Vol. 19, No. 1 (1967)
O N T H E OXIDATION-REDUCTION POTENTIAL O F T H E
AQUEOUS SOLUTIONS O F TANNIN EXTRACTS
Yoshiaki
FUKUI
Few physicochemical studies have been made on the electrical properties of aqueous so- lutions of tannin extracts. PUTNAM et a1 (') and GRASSMANN(') investigated the charge of several tannin extracts and THOMAS et U Z . ( ~ ) measured the potential difference of the tannin particles against the aqueous phase. However, no information have been available as for the oxidation-reduction potential (Eh) of tannin extract. Then, the Eh of tannins were investi- gated and the result obtained was presented in this paper.
Experimental
1. Materzals: Five tannin extracts used in this investigation were two hydrolyzable-type tannins, chestnut and myrabo, and three condensed-type ones, quebracho, gambier, and mimosa. These tannins were obtained through the courtesy of Mr HATTORI, Director of the Hyogo.ken Leather Research Institute, and Mr. KAMBARA, KAWAMATA CO. These tannin extracts were actually the same as industrial tanning materials.
2. Procedures: Tannin solutions were prepared by dissolvir~g 1.00g of tannin extract in lOOml of deionized water and filtered with a filter-paper. The tannin solutions wore titrated with N/10 HCl or N/10 NaOH solution, and then their pH and E h values were measured immediately by a Toa Electric pH meter Model HM-5A. The reading of the meter was recorded, when the potential became stable in a few minutes after the electrode was im- mersed into the titrated solution.
Resullts and Discussion
1 oxidation-reduction potentzal, E h
In general, oxidation-reduction reactions are expressed in the next equations: Red
+
a H " e Ox+
n/2 H2where Red: the reduced form, Ox : the oxidized form, n : the number of electrons, and a:
the number of hydrogen ions transferred per molecule of oxidant or reductant. For this system the oxidation-redution potentials are given by:
R T [Ox) a - n R T E = E ~ + ---- ln In [Hi-] n F [Red) n F 0.06 E = EO
+
--- I O ~ - (OX) -+
0 . 0 6 ( a - n l p H ( a t 3 0 0 ~ ) (lb) n [Red) nOLIVE 香川大学学術情報リポジトリ
46 Tech Bull Fac Agr Kagawa Univ
where
Eo:
standard oxidation-redution potential, R: gas constant, 1,987 cal/deg mole, T:absolute temperature, and F: FARADAY'S constant, 23,074 cal/volt.
When the hydrogen electrode is used as the reference electrode, the E is represented as the Eh :
here 0.06 ( a - n )
Eh=Eo -I-
n pH
From the equation (3), dEro/dpH is
2. Eh-pH plots of tannzn soultzons
The Eh obtained for the titrated tannin solutions was plotted against the corresponding pH values of these solution. The Eh-pH plots for each tannin are shown i n : ~ i ~ s . 1 and 2,
.- quebr acho
-
gambierFig 1 Eh-pH culbes for the condensed-type tannins
-
chestnut*-x- myrabo
Fig 2 Eh-pH curves for the hydrolyzable-type tannins
OLIVE 香川大学学術情報リポジトリ
Vol 19, No 1 (1967) 47
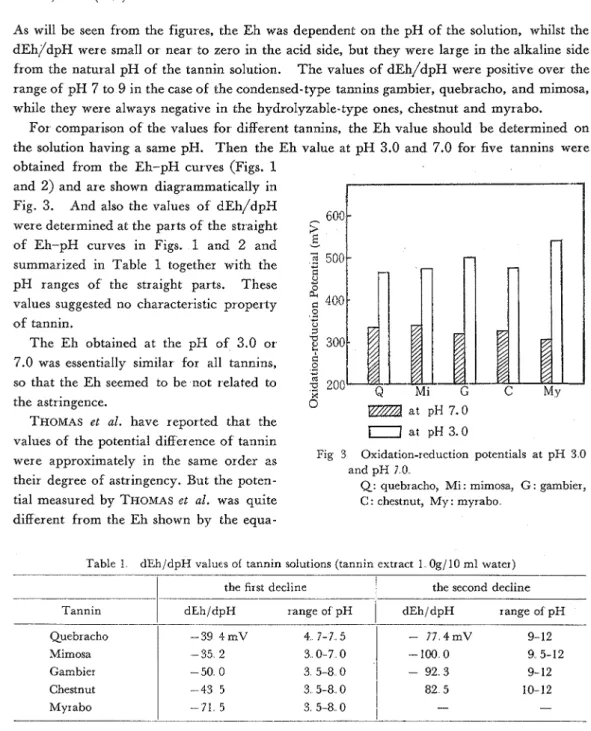
As will be seen from the figures, the Eh was dependent on the pH of' the solution, whilst the d ~ h J / d ~ ~ were small or near to zero in the acid side, but they were large in the alkaline side from the natural pH of the tannin solution. The values of' dEh/dpH were positive over the range of pH 7 to 9 in the case of the condensed-type tannins gambier, quebracho, and mimosa, while they were always negative in the hydrolyzable-type ones, chestnut and myrabo.
For comparison of the values for different tannins, the Eh value should be determined on the solution having a same pH. Then the E h value at pH 3.0 and 7.0 for five tannins were obtained from the Eh-pH curves (Figs. 1
and 2) and are shown diagrammatically in Fig. 3. And also the values of dEh/dpH
were determined at the parts of the straight 603 E of Eh-pH curves in Figs. 1 and 2 and
-
1
500 summarized in Table 1 together with the-
$ pH ranges of the straight parts. These +
values suggested no characteristic property
$
4009
of tannin. *
2
The Eh obtained at the pH of 3.0 or
f
300 7.0 was essentially similar for all tannins,I-'
so that the Eh seemed to be not related to
3
200Mi
the astr ingence.
8
a t pH 7.0 THOMAS e t al. have reported that the
at pH 3.0 values of the votential difference of tannin
Fig 3 Oxidation-reduction potentials at pH 3 0 were approximately in the same order as
and pH 7 0
their degree of astringency. But the poten- Q: quebracho, Mi : mimosa, G : gambier, tial measured by THOMAS e t al. was quite C: chestnut, My: myrabo
different from the Eh shown by the equa-
Table 1 dEh/dpH values of tannin solutions (tannin extract l Og/ 10 ml water)
I
the first decline1
the second decline Tannin1
dEh/dpH range of pHI
dEh/dpH range of pH QuebrachoMimosa Gambier Chestnut Myrabo
tion (3), because the potential arose from the double layers of tannin particles and was calculated by the following formula :
Potential diffkrence = 4,pv (300)'
KX
where , p : viscosity of water, v: velocity of migration, K : dielectric constant of' water, and X: potential drop.
48 Tech. Bull. Fac Agr.. Kagawa Univ
Summary
The oxidation-reduction potentials(Eh) of the aqueous solution of tannin extracts werc investigated. It is evident theoretically that the E h of oxidation-reduction systems involving hydrogen ions is affected by the pH value of the systems. Then the Ehs of five tannin extracts were measured at the different pH values and the Eh-pH plots were made.
The shape of Eh-pH curves showed a similar trend for all tannins. Therefore, the E h value of tannin seemed to be not related to astringency. However, in the range of pH 7 to
9, the dEh/dpH of the condensed-type tannins were positive, showing a sudden rised of the curve, while those of hydrolyzable-type ones were negative.
Moreover it was clarified by the equation that the potential difference measured by
THOMAS et al., which was considered to be related to astringency, was quite different from the oxidation-reduction potential.
(1) PUINAM, R C , BOWLES, A V : J Am Leather (1954)
Chem Ar roc 48, 241, 343 (1953) (3) THOMAS, A W
,
FOSTER, S B : 2nd Eng Chem , (2) GRASSMANN, W , SIADLER, P : Leder, 5, 206 14, 191 (1922)9 Y - 77k&$xDw@4Ly(i'J [&zCi$l:7;;lzpfL { , &jglz~-j-6@fzCiz, Z,q&!; S&.Tl,> zfls, p&jL
BZ'SI!I;. ( ~ h ) I Z T L > T D % ~ ; C A ~ ~ ~ ? : I ; ~ L L > , .F.
r
-c,
9 7- 7 ; r k ~ 3 ~ & ~ ~h IC.-X>IC L I;<?:,. ~h L(i'~cz pH D@fLDR"5$$%.,j V ~ D T , 8 & D pH C Z B U 6 Eh % # & ~ $ J C Z , * ; ; K , P H - E ~ H a f i ~ a L?:, dEh/dpH Ibf ~ - C D 9 2 = 2hsl3ldliilLMr3%, L& L?:.. L,n. L, %&ii!? Y-- ti, dEh/dpE 731 pH 7-9C-hBL>T%.-k.aL?:hr', ~ J L I ~ ~ ~ @ % T C ~ . ' ? D k . # f l ~ b I ; & t L h ~ ~ ? : , fhB, 97-~7k%$ii0> EhIiPi l i + D % & ' E k CdMi%hsl'd:L>k,E.bhQ., Ilr(&KkM&ff6 &L>h;h.6 potential difference fjs: Eh 2 3 9 :?<
B l l D b D T h 6 itr.%,%CZd: 9 - C L& L?:..(Received May 31: 1967)