAI, Okayama University, Prof. Tadao MAEKAWA, Dean of this Faculty, Prof. Sin'itir8 KAWAMURA, Prof. Akira KAJI, Assistant-prof. Takayuki TARUTANI and Assistant-prof, Teiiti NARASAKI of this Faculty, and Assis- tant-prof. Sakuz8 FUKUI, the Institute of Applied Microbiology, TGkyo University.He also greatly acknowledged to Prof J 0. LAMPEN, director of the Institute of Microbiology, Rutgers, the State University, New Brunswick, New Jersey,
U.
S. A. for kind informations of his unpublished data on the metal requirement of isomerases which he has already obtained by L. p?ntosus, and his valuable sugges- tions and keen interests.
This work was supported by Grants defrayed by the Ministry of Education (1958, 1962) to whom the author is grateful.
PART I. STUDIES ON THE PENTOSE ISOMERASES
I. Methods
I. Microorganisms.
Thirteen strains of heterofer menter s and twenty strains of homofermenter s were used throughout this experiment These microorganisms were provided from Prof. H. KATAGIRI, KyBto University and some of them from the Institute of Fer- mentation, Osaka. The collection number from the latter was expressed in parensis. Heterofer menter Lactobaczllus fcrm ntum
6 ,
L. fermsnti ATCC 9338, L. brevis ATCC 8281, L p~ntoacetzcur ATCC 368, L. gayonii ATCC 8289, L. mannitopoeus, L. bcopersicz, L. buchneri, Leuconostoc m,~s~nteroides a, Leuc. m~sent~roides ATCC 8042 (IF0 3076) and ATCC 9135 (IF0 3426), Leu. dextranicum ATCC 8086 and Leuc. citrouorum ATCC 8081Homofer menter Lactobacillus delbruckii, L lactzs, L. hlveticus ATCC 335 (IF0 3219), L. aczdophilus, L bulgaricus, L. casei, L. plantarum, L. plantarum ATCC 8008, L. arabinosur ATCC 8044, L cucumeris ( I F 0 3074), L sake' (IF0 3497), L. wortmannii ( I F 0
Studies on the Pentose Isomerases of Lactic Acid Bacteria .5
3075), L. xylosur, L. lezchmannzi ATCC 4797 (IF0 3073), Streftococcus faecalir ATCC 8043, Sc. lactir, Sc. liquefa~ciens ATCC 4532, Sc. thermofhzlur, Sc. saliuaricur ATCC 9758 and Pediococcur lzndnerz.
2. Culture.
T h e medium which was used mainly throughout this experiment was compos- ed a s follows: 1 % peptone, 1 % yeast extract, 1 % Na-acetate, 0.02 % MgSO, 7Ha0, and each 0.001 % MnS04
-
4Hz0, NaCl and FeSQ4- 7Ha0. This medium was modifi- ed with some extent on metal concentrations in the experiments after Chapter V. Malt-extract medium (sugar content 4 % ) was also used with addition of 1 % yeast extract and 2 % of CaC03 in early period of this serie. Sugars were sterilized separately and added just before inoculation. Cultures were maintained a t 37°C for 16 hours.3. Chemicals.
D-Xylose and L-arabinose were the products of Merck, and L-xylose and D- ar abinose were purchased from Mann Research Labor ator ies. Xylose used a s car
-
bon source was obtained from the Nippon Mokuzai Kagaku Co a t Yosihara, Sizuoka. Xylulose and ribulose were prepared by epimerization in pyridine under a reflux c o n d e n ~ o r ( ' ~ ~ ~ ~ ) . CM*- and DEAE-celluloses and DEAE-Sephadex were the products from Serva, Germany and from Pharmacia, Sweden, respectively. 2-Deoxyglucose, NAD and NADP were the Sigma's products, U. S. A., and crystalline xylitol was kindly provided by Mr. Yoshio MAEDA, the Section-chief of Noguchi Institute, Itabasi, T6kyo
4. Enzymatic Assay.
Cells were harvested by centrifugation or by turbine-driven Sharples centrif- uge, and enzyme was extracted by 0.01 M Tris buffer (pH 7.0) after grinding the cells with twice their weight of alumina, and then alumina was centrifuged off a t 13,000
x
g for thirty minutes. Clear supernatant was used a s crude extracts, and further purifications were carried out on these extracts.T h e reaction system was composed of 50 pmoles of Tris buffer (pH 7.0 for D-xylose isomerase and pH 8.0 for L-arabinose isomerase), 0.2 ml of M of MnSQ4 and enzyme solution in total volume of 1.8 ml, and preincubated a t 30°C for ten minutes, then 20 pmoles of pentose (D-xylose or L-arabinose) were added and reaction was proceeded a t 37°C for ten minutes.
A unit of enzyme was defined a s the quantity which produces one micromole of ketopentose from aldopentose under this condition, and specific activity was expressed a s unit of enzyme per mg of protein..
In early experiments (chapters I1 and 111), manganese ions were not added, and
*
The following abbreviations were used: ATP, adenosine triphosphate; NAD, diphosphopyridine dinucleotide ; NADP, triphosphopyridine dinucleotide ; Tr is, tris-(hydroxymethy1)-aminomethane;CM-cellulose, carboxymethyl-cellulose; DEAE-cellulose (-Sephdex), diethylaminoethyl-cellulose (-Sephadex); TCA, trichloracetic acid and EDTA, disodium salt of ethylenediamine tetraacetate
some of results were obtained with addition of 20 pmoles of sodium tetraborate. 5. Determination.
Reducing sugars were determined by the Folin-~alrnros('~) or by the Somogyi- Nel~on'~') methods. Ketopentoses were estimated by the cysteine-carbazole reac- tion(''). The color was developed a t 20°C and was read after standing for 20 or 100 minutes for ribulose or xylulose, r e ~ ~ e c t i v e l y ( ~ ~ ~ ~ ) . Another methods were also applied for this purpose, such as resocinol reaction modified by KULKA('~) and orcinol reaction(''). Protein was determined by Lowry's method(32) and phosphate by the method of FISKE and SUB BAR OW('^). Oxidation of pentose by bromine was carried out with 0.5 ml of bromine saturated water and 300 mg of BaC03 a t 25°C for 30 minutes. In this condition, 2000 pmoles of xylose was completely oxidized.
Paper chromatography was made the triple development by ascending the solvent a t room temperature, and spots were visualized by spraying either aniline- hydrogene phthalate or orcinol-TCA.
11.
Detection and Distribution of Pentose Isomerase A ~ t i v i t i e s ( ~ ~ ' ~ ~ )1. Detection of Ketopentoses.
Formation of ketopentoses from D-xylose and L-arabinose was examined with dried cells obtained from malt-extract medium. For each experiment, 15 mg of dried cells were used and borate was added to accelerate the accumulation of ketose. After incubation for on hour, reaction mixture showed the positive color by cysteine-cabazole test which spectrum had an absorption maximum a t 540 m,u (Fig.. 1). And it also showed a specific spectrum which had two absorption maxima a t 540 and 670 mp by orcinol test after oxidation by bromine (Figs. 2, 3), but they were always fully nagative when boiled bacterial preparations were used. Then it seems possibly that the reaction product would be ketopentose. Amounts of ketopentose and Br2-stable pentose from xylose were formed in almost equal a t every reaction time (Table I), and their ratios of optical densities a t two peaks of spectrum by orcinol test were found to be 0.37-0.49. These evidences might be suggested that the reaction product would be xylulose.. Similarly, ribulose was
,
0 1 I
400 500 600 700
Wavelength l m v l
Fig 1 Spectra of Ketopentose
A. Formed from xylose by L fermenturn B Formed from arabinose by L brevzr
(1) Cysteine-car bazole reaction. (2) Resorcinol reaction
Studies on the Pentose Isomerases of Lactic Acid Bacteria
W a v e l e n g t h ( m y )
Fig 2 Spectra of Reaction Product from D-
Xylose by Orcinol After Br,-Oxidation. 1 L buchnerz, 2. Leuc mesenterozdes,
3 L xylosus, 4 L brevir
500 60 0 700
Wavelength ( m y )
Fig 3 Spectra of Reaction Product from L- Arabinose by Orcinol After Br,-Oxida- tion.
1 L buchnerz, 2 L gayoniz, 3 Pc lindneri.
Table I Formation of Bromine-Stable Pentose
Incubation time (min )
.-
/
20 40 60 90 120Br,-stable pentose (p mole) 8 . 8 11.3 12. 6 12. 0 11. 6
Ketopentose (p mole)
I
6 7 11 4 13 7 13 2 12.9Leuc mesenteroider, 150 mg of dried cells, substrate; 20 p moles of D-xylose and 20 p moles of borate was added
150 mg of dried cells were ground with 300 mg of alumina, extracted with 5 ml of extractant Reaction was carried out in Tris buffer at pH 7 4 for 2 hours at 37°C
*
200 mg of dried cells were usedTable 11. Pentose Isomerase Activities of Cell-Free Extracts
-- -Organism
/
Water 3 6 5 10-xylose1
0 1 9 0 18 I 0 05 M phosphate (pH 7 4) 2 5 // 0 2 7 0 28 L fermenlum,
0 05 M Tris (pH 7 4)1
2 2 5 0 31 0 25 --1 0.05 M NaHCO, 1 2.6I
0 . 2 6 0.261
Water 2 4 D-xylose 3 52 12 3 L xylorur* 0 05 M phosphate (pH 7 4) 2 3 N 2 90 13 9 0 05 M Tris (pH 7 4) 2 9 N 2 79 10 4/
0 05 M NaHCO, 3.6 // 2.34 10. 11
water 1 51
r-arabinose 0 36 0 46 L gayonzz/
0 05 M NaHCO, 2 2 a1
0 5 3 0 56 Extractant/
Specific activity Substrate (p mole Ketose/mg protein) Iwithout with
L fermentum Leuc metenterozder L brevz r L pentoacetzcur L gayonrz L buchnerz L fermentz L xylorur PC lzndneri
Table I11 Detection of Ketopentoses
-
-Reaction time, one hour with addition of 20 p moles of borate
detected a s the reaction product from arabinose. These enzymatic activities could be extracted by water, buffers or dilute alkali after grinding the cells with levigated alumina (Table 11).
T h e activities of isomerization of pentoses by hetero- and homofermenters are summer ized in Table 111. Whereas ketopentoses were actually formed by these heterofermenters, they were scarecely formed by the most strains of homofermen- ters, such as L. delbruckii, L plantarum, L. arabznosus, L. casei, L. acidophilus and L. bulgaricus However, L. xylosus and
PC.
lindnerz showed the isomerization activities on pentoses. These differences will be elucidated in the next chapter.Organism
2. Identification of Ketopentoses.
Ketopentose formed ( p mole)
D-Xylose
I
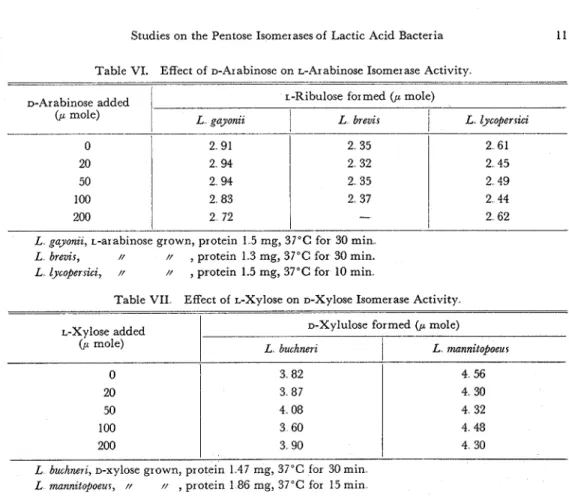
L-ArabinoseDuring the isomerization reaction, phosphorylation of pentose did not occur in the absence of ATP'64). Then the isolation of reaction product was carried out by the usual method of chromatography on a Dowex-1 (borate) column (1.5 x 14 or 20 ~ m ) ( ~ ~ ) . . Reaction mixture was composed of 150 mg of dried cells, 600 ,&moles of pentose, 300 ,&moles of MgC12, 750 ,&moles of phosphate buffer a t pH 7.4 and 300 ,&moles of sodium tetraborate in total volume of 30 ml. After incubation for 2 hours a t 37OC, the incubation mixtures were deproteinized by heating in a boiling- water bath for 5 minutes and centrifuged. T h e clear supernatant was diluted to 5 times in volume by water and chromatographed on a Dowex-1 borate column, and sugars were eluted with 0.02 M sodium tetraborate.. Consecutive fractions were collected, passed through Amberlite IR-120 (H-form), and then condensed under reduced pressure below 30°C" Borate was removed a s its volatile methyl ester by adding methanol. This procedure was repeated three times or more, and qualitative analyses were carried out with each fraction by paper chromatography. These elution patterns a r e presented in Fig. 4, and RF values of each fraction a r e listed in Table IV.
On the xylose isomerization by L. ,fermenturn, Leuc. mesenter0ide.s and L. xylo,wr, two peaks were completely separated. T h e first peak revealed a positive cysteine- carbazole reaction, and was identified a s xylulose by its RF value and by the ratio
Studies on the Pentose Isomerases of Lactic Acid Bacteria E l u a t e ( L ) '2 L 0 . 0 2 M Na tetrabomte
E
E l u a t e ( L )10.02M
Na +e+rubom+e-
c E l u a t e ( L )Fig 4. Chromatography of Pentose on Dowex-1 Borate Column
Xylose isomerase, A L fermentum. B Lew. merentermder, C L xylorur Arabinose isomerase, D L gayonzt, E Pc lzndneri
Table IV Paper Chromatography of Fractions - -- - - -- L xylorur Organism L gayonii
1
No !H,O (4:1:2) - ----p.p----p-p-p ( 9 : 1) I~ractionl R F Substrate I O H: Phenol : H,Oof : in orcinol reaction. Further elution of the column with 0.02 and 0.04
M of borate gave a second peak, and it was identified as xylose by R p value and by
orcinol spectrum although its cysteine-carbazole test was negative. Similarly, rib- D,,o : D ~ i o
(or cjnol)
Pc lzndnen
ulose was separated as the first peak and identified by positive cysteine-carbazole 0 40
L-Arabinose
1
1
0 42I
0 34test and by orcinol spectrum identical to authentic sample. Arabinose was recov- ered in the latter part of fraction. Thus, it was concluded that xylulose is formed from xylose and ribulose from arabinose by these bacteria.
3. Effect
of
Optical
Isomers.
The cell-free extracts of L. gayonii grown on glucose and of L. lycopersici grown on glucose or on D-xylose were found to react specifically on D-xylose or L-arabinose (Table V). Ketose was not produced from glucose, mannose, galactose, L-xylose, D-
Table V. Specificity of Substrate.
--
-1
Ketose formed ( p mole)Organism I
I L gayonzz
1
L lycoperrin'Carbon source
i
glucose1
glucose x y loseExtracts used (protein, mg)
I
1 32i
0 82 1 45 glucose1
0 mannose 0 galactose n-xylose1
:45 L-xylose L-ar abinose n-ar abinose n-r ibose I hamnose mannitol 0 2 4 0 0 0 0 - Reaction at 37°C for 30 minutesStudies on the Pentose Isomerases of Lactic Acid Bacteria 11
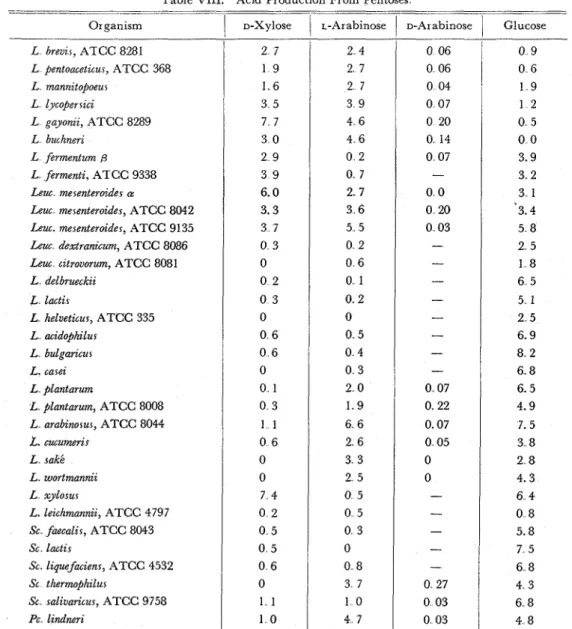
Table VI. Effect of D-Arabinose on L-Arabinose Isomerase Activity. D-Arabinose added
1
L-Ribulose formed ( p mole)(P mole) L gayonii
1
L brevir I 1 L lycoperrzciL gayonzz, L-arabinose grown, protein 1 5 mg, 37°C for 30 min L brevis, // // , protein 1 3 mg, 37°C for 30 min.
L lycoper sicz, // //
,
protein 1.5 mg, 37°C for 10 minTable VII Effect of L-Xylose on D-Xylose Isomerase Activity
-- .
-arabinose, D-ribose, rhamnose or mannitol in this condition. Moreover, D-arabinose did not reveal any inhibitory effect on L-arabinose isomerase activity (Table VI), and L-xylose did not showed any inhibitory effect on D-xylose isomerase activity (Table VII). So it was possibly suggested that these enzyme activities were the D-xylose isomerase and L-arabinose isomerase..
L-Xylose added
( P mole)
4. Summary.
D-,Xylose isomerase activity was found in the dried cells and their cell-free extracts of several strains of heterofermenters and of L. xylosus, and L-arabinose
isomerase activity in the several strains of heterofermenters and of
PC.
lindnerz. Products from D-xylose and L-arabinose were identified as xylulose and ribulose, respectively, and their optical configurations were determined as D-xyiulose and L-ribulose depend on the inability of these enzyme preparations towards their. op- tical isomers,.D-Xylulose formed ( p mole)
L buchnerz
I
L munnitopoeur 4 56 4 30 / 4 32 4 48 4 30 0 I 3 82111. Relation between Carbon Source and Pentose Isomerase Produc- tion@@
L buchnerz, D-xylose grown, protein 1 4 7 mg, 37°C for 30 min L mannztopoeur, N // , protein 186 mg, 37°C for 15 min
20 50 100 200 1. Introduction. 3 87 4 08 3 60 3 90
Our knowledge on the production of pentose isomerase by microorganisms is still obscure, and only few reports concerning this problem are available in which it is suggested that its production is adaptive to the substrate of growing culture.
K A R S T R O M ( ~ ~ ) in 1938 has first suggested to introduce two categories for enzyme production, constitutive and adaptive depend on his observations on pentose fer- mentation by bacteria. Subsequently, LAMPEN and PETER ~ O H N ( ~ ' ) reported on the
fermentation specificity for D-xylose, L-arabinose and D-ribose by L. pentorus, a homofermenter.. In lactic acid bacteria, D-xylose and L-arabinose isomerases have been shown to be produce adaptively only on each Aside from these obser.vations, the production of these isomerases by another type of bacteria, heterofermenter has yet not been discussed.
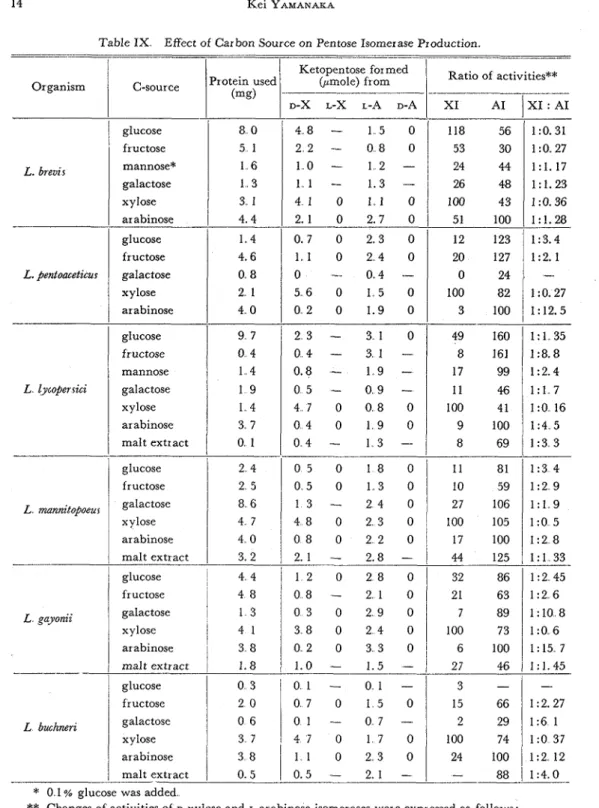
On this problem, changes of activities of isomerases were assayed in the ex- Table VIII. Acid Production From Pentoses
---- -- Organism L breuzr, ATCC 8281 L pentoacetzcur, ATCC 368 L mannztopoeur L lycoper rzci L gayonzz, ATCC 8289 L buchnerz L fermentum 6 L fermenti, ATCC 9338 Lew merenterotdes P
Leuc merenteroides, ATCC 8042 Leuc. mesenteroides, ATCC 91 35 Leuc dextranzcum, ATCC 8086 Leuc cztrovorum, ATCC 8081 L delbrueckzz & lactir L helveticus, ATCC 335 L acidophilur L bulgarzcur L . carez L plantarum L plantarum, ATCC 8008 L arabinorur, ATCC 8044 L. cucumzr L rakl. L wortmanniz L xylorur L. lezchmanniz, ATCC 4797 Sc faecalir, ATCC 8043 Sc lactzr Sc lzquefmiens, ATCC 4532 Sc thermo$hzlur Sc salivaricus, ATCC 9758 PC lzndnerz
Values are expressed mi of 0 1
1
D-Xylose 2 7 1 9 1 6 3 5 7 7 3 0 2 9 3 9 6.0 3.3 3 7 0 3 0 0 2 0 3 0 0 6 0 6 0 0 1 0 3 1 1 0 6 0 0 7 4 0 2 0 5 0 5 0 6 0 1 1 1 0 N N a O H in ten ml i-Arabinose 2 4 2 7 2 7 3 9 4 6 4 6 0 2 0 7 2 7 3 6 5 5 0 2 0 6 0 1 0 2 0 0 5 0 4 0 3 2 0 1 9 6 6 2 6 3 3 2 5 0 5 0 5 0 3 0 0 8 3 7 1 0 4 7 medium after 44 o-Arabinose1
Glucosei
0 06'
0 9 0 0 6 0 6 1 9I
1 2 0 20 0 14 0 07-
0 0 0 20 0 03 ---
- --
-- - - 0 07 0. 22 0.07 0 05 0 0 --
--
-
0 27 0 03 0 03 hours incubation. 0 5 0 0 3.9 3 2 3 1 3.4 5 8 2 5 1 8 6 5 5 1 2 5 6.9 8 2 6 8 6. 5 4.9 7. 5 3 8 2 8 4.3 6 4 0 8 5.8 7 5 6 8 4 3 6 8 4 8Studies on the Pentose Isomerases of Lactic Acid Bacteria
tracts from the cells grown on various sugars in media. 2. Acid Production from Pentoses.
Growth on pentoses (D-xylose, L - and D-arabinoses) and on glucose was deter- mined by titration with a standard alkaline solution, and values were expressed a s the ml of N/10 NaOH consumed to neutralize to pH 7.0. Cultures were made on 10 ml of medium containing 1 per cent of sugar for 44 hours' incubation. T h e results obtained a r e listed in Table VIII. Among thirty-three strains of lactic acid bacteria, nine strains of heterofermenters could grow both on D-xylose and L-arabinose, two strains of heterofermenters (L firm:ntum and L. ferm?nti) and a homofermenter L xylosus showed growth only on D-xylose, eight strains of homo- fermenters grew well on L-arabinose However, poor or no growth and no acid production were obtained with two strains of Leuconostoc (Leu dextranicum and Leu. cztrovorum) and other strains of homofermenters. D- Arabinose was not fer- mented by all strains even by those ferment L-arabinose. As already mentioned, it was confirmed, that D-arabinose was neither fermented nor isomerized by lactic acid bacteria
3. Effect of Sugar as Carbon Source on Isomerase Procuction.
Changes of activities of pentose isomerases are tested on the extracts prepared from the cells which a r e obtained from media containing various sugar a s a single carbon source, and summerized in Tables IX-XI On the strains which could not ferment pentoses, such a s L. lactis, L. helvzticus ATCC 335, L acidophilus, L. bulgarims, L. delbrkkiz, L. casei, L. lsichmannzz ATCC 4797, Sc. faecalzs ATCC 8043, Sc. lactis, Sc. lzqugasi ns ATCC 4532, Sc salivaricus ATCC 9758, the study on the production of isomerases was omitted, and isomerase activities were never found in the cells of Leuc, dextranzcum and Leu. citrovorum. Incubation was proceeded for thirty minutes and activity was expressed a s micromoles of ketopentose formed under this condi- tion. Ketose was never obtained from L-xylose or D-arabinose in all strains and in any of the conditions tested here.
In regard to the homofermenters which were able to ferment L-arabinose (L. plantarum, L. cuum?rzs, L. saki, L wortmannii and Sc. thrmophzlus), activity of L-arabinose
isomerase was found only on L-arabinose-grown cells. D-Xylose isomerase of L. xylosus and L-arabinose isomerase of PC. lindneri were found in the cells grown on glucose, fructose, mannose or pentose. However, powerful activities of D-xylose and L-arabinose isomerases were found from the cells of most strains of heterofer- menters from various sugars of medium, even in which did not contain pentose.
4. Discussiolns and Summary.
T h e specificity of pentose fermentation by bacterial cells was first demonstrat- ed by K A R S I R O M ' ~ ~ ) . He found that Lemonostoc messnter.oides (Betacoccus ambinosaceus)
was able to ferment glucose in all cases, but L-arabinose was fermented only by the cells grown on L-arabinose. So he considered the enzyme for. fermenting glu- cose to be constitutive but those for L-arabinose a s adaptive. Moreover, he also observed that the enzymes for fermehting D-xylose and L-arabinose were constitu-
The following abbreviations are used: D-X, D-xylose; L-X, L-xylose; L-A, L-arabinose; D-A, D-ar abinose ; XI, xylose isomer ase and AI, ar abinose isomerase
Table IX Effect of Carbon Source on Pentose Isomerase Production
-- Organism -- Keto~entOse formed (pmole) from - D-X L-X L-A D-A
C-source -- Ratio of activities** -
XI A1
1
XI : A1 Protein used (mg) 4 8-
1 5 0 2 2 -- 0 8 0 1 0-
1 2 - 1 1 -- 1.3-
4 1 0 1 1 0 2.1 0 2.7 0 0.7 0 2 3 0 1 1 0 2 4 0 0--
0.4 .- 5 6 0 1 5 0 0 2 0 1.9 0-
2 3-
3 1 0 0 4-
3 1-
0.8-
1 9-
0 5-
0 9-
4 7 0 0 8 0 0 4 0 1 9 0 0 4-
1 3- - I
118 56 53 30 24 44 26 48 100 43 51 100 L. brevt s L. fentometacur -- L lycofer sici 1:0.31 1 : 0 2 7 l : l . 17 1:1.23 1:0.36 1:1.28 L mannitopoeus1
glucose 8 0 fructose 5 1 mannose* 1 6 galactose 1 3 xylose 3 1 ar abinose1
4.4 glucose fructose 2 5 galactose xylose glucose fructose galactose x y lose ar abinose glucose fructose mannose galactose xylose ar abinose malt extract 0 69LL-
L gayonii1
1 4 4.6 0 8 2 1 4 0 9 7 0 4 1 4 1 9 1 4 3 7 0 1 49 160 8 161 17 99 11 46 100 41 0 5 0 1 8 11 81 0 5 0 1 3 O 0/
10 19 1 3-
2 4 27 106 4 8 0 2 3I
100 105 1: 1 35 1:8.8 1:2.4 1:l 7 1 : 0 1 6 1 : 3 4 1 : 2 9 1 : 1 9 1 : 0 5 9 100 I 1 : 4 5 8 1 3 3 arabinose1
4 0 0 8 0 2 2 01
17 100 malt extract1
3.2 2. 1-
2.8 -1
44 125 1 : 2 8 1:l 33 L buchnen glucose I 4 4 fructose I 4 8 I galactose 1 1 3*
0 1 % glucose was added**
Changes of activities of D-xylose and L-arabinose isomerases were expressed as follows: Ratio of activities = (micromoles of ketopentose formed by each extracts)/(micromoles of ketopentose formed by extracts from pentose-grown cells) x 10015 66 2 29 100 74 24 100 fructose 0 1 5 0 galactose 0 6 0 1
-
0 7 - xylose 3 7 4 7 0 1 7 0 arabinose 3 8 ' 1 1 0 2 3 0 malt extract --1
0.5 0 . 5-
2. 1 - pp 1 2 0 2 8 0 0 8-
2 1 0 0 3 0 2 9 0 1:2.27 1 : 6 1 1 : 0 3 7 1 : 2 1 2-
-88 l l : 4 . 0 -xylose 3 8 0 2 4 0 arabinose 0 2 0 3 3 0 6 1 0 0 / 1 : 1 5 7 1.0-
1.5 -4
27 46 1 1:1.45 glucoseo
3-
0 1 - 3 - -Studies on the Pentose Isomerases of Lactic Acid Bacteria 15
Table X Effect of Carbon Source on Pentose Isomerase Production I
Or gariisrn
I
C-source1
1
Protein used (mg) 1 - glucose I 0 7 fructose* 0 8 mannose I 1 5 galactose* I 0 3 Ketopentose formed(,urnole) from
1
Ratio :;tivity - -- - -- ---- - -- - - -- D-X L-X L-A D A L firmentutn L ferment1 Leuc merentermder a*
0 1 % of glucose was added. Abbreviations see in Table IXxylose 1 1 ar abinose* 0 6 maltose I 5 2 sucrose 3 8 I gluconate 3 3 malt extract 4.8 glucose 2 2 fructose
I
0 7 mannose1
xytose1
i.:
glucose fructose mannoseI
4::
2 xvlose 3 . 2tive, whereas those for glucose adaptive in L. pentoaceticu~. On a report by LAMPEN and P E ~ E R ~ O H N ( ~ ' ) , ability for fermenting glucose by L. pentosus was constitutive, while the enzymes for acting D-xylose or L-arabinose were adaptive.
On the production of pentose isomerases, D-arabinose isomerase of Escherichia coli was produced only from a medium containing D-arabinose@). L-Arabinose iso- merase was found only in the cells grown on L-arabinose-medium of L. p e n t o s ~ s ( ~ . ~ ~ . '*), A. aer~~enes(*~) and of E. co~i(~'). Similarly, D-xylose was a sole effective carbon source for production of D-xylose isomer ase from L. pentos~s(~~), Pseudonwmr hydrofihila
(219 22), E.c 01i(~l), Salmonella p p h o . ~ a ( ~ ~ ) and ~arteurellapestis(~~), and this was also same for
L-rhamnose isomerase(12' 39!. AS a few exception on this problem, L-fucose isomerase
of E. coli was found from either D-arabinose- or L-fucose-grown cells(18). GREEN
and COHEN proposed that D-arabinose isomerase and L-fucose isomerase would be the same enzyme, but further evidences on the identity of both enzymes as the same were not presented. Activity for isomerizing D-glucose to D-fructose was found only on the cells grown on xylose from Pseud. hydrophila(33), but the relation between the activities of D-xylose isomerase and D-glucose isomerase was not de- termined. On a part of this question, it will be elucidated in Part 11.
While the pentose isomerases seemed to be produced adaptively by most of bacteria, those by most strains of heterofermenters were always found even from the medium in which did not contain pentose. In contrast, the isomerase in most strains of homofermenters seems to be adaptive, it can be demonstrated only in
Table XI. Effect of Carbon Source on Pentose Isomerase Production
Organism
Protein Ketose formed (pmole) from C-sour ce
/
Ti:)
1
D-X L-X L-A D-A D-R G ML plantarum
/
malt extract L wwtmannii glucose fructose mannose ar abinose glucose fructose mannosethe pentose-containing medium. These evidences a r e seemed to be not only one
PC l~ndnerz
L xylorur
of the interesting problems on the mechanism of enzyme production but also able
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
-
2.4 0 - --
0 -- 0 0 0 0 0 0 0 0-
0 0 0 0 -- 0 - 0 0 0 0 0 2.5 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 --
1.8 0 - -- - 2 4 1 7 2 4 I. 1 L cwumemr1
2~
1
i:
mannose 2 31
arabinose/
1.01
arabinose/
glucoseto serve the useful for enzyme preparation with lesser expenses on the heterofer- menters. 0
-
0 0 0 0 0 0-
0 0 0 0 0 0 -- 0 0 0 0 0 --
2.4 0-
- -. L. arabinorur L saki. Sc thermophilur*
0.1 % of glucose was addedThe following abbreviations are used: D-R, D-ribose; G , glucose; M, mannose, and about D-X, L-X, L-A and D-A, see in Table I X
fructose mannose arabinose 0 1 -- 3 6 0 0 0 0 0
-
1 6-
0 0 0 0 1-
2 1 0 0-
0 0 -- 1 6 - 0 0 0 0 0 2 2 0 0 0 0 0-
1.5 - --
- glucose fructose mannose galactose arabinose malt extract glucose fructose mannose arabinose-
glucose fructose mannose arabinose glucose 3 2 0 0 - 0 0 0 fructose1
::I
3 3-
0-
0 - -- galactose* 0 5 0 0 0- -
-
x y lose 0.61
4.1 0 0 - ---
- 2 1 0 9 2 0 2 0 2 0 0.9 3. 5 1 9 2 4 1. 1 1.1 0 8 0 7 0.9Studies on the Pentose Isomerases of Lactic Acid Bacteria 17
IV.
Effect of Metals on Isomerase ~ r o d u c t i o n ( ~ ~ ) 1. Introduction.D-Xylose and L -ar abinose isomerases from heterofermenter s were ascertained to require manganese ions for their activities and stabilities(67). Among thirty inhibitors including chelating, oxidizing, reducing and sulfhydryl agents, activities of partially purified preparations of isomerases (ammonium sulfate 0.6 to 0.95 sat. fraction) were inhibited only by EDTA, and they were recovered by manganese ions. Activities were fully restored by the addition of manganese ions after en- zymes were inactivated by dialysis with EDTA M), so it could be confirmed that both pentose isomerases required manganese ions for their activities.
- .-
M n S 0 4 In medium ( M
Fig 5 Effect of MnSO, on Bacterial Growth (L breuzr)
Cultural medium used in our previous studies contained MnS04 a t only 0.001 per cent. This quantity of manganese ions was sufficient for bacterial growth (Fig. 5), but it seemed to be reasonable that the production of pentose isomerases would be promoted in the medium containing manganese ions in higher concentra- tion. So the effects of metals, especially of manganese ions on enzyme production were examined in cultural conditions.
2. Methods.
Mzcroorganismr Lactobacillus breuis and L. gayonii were used for the production of D- xylose isomer ase and L-ar abinose isomerase, respectively.
Culture Original medium was designed as "complete medium" and a medium from which inorganic salts were omitted is expressed a s "basal medium" in this chapter. Transfer was made from an agar stab into 8 ml of basal medium in a tube and the tube was incubated for 24 hours a t 37°C. T h e whole inoculum was transfered to 400 ml of the same basal medium with the addition of metallic salts and pentose solutions sterilized separately. As carbon source, one per cent of D-xylose was used for D-xylose isomerase production, and a mixture of 0.5 per cent each of L-arabinose and D-glucose for L-arabinose isomerase production. After incubation for 16 hours a t 37"C, cells were harvested and crude cell-free extracts were obtain-
ed by alumina-grinding. Activities were measured with or without adding MnS04 a t M in final concentration, and specific activity and total units were calculated from the activity with addition of MnS04. The amounts of protein used in each experiments from different cultural conditions ranged from 50 to 500 pg according to their activities.
Table XIII. Effect of Metallic Ions on D-Xylose Isomerase Production. Table XI1 Effect of Salt on D-Xylose Isomerase Production.
Li,S04 KzS04 %SO4 CaCl, Sr Cl, BaCl, MnSO, FeSO, Fe(NH4)2*(S04)2 Fe(SO4)3 Fe NH4(SO4), CoCI, NiCl, c u s o , ZnSO, CdSO, None MnSO,(G)* MgSO,(G)* Metal added (%) MgSO, (0 02) MnS0, (0.001) FeSO, (0.001) MnSO, (0.001)
+
FeS0, (0.001) MgSO, (0 02)+
FeSO, (0 001) Complete Basal Metal added ( 1 0 m 3 ~ ) D-Xylose consumed in medium (%) 28 0 21.7 19.9 21.3 37.7 26 6 79 8 40.5 43.3 52. 7 25 5 59 8 36 71
3 4 4 5.8 0 25 8 24. 7** 23. 7** Total protein (mg) 19 6 45.3 7. 3 39 0 19.4 51.7 9.9 Cell growth ( 0 , D,,530) Cell growth ( 0 D ,,,) 0. 41 0.80 0 35 O 79 O' 48 0.86 0. 35*
Carbon source, glucose.**
glucose consumed (%). D - X ~ l O s e consumed in medium (%) 19 9 71 6 17 4 76 7 40. 3 87.3 25.8D-X ylose isomerase formed Xylulose pmole/ml Total protein (mg) 1.6 14 5 0 1 17 1 1 5 19.9 1.7
D-X ylose isomerase formed Xylulose pmole/ml
extracts Specific Total -Mn
I
+MnI
activityI
units 3. 5 19.3 2.0 27.5 6 0 20. 1 3.8 1 0 5 4 0.6 6 4 1.3 4.1 1.0 19 8 246 1 4 7 249.9 25 7 213.1 9.6Studies on the Pentose Isomerases of Lactic Acid Bacteria
3. Effect of Metallic Ions on D-Xylose Isomerase Production.
D-Xylose isomerase of L. brevis was easily produced in the complete medium after 16 hours growth, thereafter diminished rapidly and disappeared after 40 hours' incubation@'). In this paper, therefore, the cultures grown for 16 hours were used for all the experiments.
Among the inorganic components in the complete medium, only manganese ions showed remarkable acceleration effect both on bacterial growth and on D-
Table XIV Effect of Concentration of MnSO, on D-Xylose Isomerase Production D-Xylose
(final, M)
Table X V Effect of Metallic Ions on L-Arabinose Isomerase Production : , " : , " ! n (mg) Metal added (final, 10-301) Li,SO, K,SO, MgSO, CaCle Sr Cl, BaC1, MnSO, FeSO, Fe(NH,),.(SO,), Fe2(S0,)3 FeNH4(S04)2 CoCl, NiC1, CuSO, ZnSO, CdSO, None
D-Xylose isomerase formed Xylulose r m a / m l Specific Total extracts
-1
I
-Mn,
+ M n activity units (mg) 8 3 10 3 9 6 14. 2 13.0 7 8 41 9 20 2 26 8 24 9 13 7 21 9 9 9 3 6-
- 11 8 Cell growth ( 0 D 530) 0 30 0 33 0 35 0 34 0 35 0 36 0 71 0 60 0 54 0 71 0 44 0 54 0 29 0 26 0 11 0 11 MnSO,(G)* MgSO,(G)* -*
Carbon source,L-Arabinose isomerase formed Ribulose pmole/rnl Total units -Mn
I
t M n:'
ii
5.6 0 22 3 3 glucose only 1 1 7 6 1 2 0.6 3 7 1 1 1 6 9. 7 1. 7 extractsI
T--
5 1 20.9 3 6 1.4 1 9 1 8 2 0 1.2 16 2 6 1 3 8 3 7 1 5 8 2 1 1 1 1 --
2 4 0 8 1 2 0 9 0 8 0.8 4 2 2 2 1 0 1 4 0 7 2 7 0 7 1 1-
- 1 1 1 9 2 8 2 9 2.5 1 4 21 0 7.8 4 3 4 7 1 8 8 9 1 6 2 4-
-
3 0 7 8 11 5 12 2 10 4 5 9 173 3 44 7 28 9 35 6 10 0 59 0 6 9 3 9-
-
13 2xylose isomerase production, and supplement with magnesium or ferrous ions of Mn-containing (0.001 per cent) basal medium showed neither acceleration nor in- hibitory effects. Enzyme was scarecely produced by the sole additions of ferrous (0.001 per cent) or magnesium (0.02 per cent) ions. These results were shown in Table XII. Among fourteen cations M), addition of manganese ions was the most effective not only for D-xylose isomerase production but also for the bacterial growth and D-xylose utilization, and other cations were almost negative for bacte- rial growth and for enzyme production (Table XIII) Effective concentration of man- ganese ions in the medium for isomerase production was found to be 5 x l o e 3 M (Table XIV). Specific activity of D-xylose isomerase was found to be about ten times stronger in the Mnf+-medium (5
x
M in Table XIV) than in the basal one, and total enzyme activity in the former was also thirty-six times greater than in the latter. Accelerating effect on bacterial growth by manganese ions, however, was less marked (3,6 times greater in Mn"-medium (5x
M) than in the basal one). Therefore, manganese ions were more effective for the enzyme production than for the bacterial growth. Manganese ions were seemed to be necessary more about one hundred times than that in the original medium for the maximal enzyme production.4. Effect of Metallic Ions on L-Arabinose Isomerase Production.
Similar results were also obtained on ~ . a r a b i n o s e isomerase production (Table XV). In this case, manganese ions were more effective for the enzyme production than for the bacterial growth, and cobaltous ions showed a little effect. Optimum concentration of manganese ions for producing L-arabinose isomerase of L. gayonii was found to be 1 0 - ~ - 5 x M (Table XVI).
Table XVI. Effect of Concentration of MnSO, on L-Arabinose Isomerase Production.
-f- MnSO, (final, M)
1
L-Ar abinose isomerase formed Cell growth ( 0 D 580) Ribulose pmole/ml - a c t1
Specific1
Total - M ~I
+ M ~ unitsg,":,"!n
(mg) 5. Summary.Lactobac~llus brevis and L. gayonii required manganese ions specifically for their growth. Production of pentose isomerases were greatly increased in pentose me- dium containing MnS04 a t 5 x M. This effect by manganese ions confirmed for the isomerase production than for the bacterial growth.
Studies on the Pentose Isomerases of Lactic Acid Bacteria
V.
Purification and Properties of Pentose Is~rnerase(~')1. Preparation of Isomerases.
Culture According to the results of chapter IV, yields of the pentose isomerases will be able to increase from the pentose-medium containing (1-5)
x
M of MnS04 than the previous method. Cells were obtained from this modified medium (MnS04 0.05%) after 16 hours' incubation, a t which time the enzyme activity was the highest@'). d-Xylose isomerase was obtained from the cells of L. brevzs grown on1 % D-xylose and 0 1 % glucose, and L-arabinose isomerase from the cells of L.
gayonzi grown on 0 5 % each of L-arabinose and glucose.
Pre,baration of Enzymgs. Partial purification of isomerases was carried out as follows ; Intact cells (about 8 g / 3 ~ ) was ground by alumina, and enzymes extracted by 0.05 M NaHC03 (crude extracts). One twentyth volume of 1 M-MnCiz was added to the extracts a t pH 7.0. After removed Mn-precipitates by centrifugation (Mn-treated fraction), ammonium sulfate was added, and precipitate a t 0.6 to 0 9 saturation was dissolved in 0.05 M Tris buffer (pH 7.4 for D-xylose isomerase and pH 8 0 for L-arabinose isomerase) and dialyzed against 0.0025 M T r i s buffer (pH 7.4) a t 6°C for 40 hours (second ammonium sulfate fraction). PH was adjusted to 4.8 to 5.0 with 0.05 M acetic acid, and acetone (-20°C) was added a t
-
10°C with stirring and precipitates were collected a t acetone concentrations of 0-40% and 40-60% (acetone fractions I and 11).2. Chromatography on DEAE-Cellulose.
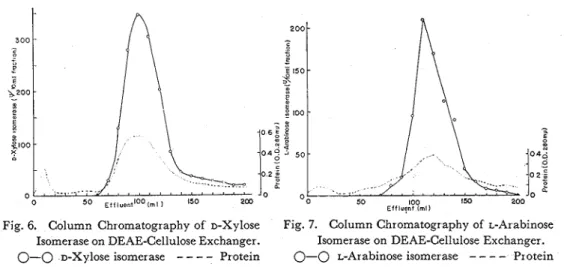
Further purification was made by chromatography on DEAE -cellulose column
(42). Enzyme (acetone fraction 11) was applied onto a column (10-14
x
0.8 cm) ofDEAE-cellulose (about 5 g) in which was equilibrated with the starting buffer. A gradient elution with KC1 was carried out a t a constant pH. Starting buffer is 0.025 M in respect to T r i s concentration and KC1 was gradually increased up to 0.2 or 0.4 M. T h e flow rate was regulated a t 0.5 ml per minutes, and effluent fractions of 20 ml were collected. D-Xylose isomerase ( 1 6 0 0 ~ ) was chromatographed a t pH
Fig. 6. Column Chromatography of D-Xylose Fig. 7. Column Chromatography of L-Arabinose Isomer ase on DEAE-Cellulose Exchanger. Isomerase on DEAE-Cellulose Exchanger.
Table XVII Preparation of Pentose Isomerase A D-Xylose isomerase (L. brevzr)
Volume Protein D-Xylose isomerase
Fraction
1
(mi)1
(mg)1
Specific ~ o t a l1
Yield ( % I activity units 1Crude extracts Mn-treated
Ammonium sulfate Ot6-0 9 sat ppt
/
10 61
541
33 4 1805/
51 0 Acetone 40-60% pptDEAE-Cellulose chromatography 1060
B L-Arabinose isomerase (L gayonii)
--
Fraction
Crude extracts Mn-treated
Ammonium sulfate 0 6-0.9 sat ppt. Acetone 4 0 6 0 % ppt
DEAE-Cellulose chromatography
Wavelength (my)
Fig. 8. UV-Spectrum of Purified Pentose Isomerases X. D - X ~ ~ O S ~ isomer ase, A. L-Ar abinose isomer ase
otein (mg)
7.0, eluted with KC1 0-0.2 M and recovered 88 per cent of the original activity. L-Arabinose isomerase (910u) was applied a t pH 8.0, eluted with KC1 0-0.4 M and 85 per cent of the original activity was recovered. Typical chromatograms a r e shown in Figs.. 6 and 7, and perparation of enzymes were summerized in Table XVII. These enzyme preparations exhibited maximal absorption spectra a t 280 m,a (Fig. 8)) and were used mainly in this experiment.
3. Properties.
O$timum Temperature The activities of both isomerases were attained maximum a t
60°C with addition of M of MnS04 in ten minutes' incubation (Fig. 9).
Optzmum p H Optimum pH for activity a t 37°C was found to be a t pH 5.8-7.0 and pH 7.8-8.0 for D-xylose and L-arabinose isomerases, respectively (Fig. 10).
L-Ar abinose isomer ase Specific Total activity units
Yield
Studies on the Pentose Isomerases of Lactic Acid Bacteria
~emperature( 'C ) Temperature (OC)
Fig. 9. Temperature-Activity Curve. A D-Xylose isomerase, B. L-Arabinose isomerase
0-0
Without M n + + 9-9 With M n t t ( 1 0 - 3 ~ ) Reaction for 10 min.P
H pHFig 10. pH-Activity Curve
A. D-Xylose isomerase (Specific activity 52 5, protein 22 pg/3ml) B L-Arabinose isomerase (Specific activity 42 0, protein 25pg/3ml)
Reaction a t 37°C for 10 min.
Optimum pHfor Enzyme Stabiliiy Effects of pH on enzyme stability were examined
by incubating the enzyme a t various pH's a t 20°C for 150 minutes. After adjust- ing pH's to 7.0 or 8.0 for D-xylose isomerase or L-arabinose isomerase respectively, remaining activities were determined and expressed a s per cent of original activity. D-Xylose isomerase was most stable a t pH 7.0-7.4 and L-arabinose isomerase a t pH 8.2 (Fig. 11).
P H pH
Fig 11 PH-Stability Curve
A. D-Xylose isomerase (Specific activity 58 4, protein 16 5pg/3ml) B. L-Arabinose isomerase Specific activity 42 0, protein 25pg/3ml)
Incubated at 20°C for 150min.
0-0
Tris buffer, $)-@ McIlvain buffer.GIucose
I
0I
0Table XVIII Substrate Specificity on Pentose Isomerases --
Isomerase
1
D-Xylose isomerase1
i-Arabinose isomeraseMannose
I
0I
0 Specific activity Galactose Mannitol Sor bit01 Dulcitol1
0I
0 78 2 132.0Protein used (pg/tube)
I
15 5 13 0Substrate (20 pmoles)
1
Ketose formed (or decreased) in 10 min at 37.C (pg) D-Xylose D-Xy lulose L-Xylose 156 - 165 0 0 0 0Studies on the Pentose Isomerases of Lactic Acid Bacteria 25
Substlate Specificity These enzyme preparations showed high specificities on the respective substrates. Except corresponding aldopentoses and their ketoses, no other ketoses were produced from the pentoses, hexoses or sugar alcohols in this reaction condition (Table XVIII).
Metal Requirement D-Xylose isomerases from Pseud. hydr~phila(~~),
L.
pento~us(~~) and from Pastsurella pe~tis(~') were reported to require manganese or magnesium ions for their activities, but magnesium ions were less effective than manganese ions. Activity of D-arabinose isomerase from E. coli BaI5 was accelerated by the addition of manganese ions(47) and the activities of L-arabinose isomerase from L. p~ntosus(~~) and L, gayonii(67) were restored by manganese, cobaltous or calcium ions after their inactivation by dialysis with EDTA. However, these enzyme preparations were seemed to be still low in purity, so it was not certain which metal activated the pentose isomerases. After dialysis of these purified enzymes in the presence of 2.5-5 x M of EDTA against 0.025 M of Tris buffer (pH 7.0) a t 5°C for 20 hours, the enzymes were fully inactivated, and their activities were recovered after addi- tion of MnS04. Additions of cobaltous, magnesium, strontium, calcium, barium, ferric or zinc ions showed only little recovery of activities (Table XIX). So it could be concluded that both pentose isomerases are the Mn++ -catalyzed enzyme.Table XIX Requirement of Metal
-
-Metal
1
D-Xylose isomer ase Xylulose formed (rg/3 ml)1
Ribulose formed (pg /3ml) L-Ar abinose isomer ase(Final, M)
I
0 10-51
0 lo+ lo-' 5 x 10-3*
Before dialysis without addition of EDTA.4. Summary.
D-Xylose and L-arabinose isomerases were purified by column chromatography on DEAE-celluIose, and some chemicaI properties of pentose isomerases are as follows :
D-Xylose isomer ase L- Arabinose isomer ase
Temperature optimum (OC) 6 0 6 0
pH optimum 5.8-7.0 7.8-8.0
pH for stability 7.0-7.4 8.2
VI. Separation of D-Xylose and L-Arabinose is om erase^(^^) 1. Introduction.
While homofermenters showed either one of the pentose isomerase activities, some of heterofermenters exhibit both isomerase activities in the same preparation a s shown in Tables V and IX. The structures of D-xylose and L-arabinose a r e different the OH-configuration only a t C-4 of these molecules, but it has not been determined whether the enzymes acting on these pentoses were different or not. From the evidences on their pH optima and distributions in many strains, i t was probably assumed that two enzymes would be different from each other. In this chapter, methods for separation of them are presented.
2. Preparation of Enzymes.
Acetone fraction I1 and purified preparations with chromatography on DEAE- cellulose which reported in chapter V were used.. T o obtaining both isomerase activities in a same preparation, cells of Lactobaczllus gayonii which had been grown in a medium containing each 0.5 % of D-xylose and glucose and 0.1 % of L-arabinose were used. This bacterium produced L-arabinose isomerase even in the glucose- or ~ ~ - x y l o s e - m e d i a ( ~ ~ ) , and enzyme were purified to second ammonium sulfate fraction a s same a s by the method reported in chapter V.
3. Heat Treatment with Manganese Ions.
D-Xylose isomerase was less stable than L-arabinose isomerase a t high tem- p e r a t ~ r e @ ~ ) . As shown in Table XX, when a n enzyme solution (ammonium sulfate fraction from the cells of L. gayonii grown on a mixtures of D-xylose, L-arabinose and glucose) was treated a t 60°C for ten minutes in the presence or absence of 1 0 , - 3 ~ of MnS04, the activity of L-arabinose isomerase remained without loss of activity in the presence of manganese ions, while D-xylose isomerase was com- pletely inactivated by these treatments.
50°C, 5 min
/
-Mn* f Mn** 34 1 3 9 2 54 2 77 31
1 : 1 5 9 1 : 1 9 7 Table XX. Heat Treatment of Pentose Isomerases.-Mn* 0 34 1
-
6OoC, 10 min f Mn** 0 74 7
1
-
*
IO-$M of MnSO, added for activity measurement Heat treatment**
1 0 - 3 ~ of'MnSO4 added before heat treatment4. Fractionation by Acetone at Various pH's. Isomerase remained (u/ml) D-Xylose L-Arabinose
The second ammonium sulfate fraction containing both isomerases was frac- tionated by acetone of 0,-,40% and 40-,60% a t pH 4.8-5.4 (Table XXI). While L-arabinose isomerase was easily precipitated by 40% acetone a t pH 5.0, D-xylose
Ratio of isomerase activities (Xylose isomerase :
- isomer ase isomerase ar abinose isomer ase)
Studies on the Pentose Isomerases of' Lactic Acid Bacteria 27
Table X X I Separation of Pentose Isomerase by Acetone
isomerase was mainly remained in solution and could be precipitated by further addition of acetone to 60%. However, both isomerases could not be separated completely from each other by this method.
5. Separation by Chromatography on DEAE-Cellulose Column.
Conditions for column chromatography on DEAE-cellulose were examined in Initial
pH
E f f l u e n t ( m i
f
Fig 12 Column Chromatogaraphies of D-XyIose Isomerase on DEAE-Cellulose Exchanger
-
D-Xylose isomerase,-
--
- ProteinColumn 0 8 x 9 0 cm; Gradient elution of KC1 0 to 0 4 M at pH 7 0 @--g, pH 7 4 0-0,
and pH 8 0
A-a
5 4
1
40-60%1
1 1 21
48.1 5401
55 5 62 1L-Ar abinose isomerase Specific Total activity unit Fraction
I
Proteinby acetone
/
(mg)D-Xylose isomer ase Specific Total activity unit
E f f l u e n t ( m i )
Fig. 13 Column Chromatographies of L-4rabinose Isomerase on DEAE-Cellulose Exchanger. -- L-Arabinose isomerase, -
- - -
Protein See in Fig 12order to separate the two enzymes completely. In preliminary experiments, chro- matographies were run a t pH 7.0, 7.4 and 8.0 with the gradient elution of KC1 0 to 0.4 M. Elution patterns of two isomerases are summarized in Figs. 12 and 13.
D-Xylose isomerase could be eluted faster a t a lower pH under these condi- tions, but L-arabinose isomerase was always found in the same fraction. When two enzymes were chromatographed a t pH 7.4, D-xylose and L-arabinose isomerases were found in the first and second peaks, respectively, but the separation of both
I&-0 0 2 5 u Tr!s outter ( p H 7 0 ) 1~.0025MTnsbuffer(pH80)+ K C I 0 - o 2~ gradient K C I 0 1 0 4 u g r a d l e n t
Effluent ( m i )
Fig 14. Separation of Pentose Isomerases on DEAE-Cellulose Column
Studies on the Pentose Isomerases of Lactic Acid Bacteria 29
enzymes was not complete with oveilapping of peaks. Therefore, a method which combined two steps of gradient elution was established. An enzyme preparation containing both isomerases was adsorbed on a column a t pH 7.0, and then eluted with T r i s buffer a t pH 7.0 with a gradient of KC1 from 0 to 0 2 M (total eluate 150 ml). D-Xylose isomer ase was recovered but L-ar abinose isomer ase was not eluted in this first elution After column was washed with 10 ml of 0.025 M Tris buffer a t pH 8.0 containing KC1 0.1 M, second gradient elution was begun a t pH 8.0 with a gradient of KC1 0-1 to 0.4 M. Then L-arabinose isomerase was found in the second peak without contamination of D-xylose isomerase (Fig. 14). Recoveries were found to be 80.3 and 76 7% for D-xylose and L-arabinose isomerases, respect- ively. Then it can be concluded that both pentose isomerases are quite separate enzymes regardless the similarity of their reaction sequences
6. Summary.
D-Xylose isomerase and L-arabinose isomerase are different enzymes which can be separated from each other with acetone fractionation a t pH 4.8,-5.0, heat treatment or chromatography on a column of DEAE-cellulose.
PART 11. STUDIES ON THE GLUCOSE ISOMERASE
I. Production
of
D-Glucose Isomerase from Heterolactic AcidBacteria(70)
The conversion of D-glucose to D-fructose by bacteria was first demonstrated by MARSHALL and KOOI in the cells or their sonic extracts of Pseudomonas hydrofhila which were grown on D-xylose, and its activity was found to be required arsenate in the reaction system(33). Recently, TSUMURA and SATO obtained similar results with D-xylose-grown cells of Aerobacter c l ~ a c a e ( ~ ~ - ~ ~ ) . Similar activity was also found in Eschrichia freundii and E. intermediae by NATAKE and Y O S I M U R A ( ~ ~ , ~ ~ ) The glucose isomerizing activity of these bacteria were reported to be required arsenate in the reaction system. These facts suggested that the glucose isomerizing activity was found only with araenate, however, arsenate was not required for D-xylose iso- merase. But little is known about the relations between the D-xylose isomerase and the glucose isomerizing activity.
As the glucose isomerizing activity has not been detected in the D-xylose iso- merase preparations from lactic acid
,
fu rther studies have been made on this problem, and the glucose isomerizing activity was found in the cell- free extracts of ten strains of heterolactic acid bacteria grown on D-xylose. After purified it to 8 fold in specific activity, i t was named a s the "D-glucose isomerase" which was able to convert D-glucose to D-fructose and vice-versa without arsenate. Fructose was identified by paper chromatography after isolated a s calcium-fructose complex. No other ketoses or reducing sugars could not be detected by paper chromatography in this reaction mixture.In this chapter, the production of D-glucose and D-xylose isomerases from heter ofer menter s was discussed.
2. Methods.
Culture The complete medium was composed of each one per cent peptone, yeast extract and sodium acetate, and 0.1 per cent MnS04. 4H20, 0..02 per cent MgS04. 7H20 and 0.001 per cents NaCl and Peso4. 7H20. The medium which the addition of inorganic salts was omitted is expressed as the basal medium.
Microorgansims were cultured a t 37°C for sixteen hours and cell-free extracts were prepared by the method noted in previous chapters.
Activity Measurement Activity of D-glucose isomerase was determined as follows: The incubation system was composed of 0.5 ml of 0.05 M Tris buffer a t pH 7.0, 0.3 ml of lo'-' M MnS04, enzyme solution and 1.0 ml of 2 M D-glucose solution in total volume of 3.0 ml. After incubation a t 40°C for 10 minutes, glucose solution was added and reaction proceeded a t 50°C for thirty minutes. Fructose was determined by cysteine-carbazole method a t 40°C for thirty minutes. Definition of enzyme unit is same as that of D-xylose isomerase under these conditions.
3. Effects
of
Cultural Conditions and Carbon Sources.As already noted, the maximum production of D-xylose isomerase of Lactoba-
cillus brevip was obtained after 16 hours' incubation, D-glucose isomerase was also
Cultuml t i m e ( h r )
Fig 15 Cultural Hours on the Production of Isomerases
0-0
D-Xylose isomerase, 0--@ D-Clucose isomerase, - - - - Cell growth (0 D at 530 mp) L brevzr , 0 4 L mediumTable XXII. Eff'ect of Cultural Condition..
I
CellI
XyloseI
ExtractsI
D-Xylose isomerase/
D-Glucose isomerase Total Specific Total activity unit activity unit Static1
0 851
84 41
34 91
10 2 3571
0 7 18 5 Shaking1 0 5 0/
5 2 3 1 2 , 61
1 0 2 128 0 , 2 1Studies on the Pentose Isomerases of Lactic Acid Bacteria 3 1
Table XXIII Effects on Concentration of Xylose and Glucose Carbon source ( % ) Sugar consumed (%)
I
-D-Xylose Glucose D-Xylose Glucose
L breuir, 0..4 L medium.
produced by this bacterium from D-xylose-medium after 16 to 20 hours growth, and disappeared after 40 hours (Fig. 15), and amounts of two isomerases were nearly proportional a t every cultural stage. Yield of enzyme was decreased in shaking culture (Table XXII). D-Xylose was found to be the only active carbon source for the productions of D-glucose isomerase and of D-xylose isomerase, but D-glucose, D-fructose, and their mixture, mannose, galactose, L-arabinose, maltose and sucrose were fully ineffective. D-Xylose was needed in 0.5 to 1.0% for this enzyme production, and addition of 0.1 to 0.5% of glucose to xylose..medium accel- erated this enzyme production (Table XXIII).
4. Effects of Metallic Ions.
For bacterial growth and for the production of D-xylose isomerase, manganese ions were established to be essential in the cultural medium(74). Manganese ions
Table XXIV. Effect of Metallic Ions..
- -None 0 5 2 3 1 0 2 9 90 0 1 3 MgSO4 0 55 I 3 4 5
1
3 6 0 2 5 MnS04 1 11 I 157 2 7 5 1180 156 CoCI, 0 53 33 4 3 5 115 0 6 17 FeSO, 0 60 37 7 4 2 156 1 0 1 2 FedS04)3 0.87 45.0,
3.2 1451
0.2 8L breuzr, 0 8 L of basal medium, Carbon source, 1 % D-xylose t 0 1 % glucose ~~~~l added
(final, 1 0 - 3 ~ )
I
cell growth Extracts
1
D - x ~ l O s e isomerase proteinSpecific Total
1
activity UD-Glucose isomer ase Specific Total activity U