(注意)この論文には正誤表があります
香川大学農学部学術報告 第45巻第2号 正誤表 URL
http://www.lib.kagawa-u.ac.jp/metadb/up/AN00038339/AN00038339_45_2_e.pdf
Notice
Technical Bulletin of Faculty of Agriculture, Kagawa University Vol.45 No.2 Errata
URL
Tech Bull Fac Agr Kagawa Univ , Vol 45, No 2, 11 1-114, 1993
E F F E C T O F MUCOPOLYSACCHARIDES ON CHONDROITINASE
PRODUCTION AND RAPID ISOLATION O F CHONDROITINASE
Kaoru TAKEGAWA, Kenji IWAHARA and Shojiro IWAHARA
A gram-positive bacterium, strain no C28, produced a high level of extracellular chondroitinase when cells were grown in a medium containing chondroitin sulfate Production of the enzyme was also induced by hyaluronic acid and chondroitin, but not by dermatan sulfate, heparan sulfate and heparin To purifiy the enzyme, the bacterium was first grown in a glucose medium, and then the cells were transferred to an inducing medium containing only chondroitin sulfate T h e culture fluid obtained using the inducing method showed a higher specific enzyme activity than the culture fluid obtained by the growing method The enzyme purified from the enzyme-rich culture fluid obtained by the inducing method was found to be homogeneous after only one purification step
Introduction
In an earlier paper"), we reported that a Gram-positive bacterium, strain no C28, identified as either an Aureobacterzum or a Curtobacterzum, produced a high level of chondroitinase (EC
4 2 2 4 or 4 2 2 5) into the culture medium, and the purification, properties, and substrate specificity of the purified chondroitinase were also described During study of the production of the enzyme, we found that the bacterium highly induced chondroitinase in culture fluid when grown in medium containing chondroitin sulfate In this report, we describe the induction of an extracellular chondroitinase from the bacterium grown on various mucopolysaccharides We also report the rapid purification of the enzyme using the inducing method
Materials and Methods
Chemicals Chondroitin sulfate-A, dermatan sulfate, chondroitin sulfate-C, hyaluronic acid, and authentic 4,5-unsaturated disaccharides were purchased from Seikagaku Kogyo Co Heparin and heparan sulfate were obtained from Sigma Chemical Co DEAE-Toyopearl 650 M was purchased from Tosoh Co , Tokyo Molecular weight markers were obtained from Pharmacia Fine Chemical Co All other chemicals were of analytical grade
Microorganism and cultivation A bacterium, strain No C28 isolated from a soil sample, was used throughout this study(') The maintenance medium comprised 0 5% chondroitin sulfate (Wako Pure Chemical Co ), 0 5% peptone, 0 5% yeast extract, 0 5% NaCl and 2% agar The pH was adjusted to 7 0 This medium was also used for liquid cultures
Analytical methods Chondroitinase activity was assayed with the substrate chondroitin
(2)
sulfate-A as described previously(') Protein was determined by the method of Lowry et a1 ,
which crystalline ovalbumin as the standard Neutral sugars were assayed by means of the phenol-H2SOI rea~tion'~) The reducing power of sugars was measured by the method of
112 Tech Bull Fac Agr Kagawa Univ, Vol 45, No 2, 1993
Nelson and ~ o m o ~ ~ i ' ~ ~ ) Uronic acid was measured by the meta-hydroxydiphenyl method@) with D-glucuronic acid as the standard
Results and Discussion
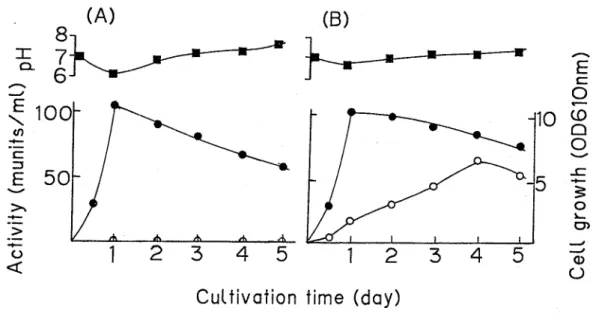
Enzyme production in the supernatant at different stages of growth was investigated when the bacterium was grown in medium with or without 0 5% chondroitin sulfate A The bacterium was cultured in a liquid medium composed of 0 5% peptone, 0 5% yeast extract, 0 5% NaC1, and with or without 0 5% chondroitin sulfate A, pH 7 0 Both culture fluids showed similar profiles for pH and cell growth (Fig 1) High chondroitinase activity was found in the supernatant of a culture grown in chondroitin sulfate medium Maximum enzyme activity was observed after 3 to 4 days On the other, no chondroitinase activity was detected in the culture fluid without chondroitin sulfate
Cultivation time (day)
Fig 1 Growth and enzyme production in cultures of strain No C28
The bacterium was cultured in 500-ml Ehrenmeyer flasks without (A) or with (B) 0 5% chondroitin sulfate at 28°C Symbols : @ , growth;
0
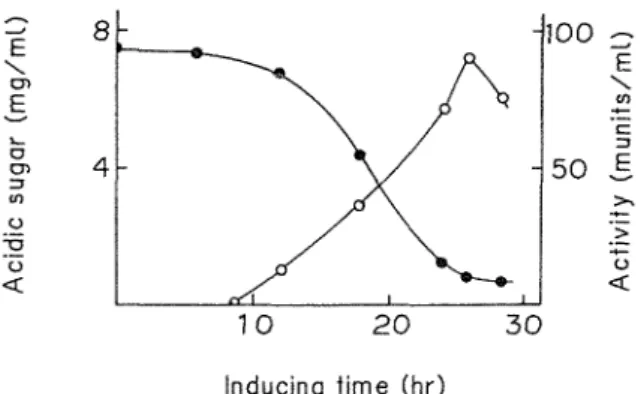
, chondroitinase; W , pHTo investigate further the induction of chondroitinase production by chondroitin sulfate, washed cells of the bacterium grown on glucose medium without chondroitin sulfate A in 10 mM potassium phosphate buffer (pH 7 0) Then the inducing cultivation was carried out with shaking at 28
C ,
and the chondroitinase activity was determined The chondroitinase activity increased in proportion with decreasing amount of chondroitin sulfate A in the medium, although cell growth was not significantly affected (Fig 2) Maximum enzyme activity was observed after 24 to 28 h of inducing cultivationWashed cells of the bacterium were transferred to the media containing various mucopolysaccharides (final 075%), and the enzyme activity in each culture fluid was investigated after 26 h of cultivation Besides the addition of chondroitin sulfate A and C (90
I<. TAKEGAWA et al. : Production of bactrial chondroitinase 113
munits/ ml), the enzyme was induced with the addition of hyaluronic acid (25 munits/ml) and chondroitin (75 munits/ ml). On the other hand, dermatan sulfate, heparin, heparan sulfate did not induce the enzyme. These results showed that the bacterium induced the enzyme when incubated with a medium containing mucopolysaccharides which the purified enzyme was able to degra- de'".
T h e culture fluid obtained using the inducing method described above showed a higher (more than 7-fold) specific activity (0.49 units/mg protein) than the culture fluid obtained by t h e growing method (0.067 units/ mg)"'. Therefore, w e examined the purificat- ion of the enzyme produced by the inducing method. T h e culture fluid (20 ml) was applied to a DEAE-Toyopearl 650 M column (1.5 x 4.5 c m ) equilibra- ted with 10 n1M phosphate buffer (pH 8.2). T h e column was washed with the same buffer and then eluted with a linear gradient of 0 to 0.5 M NaCl in the same buffer (150 ml). T h e active fraction were pooled and lyophilized.
Inducing time (hr)
Fig. 2. Effect of chondroitin sulfate on chondroitinase production Washed cells grown in the culture medium without chondroitin sulfate for 24 h were added to 3 rnl of medium containing 0.75 %
chondroitin sulfate in 10 mM phosphate buffer (pH 7.0). Symbols : @ , acidic sugar; and
0
,chondroitinase.
T h e lyophilized enzyme solution was
A
B
C
dissolved in wat-polyacrylamide rmed on a 10
and to Fig.3. The one step purification of chondroitinase gel, which was perfo- illustrated by SDS-polyacrylamide gal electrophor-
% polyacrylamide gel esis.
containing 0.1 % SDS(~'. T h e purified Samples in lanes are as follows: lane A, molecular chondroitinase migrated a s a single weight markers; lane B, culture fluid of the protein band a t a position correspondi- inducing medium; lane C, chondroitinase after ng to a molecular weight of about DEAE-Toyopearl column chromatography. 83,000 (Fig. 3C). These results showed
that the inducing method a s described in this paper was very effective for the purification of the C28 condroitinase, since the purified enzyme w a s obtained within a few days.
There have been many reports concerning the induction of glycosidases that act on polysaccharides such a s starch, cellulose, mannan, glucan and
hiti in'^'^),
but there are no reports of the induction of mucopolysaccharide lyases. However, chondroitinase-producing bacteria such a s Flavobacterium heparinum, Proteus vulgaris and Arthrobacter aurescence were ali114 Tech Bull Fac Agr Kagawa Univ, Vol 45, No 2, 1993
grown (10.11)
in a medium containing chondroitin sulfate Therefore, t h e s e chondroitinase may b e inducing enzymes T h e induction mechanism of t h e bacterial chondroitinases is currently being investigated
References
(1) TAKEGAWA, K , IWAHARA, K and IWAHARA, S :
Purification and properties of chondroitinase produced by a bacterium isolated from soil J Ferment Bzoeng , 72, 128-131 (1991) (2) LOWRY, 0 H , ROSEBROUGH, N J , FARR, A L,
and RANDALL, R J . Protein measurement with the Folin phenol reagent J Bzol Chem, 193, 265-275 (1951)
(3) Du~ors, M , GILLES, K A , HAMILTON, J K ,
REBERS, P A and SMITH, F : Colorimetric method for determination of sugars and related substances Anal Chem , 28, 350-356 (1956) (4) SOMOGYI, M . Notes on sugar determination J
Bzol Chem , 230, 399-408 (1952)
(5) NELSON, N . A photometric adaptation of the Somogyi method for the determination of glucose J Bzol Chem , 153, 375-380 (1944) (6) BLUMENKRANZ, N and ASOBE-KANSEN, G : New
method for quantitative determination of uronic acids Anal Bzochem , 54, 484-489 (1973) (7) LAEMMLI, U K . Cleavage of structural proteins
during the assembly of the head of bacteriopha- ge T4 Nature, 227, 680-685 (1970)
(8) MANN, J W , JEFFRIES, T W and MACMILLAN,
J D : Production and ecological significance of yeast cell wall-degrading enzymes from Oersk- ovia Appl Envzron Mzcrobzol , 251, 2005- 2014 (1976)
(9) 'TANAKA, H , ITAKURA, K and 'TODA, K :
Concerted induction of P -glucanases of Baczllus czrculans WL,12 in responce to various yeast glucans Agrzc Bzol Chem , 42, 1631 -
1636 (1978)
(10) YAMAGATA, T , SAITO, H , HABUCHI, 0 and SUZUKI, S . Purification and properties of bacterial chondroitinase and chondrosulfatases J Bzol Chem , 243, 1523- 1535 (1968)
(ll) HIYAMA, K and OKADA, S : Crystallization and some propelties of chondroitinase from Arthr- obacter aurercens I Bzol Chem , 250, 1824- 1828 (1975)